Abstract
Carbasugars are a wide group of carbohydrate mimetics in which the ring oxygen is replaced by a methylene group. The high importance of these compounds is related to their interesting biological and pharmacological properties which are the matter of current studies. In our work, a concise synthesis of carbasugars from naturally occurring D-pentoses is presented. The one-pot seleno-Michael reaction connected with intramolecular aldol reaction is a key step of the carbasugar core asymmetric synthesis. Further transformation of obtained carbasugar moiety led to different bioactive compounds. Tandem seleno-Michael reaction conjugated with oxidation/elimination step of in situ generated nucleophile was described a few years ago in the intermolecular variant. In our work, we present the first example of this reaction in an intramolecular way which leads to a previously inaccessible cyclic product of Morita–Baylis–Hillman reaction. Conducted experiments allowed us to obtain cyclic products with high yields and good diastereoisomeric excesses.
1. Introduction
Tandem reactions or domino reactions are an extremely important and useful group of transformations, due to the fact that they allow obtaining multifold desirable organic molecules in a convenient and economical way [1,2]. For many years, thiolates have been widely used as good nucleophiles in reactions with α,β-unsaturated esters producing Michael adducts with quantitative yields [3]. This reaction proceeds through a transition state in which β-thio-enolate is formed, which after protonation leads to the formation of a Michael product. When the transition β-thio-enolate cannot be protonated due to the aprotic environment, it is possible to use it in the aldol reaction. This approach was proposed by Kamimura in 1998 and presented as a new kind of tandem Michael/aldol reaction (Scheme 1) [4].
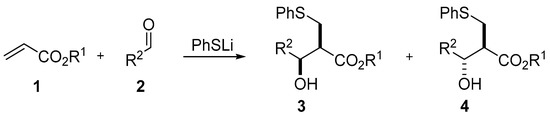
Scheme 1.
Tandem thio-Michael/aldol reaction.
Further research into the tandem thio- or seleno-Michael/aldol reaction led to the development of an intramolecular variant of this reaction. Ono, in his work, showed a highly diastereoselective method of obtaining derivatives of 2-hydroxycyclohexane-1-carboxylate esters using thiolates as initiators of the tandem reaction (Scheme 2) [5].
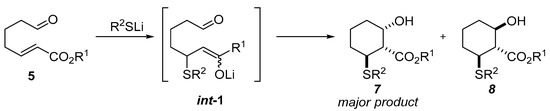
Scheme 2.
Tandem Michael/aldol cyclization reaction.
The authors observed that after Barton–McCombie deoxygenation of tandem reaction product only a trans-phenylsulfanyl ester was obtained which indicates the stereospecificity of the whole process. A few years later, the same Japanese group published a work in which using chiral lithium thiolate carried out the said cyclization reaction in an asymmetric manner (Scheme 3) [6].
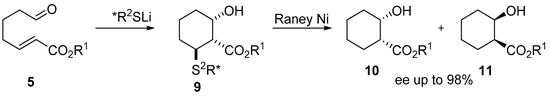
Scheme 3.
Asymmetric tandem Michael/aldol cyclization.
Further research in the field of tandem Michael reaction prompted researchers to look for much simpler reaction systems that would not require the troublesome use of thiolates. In 2009, an article was presented in which using in situ generated lithium n-butyl chalcogenolate as the initiator of the intermolecular tandem reaction (Scheme 4) was shown [7].
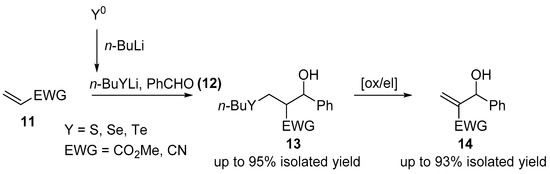
Scheme 4.
Lithium n-butyl chalcogenolates induced three components tandem reaction.
The developed protocol allowed researchers to obtain products of tandem reactions with near-quantitative yields. Additionally, after oxidation and elimination of selenium in mild conditions, they allowed researchers to obtain Morita–Baylis–Hillman-type products. Further works of Sousa [8] and Dos Santos [9] allowed to extend the range of substrates scope used and significantly shorten the reaction time even up to 30 min making this method highly usable.
In our work from 2018, we noticed the potential of chalcogenolates generated in this way. After noticing that α,β-unsaturated 7-oxoesters are not cyclized under classic Morita–Baylis–Hillman reaction conditions catalyzed by tertiary amines or phosphines, we proposed the use of in situ generated lithium n-butylselenolate as the initiator of the tandem reaction (Scheme 5) [10].
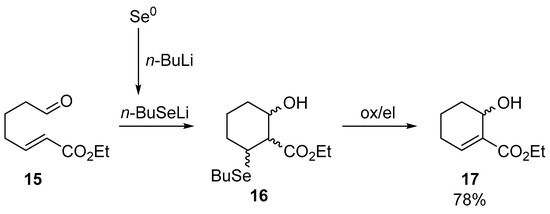
Scheme 5.
Lithium n-butylselenolate induced intramolecular Michael/aldol reaction and oxidation/elimination step.
Overcoming the limitation of the classic variant of Morita–Baylis–Hillman [11] reaction allows projecting a novel way of obtaining carbasugars (Figure 1). Our approach to the carbasugar synthesis assuming disconnection between C1 and C6 atoms and creation of a new C-C bond involving tandem seleno-Michael reaction.

Figure 1.
Key disconnection for novel carbasugar synthesis method.
Carbasugars are a wide group of carbohydrate mimetics where the ring oxygen is replaced by a methylene group [12]. This change has no significant impact on structure (bonds length, torsion angles, conformation) but strongly affects biological activity (pharmacokinetics, molecule–enzyme interactions), which is caused by a lack of hemiacetal functionality. The high importance of these compounds is related to their interesting biological and pharmacological properties which are the matter of current studies. N-alkyl derivatives of β-valienamine such as N-octyl-β-valienamine or N-octyl-4-epi-β-valienamine are potent inhibitors of lysosomal enzymes and find application in the treatment of Gaucher disease. Other carbasugars like voglibose or acarbose (which contains (+)-valienamine subunit) are used in the treatment of diabetes type II and oseltamivir, which is sold under trade name Tamiflu™, has significant anti-influenza activity and is currently used to prevent the development of this disease (Figure 2) [13].
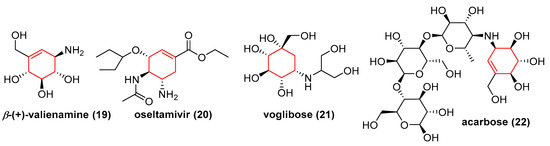
Figure 2.
Example of some unsaturated and saturated carbasugars.
Pericosines (A-E) are a subclass of carbasugars which are isolated from the fungus Perconia byssoides (OUPS–N133), originally separated from sea hare, Aplysia kurodai (Figure 3). They have been shown to display a wide range of interesting biological activities such as significant cytotoxicity against P388 lymphocytic human cancer cells, growth inhibition of tumor cell lines HBC-5 and SNB-75 and inhibition of some enzymes including human topoisomerase II or protein kinase EGFR [14].
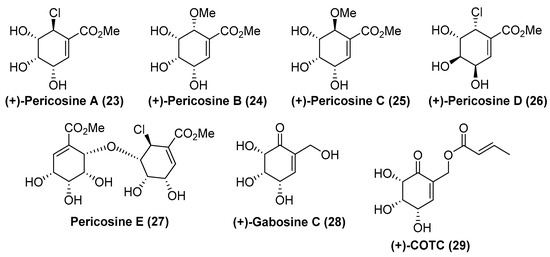
Figure 3.
Structures of naturally occurring Pericosines, (+)-Gabosine C (28) and (+)-COTC (29).
Carbohydrates, especially monosaccharides, are excellent starting materials for the total synthesis of various natural and valuable synthetic compounds. Their availability is usually very high, prices are low and the chemistry of carbohydrates is well known. An application of monosaccharides to carbohydrate mimetic synthesis seems to be a natural choice [15]. General synthesis of carbasugars moieties is employing Grubbs cross-metathesis reaction [16,17], aldol-type cyclization [18], Corey–Fuchs [19] reaction and others [12]. Total synthesis of Gabosines has been recently reviewed by Mac and co-workers [20].
2. Results
2.1. Retrosynthesis of Carbasugars via Tandem Seleno-Michael/aldol Reaction As a Key Step
Our proposition for carbasugar synthesis via intramolecular seleno-Michael aldol reaction starting from preparation of precursors from commercial and readily available D-pentoses (Scheme 6) [10].
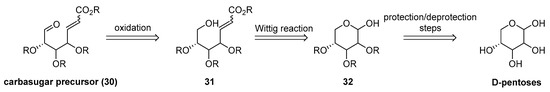
Scheme 6.
Retrosynthesis of carbasugar precursor.
Protection/deprotections steps, followed by Wittig reaction with appropriate phosphorane ylide and oxidation of remaining hydroxyl group, lead to functionalized ω-oxo-α,β-unsaturated esters. After cyclization in previously optimized tandem seleno-Michal/aldol reaction conditions and oxidation/elimination step, carbasugar core moiety can be functionalized in natural products direction (Scheme 7)
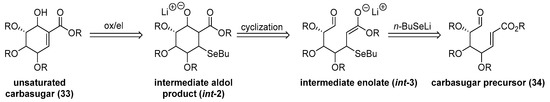
Scheme 7.
Retroanalysis of cyclization step.
2.2. Application of D-Xylose in Total Synthesis of β-(+)-Valienamine (19)
The synthesis of tri-O-benzyloxylopyranoses (35) was completed in five steps from commercially available d-xylose in a 69% overall yield. The Wittig reaction of partially protected D-xylose and phosphorane ylide gave primary alcohol (36) as a mixture of E/Z isomers in 65% yield. Oxidation of the remaining hydroxyl group under Swern oxidation conditions gave compound 37 in 90% yield. Then, compound 37 was subjected to the Michael/aldol reaction with in situ generated n-butyllithium selenolate. The crude mixture of products was treated with hydrogen peroxide and pyridine, and heated to 50 °C to give a mixture of cyclic Morita–Baylis–Hillman-type products (anti-39 and syn-39. After the separation of diastereoisomers, the main product was treated with DIBAL-H to give corresponding diol (40). Protection of two hydroxyl groups with benzyl ethers followed by selective introduction of the amino group with chlorosulfonyl isocyanate lead to protected β-(+)-valienamine. Deprotection of all benzyl ether with boron trichloride provides β-(+)-valienamine (19) with 8% total yield (Scheme 8).
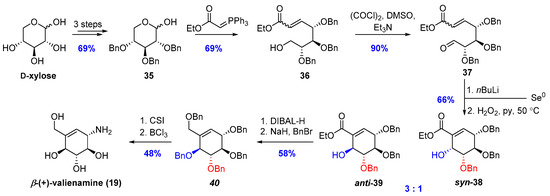
Scheme 8.
Total synthesis of β-(+)-valienamine (19).
2.3. Total Synthesis of Methyl (-)-Shikimate (43) and Their Derivatives
Synthesis of (-)-shikimic acid derivative carbasugars was initiated from commercially available D-lyxose and the first steps were similar to described in the previous paragraph for D-xylose. Carbasugar precursor (41) obtained in five steps was cyclized by tandem seleno-Michael/aldol reaction protocol to give two diastereoisomers of unsaturated carbasugars (42) with anti-isomer as a major one. Further modification of carbasugar moiety leads to different natural products structure. Deoxygenation of a secondary hydroxyl group, base hydrolysis of ester and deprotection with boron trichloride produces methyl (-)-shikimate (43). Diastereoisomer separation and DIBAL-H reduction produces protected (-)-MK7607 (44). A similar protocol for the D-xylose derivatives leads to the unnatural enantiomer of valienamine (45) and a few-step transformation including oxidation of secondary hydroxyl group produces protected (-)-gabosine E (46) (Scheme 9) [21].
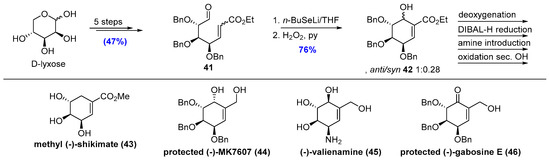
Scheme 9.
Total synthesis of D-lyxose derivate carbasugars.
2.4. Application of d-Ribose in Total Syntheses of Carbasugars
Receiving carbasugars from d-ribose is much more challenging. After three protection/deprotection steps, an inseparable mixture of furanoses (48) and pyranoses (47) was obtained. This problem was resolved after the Wittig reaction with tert-butyldimethylsilyl chloride as a distinguishing agent. After cyclization and oxidation/elimination step, carbasugar (52) was converted to protected (+)-COTC (55) in three steps with 34 yields. Two obtained diastereoisomers of 52 are inseparable before the methylation step. After methylation is stored in a sealed tube with methyl iodide and silver oxide, protected (+)-Pericosines B (56) and C (57) were separated and benzyl ethers were removed with boron trichloride to give (+)-Pericosine B (24) and (+)-Pericosine C (25) (Scheme 10) [22].
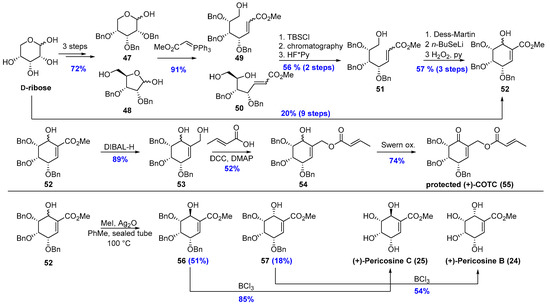
Scheme 10.
Total Syntheses of (+)-Pericosines C (25) and B (24), and protected (+)-COTC (55).
3. Conclusions
Tandem seleno-Michael/aldol reaction could be a useful tool in the synthesis of the carbocyclic core of carbasugars. The application of simple d-pentoses as a cheap and readily available starting material leads to the availability of several natural compounds including aminocarbasugars and methyl (-)-shikimate derivatives.
Author Contributions
Conceptualization, S.B.; methodology, S.B.; software, P.B.; validation, P.B.; formal analysis, S.B.; investigation, P.B and N.B.; resources, S.B.; data curation, P.B.; writing—original draft preparation, P.B.; writing—review and editing, S.B.; visualization, P.B.; supervision, S.B.; project administration, S.B.; funding acquisition, S.B. and P.B. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support from the Polish National Science Centre (Grant No. 2015/17/D/ST5/01334 & 2018/31/N/ST5/03503) is gratefully acknowledged. The research was carried out with the equipment purchased thanks to the financial support of the European Regional Development Fund in the framework of the Polish Innovation Economy Operational Program (contract no. POIG.02.01.00-12-023/08).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nicolaou, K.C.; Edmonds:, D.J.; Bulger, P.G. Cascade reactions in total synthesis. Angew. Chem. Int. Ed. 2006, 45, 7134–7186. [Google Scholar] [CrossRef]
- Tietze, L.F. Domino reactions in organic synthesis. Chem. Rev. 1996, 96, 115–136. [Google Scholar] [CrossRef]
- Miyata, O.; Shinada, T.; Ninomiya, I.; Naito, T.; Date, T.; Okamura, K.; Inagaki, S. Stereospecific Nucleophilic Addition Reactions to Olefins. Addition of Thiols to α,β-Unsaturated Carboxylic Acid Derivatives. J. Org. Chem. 1991, 56, 6556–6564. [Google Scholar] [CrossRef]
- Kamimura, A.; Mitsudera, H.; Asano, S.; Kidera, S.; Kakehi, A. Stereoselective thio-Michael/aldol tandem reaction to α,β-unsaturated esters. J. Org. Chem. 1999, 64, 6353–6360. [Google Scholar] [CrossRef]
- Ono, M.; Nishimura, K.; Nagaoka, Y.; Tomioka, K. The conjugate addition-aldol tandem reaction of α,β-unsaturated esters catalyzed by lithium benzenethiolate. Tetrahedron Lett. 1999, 40, 1509–1512. [Google Scholar] [CrossRef]
- Nishimura, K.; Tsubouchi, H.; Ono, M.; Hayama, T.; Nagaoka, Y.; Tomioka, K. Asymmetric Michael-aldol tandem cyclization of ω-oxo-α,β-unsaturated esters with 10-mercaptoisoborneol methyl ether. Tetrahedron Lett. 2003, 44, 2323–2326. [Google Scholar] [CrossRef]
- Keppler, A.F.; Gariani, R.A.; Lopes, D.G.; Comasseto, J.V. Lithium butylchalcogenolate induced Michael-aldol tandem sequence: Easy and rapid access to highly functionalized organochalcogenides and unsaturated compounds. Tetrahedron Lett. 2009, 50, 2181–2184. [Google Scholar] [CrossRef]
- Sousa, B.A.; Dos Santos, A.A. A facile, versatile, and mild morita-baylis-hillman-type reaction for the modular one-pot synthesis of highly functionalized MBH adducts. Eur. J. Org. Chem. 2012, 2012, 3431–3436. [Google Scholar] [CrossRef]
- Sousa, B.A.; Keppler, A.F.; Gariani, R.A.; Comasseto, J.V.; Dos Santos, A.A. Metallic chalcogenolates mediated modular Michael-aldol cascade reaction: An easy route to multi-functionalized chalcogenides and Morita-Baylis-Hillman adducts. Tetrahedron 2012, 68, 10406–10413. [Google Scholar] [CrossRef]
- Banachowicz, P.; Mlynarski, J.; Buda, S. Intramolecular Tandem Seleno-Michael/Aldol Reaction: A Simple Route to Hydroxy Cyclo-1-ene-1-carboxylate Esters. J. Org. Chem. 2018, 83, 11269–11277. [Google Scholar] [CrossRef]
- Wei, Y.; Shi, M. Recent advances in organocatalytic asymmetric morita–baylis–hillman/aza-morita–baylis–hillman reactions. Chem. Rev. 2013, 113, 6659–6690. [Google Scholar] [CrossRef]
- Arjona, O.; Gómez, A.M.; López, J.C.; Plumet, J. Synthesis and conformational and biological aspects of carbasugars. Chem. Rev. 2007, 107, 1919–2036. [Google Scholar] [CrossRef]
- Bras, N.F.; Cerqueira, N.M.; Ramos, M.J.; Fernandes, P.A. Glycosidase inhibitors: A patent review (2008–2013). Expert Opin. Ther. Pat. 2014, 24, 1–18. [Google Scholar] [CrossRef]
- Yamada, T.; Iritani, M.; Ohishi, H.; Tanaka, K.; Minoura, K.; Doi, M.; Numata, A. Pericosines, antitumour metabolites from the sea hare-derived fungus Periconia byssoides. Structures and biological activities. Org. Biomol. Chem. 2007, 5, 3979–3986. [Google Scholar] [CrossRef]
- Yang, X.; Yuan, P.; Shui, F.; Zhou, Y.; Chen, X. A divergent strategy to synthesize gabosines featuring a switchable two-way aldol cyclization. Org. Biomol. Chem. 2019, 17, 4061–4072. [Google Scholar] [CrossRef]
- Babu, D.C.; Rao, C.B.; Venkatesham, K.; Selvam, J.J.P.; Venkateswarlu, Y. Toward synthesis of carbasugars (+)-gabosine C, (+)-COTC, (+)-pericosine B, and (+)-pericosine C. Carbohydr. Res. 2014, 388, 130–137. [Google Scholar] [CrossRef]
- Muniraju, C.; Rao, J.P.; Rao, B.V. Stereoselective synthesis of (+)-pericosine B and (+)-pericosine C using ring closing metathesis approach. Tetrahedron Asymmetry 2012, 23, 86–93. [Google Scholar] [CrossRef]
- Yuan, P.; Liu, X.; Yang, X.; Zhang, Y.; Chen, X. Total Syntheses of (+)-Gabosine P, (+)-Gabosine Q, (+)-Gabosine E, (-)-Gabosine G, (-)-Gabosine I, (-)-Gabosine K, (+)-Streptol, and (-)-Uvamalol A by a Diversity-Oriented Approach Featuring Tunable Deprotection Manipulation. J. Org. Chem. 2017, 82, 3692–3701. [Google Scholar] [CrossRef]
- Sardinha, J.; Rauter, A.P.; Sollogoub, M. First synthesis of 5-fluoro-(+)-MK7607, its 1-epimer and 6-deoxy derivative. Tetrahedron Lett. 2008, 49, 5548–5550. [Google Scholar] [CrossRef]
- Mac, D.H.; Chandrasekhar, S.; Grée, R. Total synthesis of gabosines. Eur. J. Org. Chem. 2012, 5881–5895. [Google Scholar] [CrossRef]
- Banachowicz, P.; Buda, S. Gram-scale carbasugar synthesis: Via intramolecular seleno -Michael/aldol reaction. RSC Adv. 2019, 9, 12928–12935. [Google Scholar] [CrossRef]
- Biduś, N.; Banachowicz, P.; Buda, S. Application of a tandem seleno-michael/aldol reaction in the total syntheses of (+)-Pericosine B, (+)-Pericosine C, (+)-COTC and 7-chloro-analogue of (+)-Gabosine C. Tetrahedron 2020, 76, 131397. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).