Integrating Diphenyl Diselenide and Its Mehg+ Detoxificant Mechanism on a Conceptual DFT Framework †
Abstract
1. Introduction
2. Methods
2.1. Experimental
2.2. Computational Methods
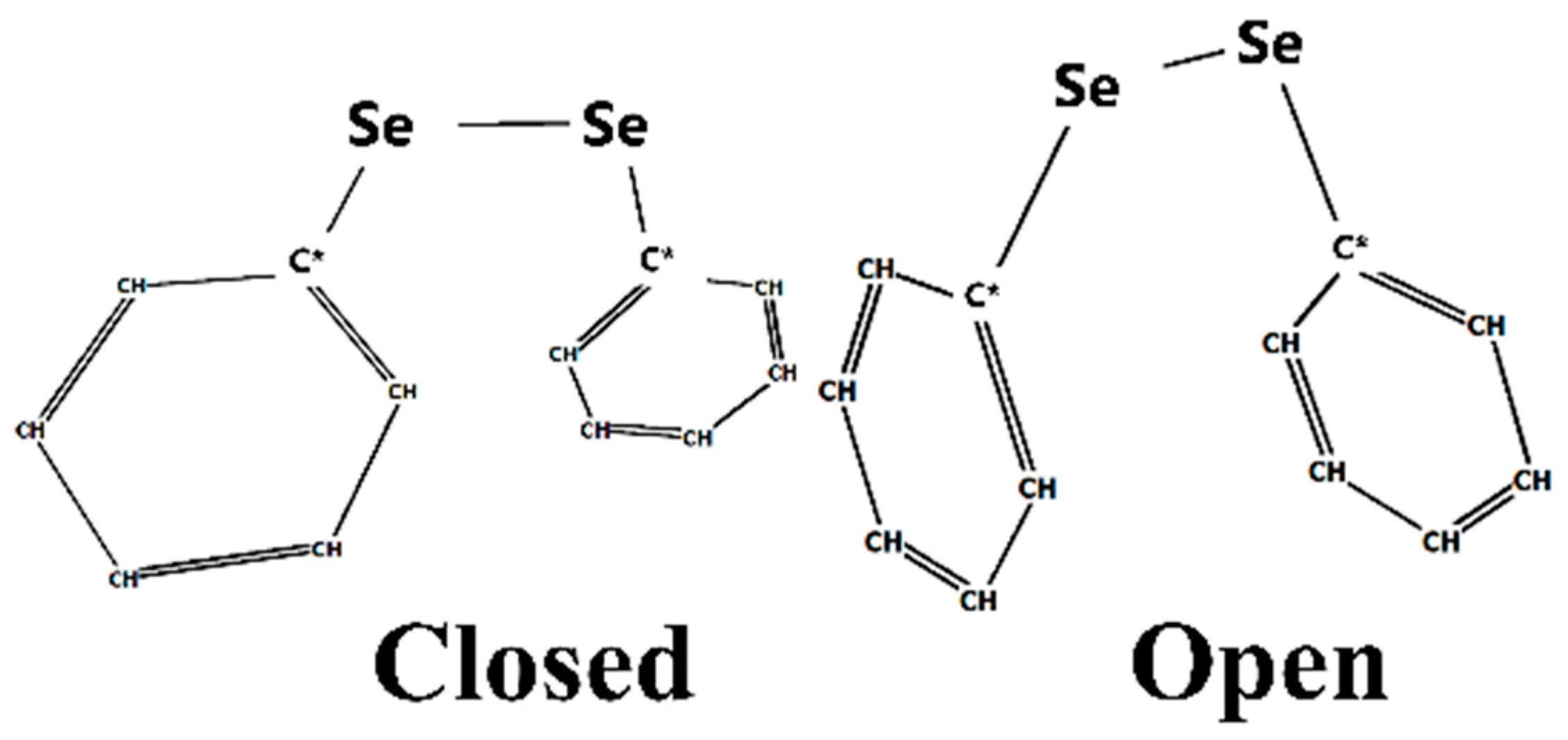
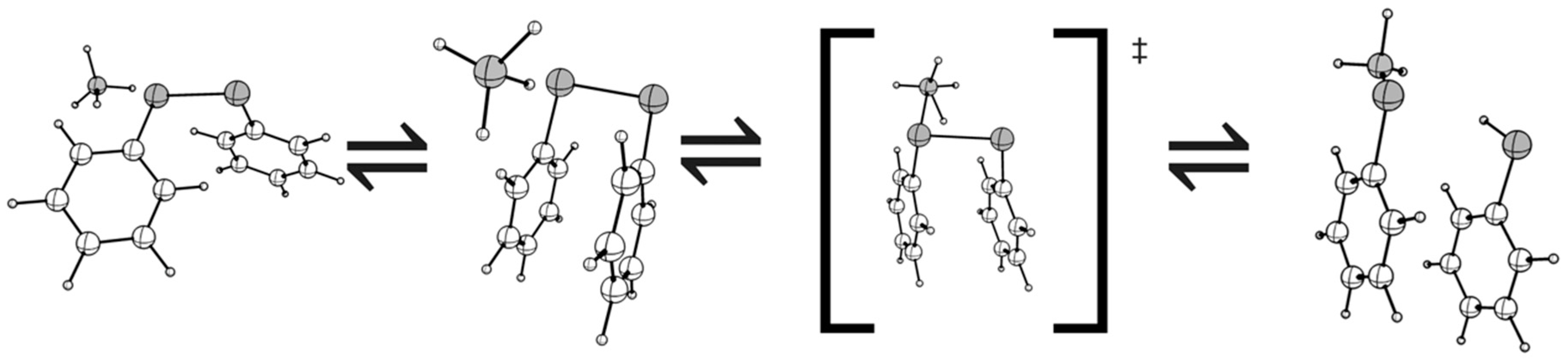
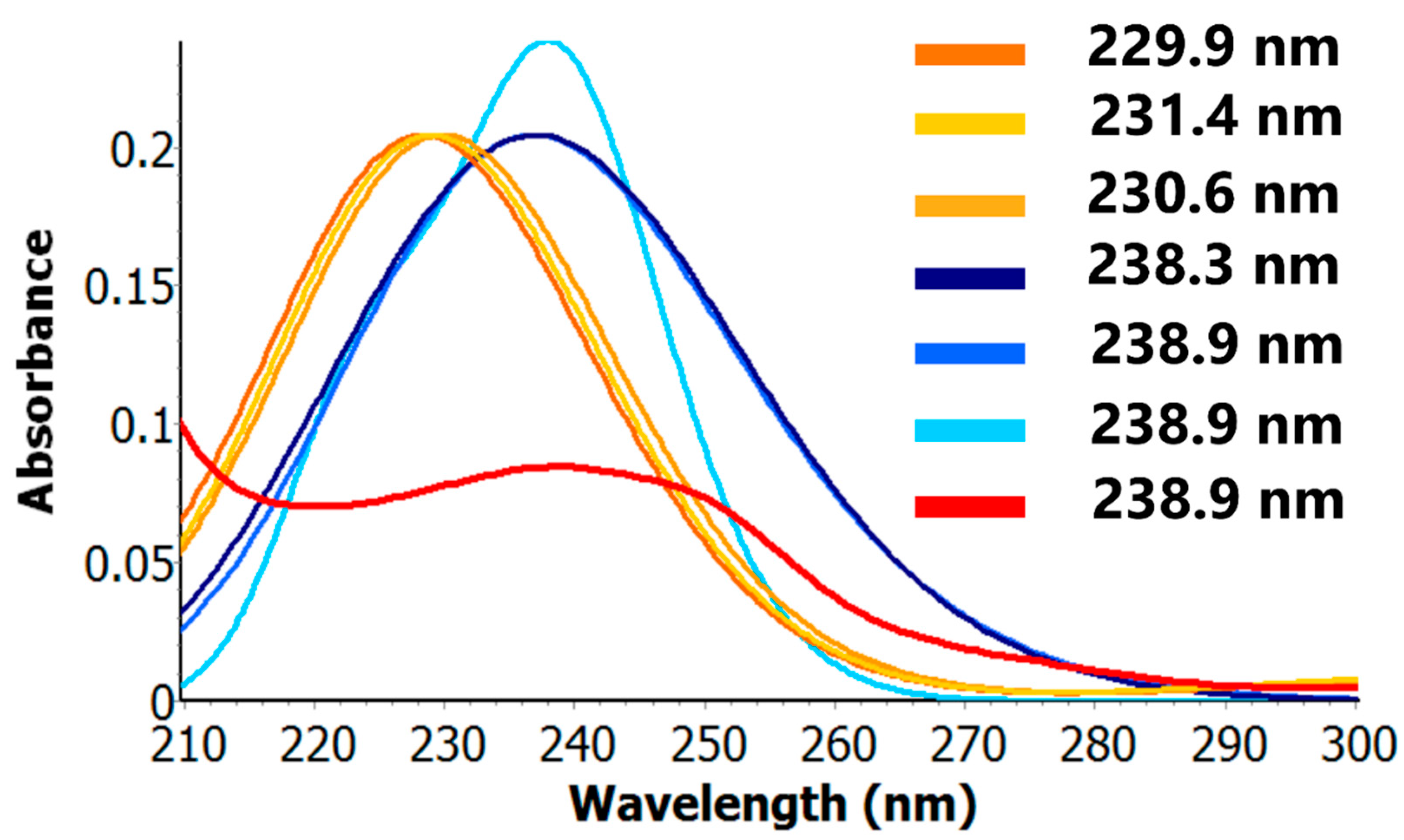
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Farina, M.; Aschner, M. Glutathione antioxidant system and methylmercury-induced neurotoxicity: An intriguing interplay. Biochim. Biophys. Acta-Gen. Subj. 2019, 1863, 129285. [Google Scholar] [CrossRef] [PubMed]
- Farina, M.; Rocha, J.B.T.; Aschner, M. Mechanisms of methylmercury-induced neurotoxicity: Evidence from experimental studies. Life Sci. 2011, 89, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Glaser, V.; Moritz, B.; Schmitz, A.; Dafré, A.L.; Nazari, E.M.; Rauh Müller, Y.M.; Feksa, L.; Straliottoa, M.R.; De Bem, A.F.; Farina, M. Protective effects of diphenyl diselenide in a mouse model of brain toxicity. Chem. Biol. Interact. 2013, 206, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Glaser, V.; Martins, R.D.P.; Vieira, A.J.H.; Oliveira, E.D.M.; Straliotto, M.R.; Mukdsi, J.H.; Torres, A.I.; De Bem, A.F.; Farina, M.; Da Rocha, J.B.T.; et al. Diphenyl diselenide administration enhances cortical mitochondrial number and activity by increasing hemeoxygenase type 1 content in a methylmercury-induced neurotoxicity mouse model. Mol. Cell. Biochem. 2014, 390, 1–8. [Google Scholar] [CrossRef]
- Madabeni, A.; Dalla Tiezza, M.; Omage, F.B.; Nogara, P.A.; Bortoli, M.; Rocha, J.B.T.; Orian, L. Chalcogen–mercury bond formation and disruption in model Rabenstein’s reactions: A computational analysis. J. Comput. Chem. 2020, 41, 2045–2054. [Google Scholar] [CrossRef]
- Branco, V.; Carvalho, C. The thioredoxin system as a target for mercury compounds. Biochim. Biophys. Acta-Gen. Subj. 2019, 1863, 129255. [Google Scholar] [CrossRef]
- Meinerz, D.F.; Branco, V.; Aschner, M.; Carvalho, C.; Rocha, J.B.T. Diphenyl diselenide protects against methylmercury-induced inhibition of thioredoxin reductase and glutathione peroxidase in human neuroblastoma cells: A comparison with ebselen. J. Appl. Toxicol. 2017, 37, 1073–1081. [Google Scholar] [CrossRef]
- Nogara, P.A.; Oliveira, C.S.; Schmitz, G.L.; Piquini, P.C.; Farina, M.; Aschner, M.; Rocha, J.B.T. Methylmercury’s chemistry: From the environment to the mammalian brain. Biochim. Biophys. Acta-Gen. Subj. 2019, 1863, 129284. [Google Scholar] [CrossRef]
- de Freitas, A.S.; Funck, V.R.; dos Santos Rotta, M.; Bohrer, D.; Mörschbächer, V.; Puntel, R.L.; Nogueira, C.W.; Farina, M.; Aschner, M.; Rocha, J.B.T. Diphenyl diselenide, a simple organoselenium compound, decreases methylmercury-induced cerebral, hepatic and renal oxidative stress and mercury deposition in adult mice. Brain Res. Bull. 2009, 79, 77–84. [Google Scholar] [CrossRef]
- Oliveira, C.S.; Nogara, P.A.; Ardisson-Araújo, D.M.P.; Aschner, M.; Rocha, J.B.T.; Dórea, J.G. Neurodevelopmental Effects of Mercury. Adv. Neurotoxicol. 2018, 2, 27–86. [Google Scholar]
- Zaccaria, F.; Wolters, L.P.; Fonseca Guerra, C.; Orian, L. Insights on selenium and tellurium diaryldichalcogenides: A benchmark DFT study. J. Comput. Chem. 2016, 37, 1672–1680. [Google Scholar] [CrossRef] [PubMed]
- Bortoli, M.; Tiezza, M.D.; Muraro, C.; Saielli, G.; Orian, L. The 125Te chemical shift of diphenyl ditelluride: Chasing conformers over a flat energy surface. Molecules 2019, 24, 1250. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, B.C.; Alvim, J.C.; da Silva, F.D.; Nogara, P.A.; Olagoke, O.C.; Aschner, M.; Oliveira, C.S.; Rocha, J.B.T. High level of methylmercury exposure causes persisted toxicity in Nauphoeta cinerea. Environ. Sci. Pollut. Res. 2020, 27, 4799–4813. [Google Scholar] [CrossRef] [PubMed]
- Bortoli, M.; Zaccaria, F.; Tiezza, M.D.; Bruschi, M.; Guerra, C.F.; Matthias Bickelhaupt, F.; Orian, L. Oxidation of organic diselenides and ditellurides by H2O2 for bioinspired catalyst design. Phys. Chem. Chem. Phys. 2018, 20, 20874–20885. [Google Scholar] [CrossRef] [PubMed]
- Powell, M.F.; Bruice, T.C. Hydride vs. Electron Transfer in the Reduction of Flavinand Flavin Radical by 1,4-Dihydropyridines. J. Am. Chem. Soc. 1983, 105, 1014–1021. [Google Scholar] [CrossRef]
- Hori, Y.; Ida, T.; Mizuno, M. A comparative theoretical study of the hydride transfer mechanisms during LiAlH4 and LiBH4 reductions. Comput. Theor. Chem. 2016, 1076, 86–93. [Google Scholar] [CrossRef][Green Version]
- Jónsson, H.; Mills, G.; Jacobsen, K.W. Nudged Elastic Band Method for Finding Minimum Energy Paths of Transitions. In Classical and Quantum Dynamics in Condensed Phase Simulations; Berne, B.J., Ed.; World Scientific: Singapore, 1998. [Google Scholar]
- Runge, E.; Gross, E.K.U. Density-functional theory for time-dependent systems. Phys. Rev. Lett. 1984, 52, 997. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density Functional Approach to the Frontier-Electron Theory of Chemical Reactivity. J. Am. Chem. Soc. 1984, 106, 4049–4050. [Google Scholar] [CrossRef]
- Méndez, F.; Gázquez, J.L. Chemical Reactivity of Enolate Ions: The Local Hard and Soft Acids and Bases Principle Viewpoint. J. Am. Chem. Soc. 1994, 116, 9298–9301. [Google Scholar] [CrossRef]
- Berger, G. Using conceptual density functional theory to rationalize regioselectivity: A case study on the nucleophilic ring-opening of activated aziridines. Comput. Theor. Chem. 2013, 1010, 11–18. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 21, 73–78. [Google Scholar] [CrossRef]
- Neese, F. The ORCA Program System: Software Opdate—Version 4.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2018. [Google Scholar] [CrossRef]
- Swart, M.; Ehlers, A.W.; Lammertsma, K. Performance of the OPBE exchange-correlation functional. Mol. Phys. 2004, 102, 2467–2474. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Van Lenthe, E.; Baerends, E.J.; Snijders, J.G. Relativistic total energy using regular approximations. J. Chem. Phys. 1994, 101, 9783–9792. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Creve, S.; Eriksson, L.A.; Vanquickenborne, L.G. Calculation of the hyper® ne constants of phosphorus-containing radicals. Mol. Phys. 1997, 91, 537–550. [Google Scholar] [CrossRef]
- Roos, G.; Geerlings, P.; Messens, J. Enzymatic catalysis: The emerging role of conceptual density functional theory. J. Phys. Chem. B 2009, 113, 13465–13475. [Google Scholar] [CrossRef]
- Fukui, K. The Path of Chemical Reactions—The IRC Approach. Acc. Chem. Res. 1981, 14, 363–368. [Google Scholar] [CrossRef]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, G.R. Clearance approaches in pharmacology. Pharmacol. Rev. 1987, 39, 1–47. [Google Scholar] [PubMed]
- Allen, F.H. The Cambridge Structural Database: A quarter of a million crystal structures and rising. Acta Crystallogr. Sect. B Struct. Sci. 2002, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge structural database. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omage, F.B.; Oliveira, C.S.; Orian, L.; Rocha, J.B.T. Integrating Diphenyl Diselenide and Its Mehg+ Detoxificant Mechanism on a Conceptual DFT Framework. Chem. Proc. 2020, 2, 26. https://doi.org/10.3390/ECCS2020-07577
Omage FB, Oliveira CS, Orian L, Rocha JBT. Integrating Diphenyl Diselenide and Its Mehg+ Detoxificant Mechanism on a Conceptual DFT Framework. Chemistry Proceedings. 2020; 2(1):26. https://doi.org/10.3390/ECCS2020-07577
Chicago/Turabian StyleOmage, Folorunsho Bright, Cláudia S. Oliveira, Laura Orian, and Joao Batista Teixeira Rocha. 2020. "Integrating Diphenyl Diselenide and Its Mehg+ Detoxificant Mechanism on a Conceptual DFT Framework" Chemistry Proceedings 2, no. 1: 26. https://doi.org/10.3390/ECCS2020-07577
APA StyleOmage, F. B., Oliveira, C. S., Orian, L., & Rocha, J. B. T. (2020). Integrating Diphenyl Diselenide and Its Mehg+ Detoxificant Mechanism on a Conceptual DFT Framework. Chemistry Proceedings, 2(1), 26. https://doi.org/10.3390/ECCS2020-07577







