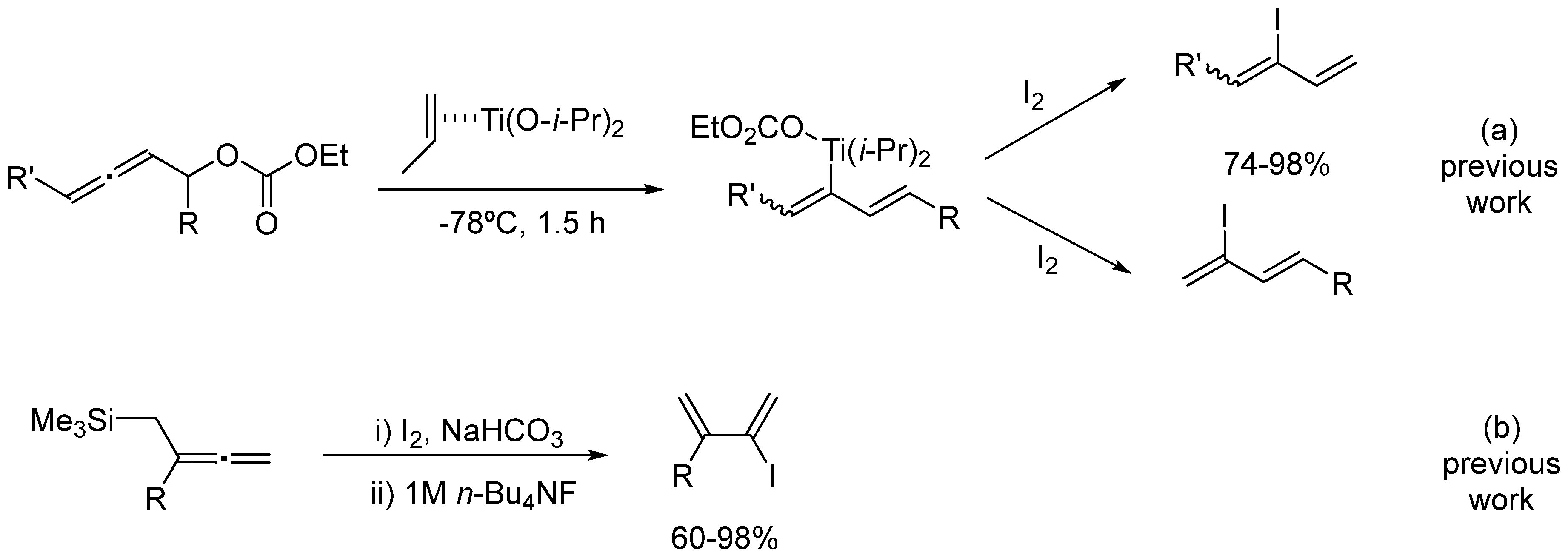
Zn–Catalyzed Direct Synthesis of 3-Iodo-1,3-dienes from α-Allenols †
Abstract
:1. Introduction
2. Experimental Section
2.1. General Procedure for the Preparation of α-Allenols 2a–h
2.2. General Procedure for the Preparation of 3-Iodo-1,3-dienes 3a–h
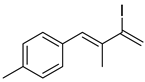
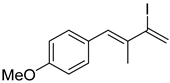
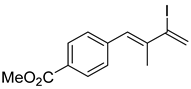

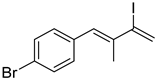
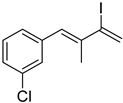
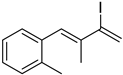
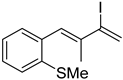
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Schuster, H.F.; Coppola, G.M. Allenes in Organic Synthesis; John Wiley & Sons: New York, NY, USA, 1984. [Google Scholar]
- Patai, S. The Chemistry of Ketenes, Allenes and Relates Compounds Part 1; John Wiley & Sons: New York, NY, USA, 1980. [Google Scholar]
- Alcaide, B.; Almendros, P. Progress in allene chemistry. Chem. Soc. Rev. 2014, 43, 2886–2887. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Shirley, H.J.; Aitken, H.R.; Schulte, T.; Shöhnel, T.; Hume, P.A.; Brimble, M.A.; Furkert, D.P. Intermolecular Diels–Alder Cycloaddition/Cross-Coupling Sequences of 2-Bromo-1, 3-butadienes. Org. Lett. 2020, 22, 1022–1027. [Google Scholar] [CrossRef] [PubMed]
- Clayden, J.; Greeves, N.; Warren, S. “Organometallic Chemistry” Organic Chemistry, 2nd ed.; Oxford University Press Inc.: New York, NY, USA; Oxford, UK, 2012. [Google Scholar]
- Coleman, R.S.; Walczak, M.C. Tandem Stille/Suzuki− Miyaura coupling of a hetero-bis-metalated diene. rapid, one-pot assembly of polyene systems. Org. Lett. 2005, 7, 2289–2291. [Google Scholar] [CrossRef] [PubMed]
- Bradford, T.A.; Payne, A.D.; Willis, A.C.; Paddon-Row, M.N.; Sherburn, M.S. Cross-coupling for cross-conjugation: Practical synthesis and Diels− Alder reactions of [3] dendralenes. Org. Lett. 2007, 9, 4861–4864. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Meng, B.; Huang, X.J. Halohydroxylation of 1-cyclopropylallenes: An efficient and stereoselective method for the preparation of multisubstituted olefins. Org. Chem. 2008, 73, 6895–6898. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, T.; Esumi, T.; Iwabuchi, Y.; Irie, H.; Hatakeyama, S. Preparation of 2-Iodo-1, 3-butadienes from 1-Trimethylsilyl-2, 3-butadienes and their Functionalizations. Tetrahedron Lett. 1998, 39, 43–46. [Google Scholar] [CrossRef]
- Froese, J.; Reed, J.; Hudlicky, T. Palladium-catalyzed carbonylation of halo arene-cis-dihydrodiols to the corresponding carboxylates. Access to compounds unavailable by toluene dioxygenase-mediated dihydroxylation of the corresponding benzoate esters. Org. Biomol. Chem. 2014, 12, 7810–7819. [Google Scholar] [CrossRef] [PubMed]
- Wade, L.C. Química Orgánica, 5th ed.; Pearson Educación, S.A.: Madrid, Spain, 2004. [Google Scholar]
- Grob, C.A.; Spaar, R. Mesomeric Vinyl Cations. Part II. Solvolysis of 2-bromo-1, 3-butadienes. Helvetica Chim. Acta 1970, 53, 2119–2129. [Google Scholar] [CrossRef]
- Okamoto, S.; Sato, H.; Sato, F. Highly efficient synthesis of alka-1, 3-dien-2-yltitanium compounds from alka-2, 3-dienyl carbonates. A new, practical synthesis of 1, 3-dienes and 2-iodo-1, 3-dienes. Tetrahedron Lett. 1996, 37, 8865–8868. [Google Scholar] [CrossRef]
- Alcaide, B.; Almendros, P.; Aragoncillo, C.; Redondo, M.C. Stereoselective Synthesis of 1,2,3-Trisubstituted 1,3-Dienes through Novel [3,3]-Sigmatropic Rearrangements in α-Allenic Methanesulfonates: Application to the Preparation of Fused Tricyclic Systems by Tandem Rearrangement/Diels−Alder Reaction. Eur. J. Org. Chem. 2005, 98–106. [Google Scholar] [CrossRef]
- Alcaide, B.; Almendros, P.; Rodríguez-Acebes, R. Efficient entry to diversely functionalized spirocyclic oxindoles from isatins through carbonyl-addition/cyclization reaction sequences. J. Org. Chem. 2006, 71, 2346–2351. [Google Scholar] [CrossRef] [PubMed]
- Isaac, M.B.; Chan, T.-H. Indium-mediated coupling of aldehydes with prop-2-ynyl bromides in aqueous media. J. Chem. Soc. Chem Commun. 1995, 1003. [Google Scholar] [CrossRef]
- Chou, T.-H.; Yu, B.-H.; Chein, R.-J. ZnI2/Zn (OTf)2-TsOH: A versatile combined-acid system for catalytic intramolecular hydrofunctionalization and polyene cyclization. Chem. Commun. 2019, 55, 13522–13525. [Google Scholar] [CrossRef] [PubMed]
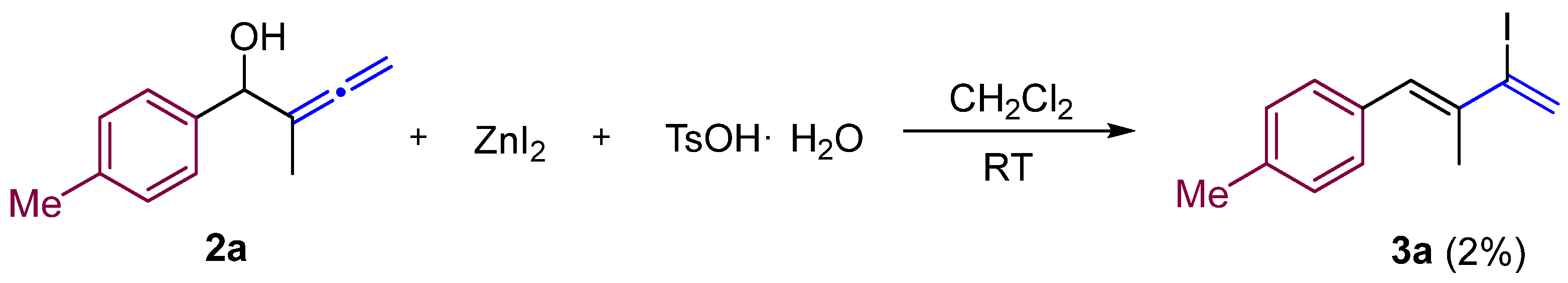


| Entry | ZnI2 (Equiv.) | TsOH·H2O (Equiv.) | Yield (%) |
|---|---|---|---|
| 1 | 0.025 | 0.025 | 2 |
| 2 | 0.1 | 0.1 | 33 |
| 3 | 1.2 | 0.1 | 37 |
| 4 | 1.2 | - | 48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toledano-Pinedo, M.; Peñín, B.; Campo, T.M.d.; Almendros, P. Zn–Catalyzed Direct Synthesis of 3-Iodo-1,3-dienes from α-Allenols. Chem. Proc. 2021, 3, 113. https://doi.org/10.3390/ecsoc-24-08091
Toledano-Pinedo M, Peñín B, Campo TMd, Almendros P. Zn–Catalyzed Direct Synthesis of 3-Iodo-1,3-dienes from α-Allenols. Chemistry Proceedings. 2021; 3(1):113. https://doi.org/10.3390/ecsoc-24-08091
Chicago/Turabian StyleToledano-Pinedo, Mireia, Beatriz Peñín, Teresa Martínez del Campo, and Pedro Almendros. 2021. "Zn–Catalyzed Direct Synthesis of 3-Iodo-1,3-dienes from α-Allenols" Chemistry Proceedings 3, no. 1: 113. https://doi.org/10.3390/ecsoc-24-08091
APA StyleToledano-Pinedo, M., Peñín, B., Campo, T. M. d., & Almendros, P. (2021). Zn–Catalyzed Direct Synthesis of 3-Iodo-1,3-dienes from α-Allenols. Chemistry Proceedings, 3(1), 113. https://doi.org/10.3390/ecsoc-24-08091