Abstract
Chagas disease, considered by the World Health Organization as a neglected tropical disease, is responsible for the deaths of more than 10,000 people annually. The main drugs used to overcome the disease, Benzonidazole and Nifurtimox, besides being old, have limitations regarding the adverse effects related to the treatment time and, consequently, their toxicity. Therefore, the need for a new drug to be used against this disease becomes evident. The classes of organoselenium and aromatic heterocycles 1,2,3-triazoles are promising for the issue of the profile of both classes for further evaluation against Trypanossoma cruzi, since the known chemistry and antiparasitic activity of both have already been described. In this work, the molecular hybridization technique was used in order to combine the individual bioactive protozoan that causes Chagas disease. The methodology used was based on works described in the literature. Initially, benzene azides were synthesized from commercial anilines, while ethynyl(phenyl)selane came from different aromatic diselenides. With these intermediates, a 1,3-dipolar cycle-addition was performed to obtain the new target molecules 1-phenyl-4-(phenylselanyl)-1H-1,2,3-triazoles, with moderate to good yields ranging from 52 to 75%. The characterization of the final molecules is in process and, when finished, they will be sent for evaluation of biological activity. It is possible to conclude that the method used is simple and easy to access, an important factor for potential drugs against neglected diseases. After the assessment of bioactivity, it will be possible to identify the efficiency of these substances, as well as, if necessary, the optimization of their structure.
1. Introduction
Chagas disease, caused by the protozoan Trypanossoma cruzi (T. cruzi), is considered by the World Health Organization to be a neglected tropical disease. Despite this, it is estimated that this disease affects between 6 and 8 million people worldwide, and it is responsible for the deaths of 10,600 to 12,500 people annually [1]. The drugs currently used for its treatment are Benzonidazole (1) and Nifurtimox (2), two derivatives of nitrogenous heterocycles (Figure 1).
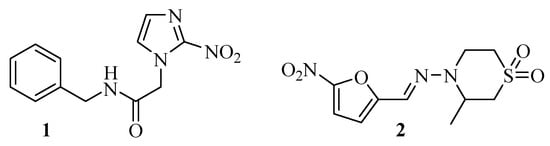
Figure 1.
Chemical structures of Benzonidazole (1) and Nifurtimox (2).
However, they have been used for more than 40 years as the only viable treatment options. Furthermore, their maximum efficiency is found only in the acute phase of the infection, and the long treatment time results in high toxicity [2]. In this context, motivated by the neglect of the Chagas disease, even with a significant number of deaths caused by it, as well as the few options of drugs for its treatment, and mainly because it still has no cure, some research groups have been dedicated to developing new alternatives to fight this disease that affects the most vulnerable population around the world. Some of these studies focused on organoselenium compounds and nitrogenous heterocycles.
Selenium was discovered in the 19th century; however, due to its strong odor and supposed toxicity, this element did not attract attention at that time. Nevertheless, in the 1950s, it was discovered that selenium is an essential element in the animal diet [3]. Today, it is known that selenium has an antioxidant function, being part of the active site of the Glutathione Peroxidase (GPx), an important enzyme that works in the reduction in reactive oxygen species (ROS) [4]. In this context, organoselenium compounds have already been described as antioxidants, apoptosis inducers, chemopreventors of cancers in various organs (the brain, liver, skin and prostate, for example), antimicrobial, antihypertensive, anti-HIV and anti-protozoan [5]. As an example of interest for this work, in 2017, Chao and collaborators developed a series of selenocyanates with inhibitory activity of an important enzyme in the growth of T. Cruzi, which were extremely selective substances and strong candidates for antiparasitic agents [6].
On the other hand, nitrogen-containing aromatic heterocycles, especially 1,2,3-triazoles, have been widely described in the literature because of their several pharmacological activities, such as antiplatelet [7], antimicrobial [8], antiviral [9], anti-inflammatory [10] and anti-allergic activities, among others. Their extensive pharmacological activity, together with the fact that they do not have major adverse effects, makes their importance undoubted in the medical field.
Therefore, in view of the need for new drugs for the treatment of Chagas disease, which are more active and selective, the objective of this work is the synthesis of a new selenium containing 1,2,3-triazoles (Figure 2) for evaluation against Trypanossoma cruzi.
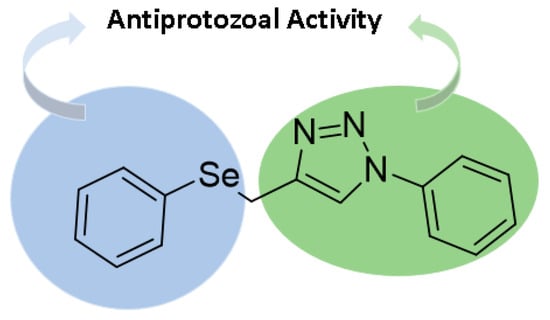
Figure 2.
General structure of selenium containing 1,2,3-triazoles.
2. Materials and Methods
The structural determination of the synthesized substances was performed using instrumental methods of hydrogen nuclear magnetic resonance spectroscopy (1H NMR) and by infrared (IR) spectroscopy. The NMR spectra were obtained in a Varian VNMRS 500 MHz or Varian VNMRS 300 MHz device, and the chemical shifts were determined using TMS as an internal reference. The spectra in the infrared region were obtained using a Perkin–Elmer spectrophotometer, model 1420 with double beam in a beam over KCl windows. The absorption values were expressed as a wave number, using the reciprocal centimeter (cm−1) as the unit.
The reaction monitoring process was carried out using thin-layer chromatography (TLC) with silica gel plates 60-F-254, 0.2-mm thick (reference Merck, Darmstadt, Germany). For the purification of the substances by column chromatography, 230–400 MeshASTM flash silica gel 60 (reference Merck, Darmstadt, Germany) was used.
2.1. Synthesis of Azides
The synthesis of azidobenzene compounds, 4a, 4b, 4c and 4d, started from commercial anilines using the same methodology already described in the literature [11]. Aniline was dissolved in hydrochloric acid (6 M HCl) in an ice bath, with the temperature controlled between 0 and 5 °C. Sodium nitrite (NaNO2) dissolved in water was added dropwise. The reaction mixture was kept under stirring for 30 min. Then, sodium azide (NaN₃), dissolved in water, was added dropwise. After the addition, the system was stirred for two hours at room temperature and extracted with ethyl acetate (3 × 15 mL), dried over anhydrous sodium sulfate and concentrated under vacuum. The crude product was used without purification.
2.2. Synthesis of the Alkynes
The ethynyl (phenyl) selane compounds, 6a, 6b, 6c and 6d were synthesized from the corresponding diselenides, which were previously synthesized. Based on a methodology described in the literature [12], a solution containing the diphenyl diselenide in THF under a nitrogen atmosphere was added dropwise in an ethanolic suspension of sodium boron hydride (NaBH4). The reaction mixture was kept under stirring at 0 °C, and propargyl bromide, in THF, was added. After 25 min, the reaction was poured into water and the organic phase was extracted using diethyl ether. The combined organic phases were dried over anhydrous sodium sulfate and concentrated under vacuum. The reaction was purified using flash chromatography using hexane as the eluent.
2.3. Synthesis of Triazoles Containing Selenium
From a methodology of the cycloaddition of azides and alkynes already described in the literature [13], the 1-phenyl-4-(phenylselanyl)-1H-1,2,3-triazoles, 7a, 7b and 7c were synthesized Azide and alkali were added in a suspension of water and dichloromethane. To the system, under stirring, copper sulfate and sodium ascorbate were added. The mixture was kept under stirring and at room temperature. The progress of the reactions was monitored by TLC. After the end of the reaction, extraction was done using dichloromethane. The organic phases were washed with brine, a saturated sodium bicarbonate solution, and dried with anhydrous sodium sulfate. The product was concentrated using vacuum and purified by recrystallization.
3. Results and Discussion
Initially, using a methodology already described in the literature [11], the synthesis of azides was carried out from commercial anilines. Through a reaction of diazonium salt formation, followed by substitution by the azide group the 4a–d products were obtained with quantitative yields (Scheme 1).
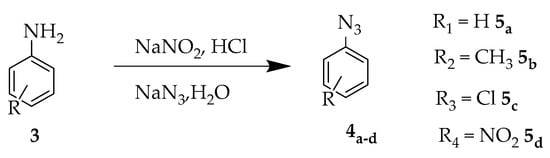
Scheme 1.
Synthesis of azides 5a–d.
The obtained products, oily compounds, were characterized by infrared (IR) spectroscopy, and to exemplify the IR spectrum of compound 4b, illustrated in Figure 3. The inclusion of donor and withdrawer groups was carried out for further evaluation of their influence on biological activity.
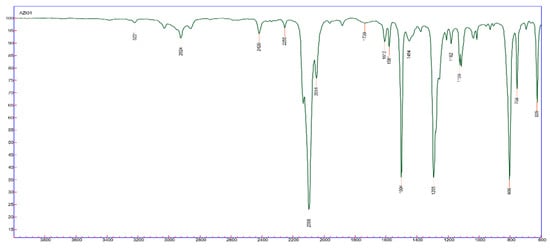
Figure 3.
Infrared spectroscopy for substituted methyl azide—4b.
From the IR, it is possible to see the band in 2098 cm−1 characteristic of the azide group (N3) [11], which proves, therefore, the formation of the desired product.
The molecules containing selenium were synthesized starting from different, previously obtained, diphenyl diselenides, which underwent cleavage by the reducing agent, giving rise to the nucleophile responsible for replacing the bromine atom in the propargyl bromide (Scheme 2) [12].
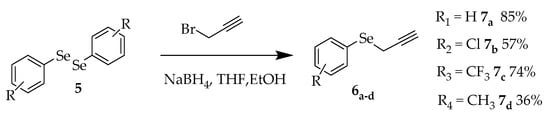
Scheme 2.
Synthesis of alkynes functionalized with selenium 6a–d.
The four structures were obtained as yellow oils and had yields that varied between 36 and 85%. The best result, with an 85% yield, was shown in compound 7a. The presence of a donor group decreases the yield, but the synthesis of compounds with donor groups and electron withdrawers will be important for the evaluation of biological activity. The selenofunctionalized alkynes were characterized by 1H NMR, as shown in Figure 4.
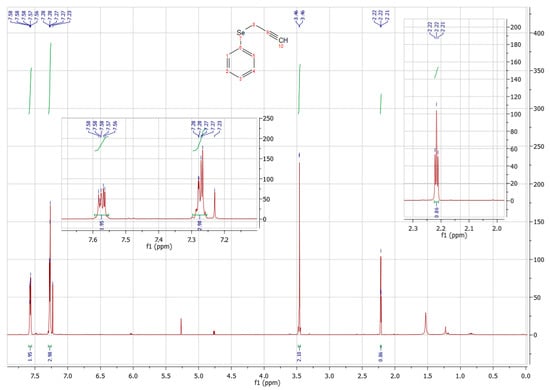
Figure 4.
1H-NMR spectrum of compound 6a (300 MHz, CD3Cl, 25 °C).
In this spectrum, we can see that, according to the integrals of each signal, there is a total of eight hydrogens that correspond to the eight hydrogens that are expected in the molecule in question. There are two multiplets at 7.58 and 7.27 ppm, with integrations of two and three hydrogens, respectively, totaling the aromatic H of the molecule. Then, there is a doublet at 3.46 ppm, integrating two hydrogens, referring to the 2 H of CH2 of the substance. Finally, the triplet in 2.22 ppm, integrating 1 H, characteristic of the H of the CH of the triple bond [12].
The last step to synthesize the target is the stage of formation of triazole from the previously synthesized anilines and selenoalkines. The seven 1,3-dipolar addition cycle reactions were performed to obtain the final products 7a–g (Scheme 3).
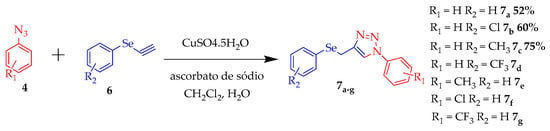
Scheme 3.
Synthesis of triazoles containing selenium.
Only three compounds have yields, which vary from 52 to 75%, where it is possible to observe an increase in income with an electron donor group. The reactions obtaining 7d-g were accompanied by thin-layer chromatography (TLC), were purified by chromatographic column, and unfortunately, due to the COVID-19 pandemic, the authors could not perform the characterization for further verification of their structure, thus it was not possible to calculate yields. To date, compounds 7a, 7b and 7c were analyzed by 1H NMR spectroscopy, taking as an example the 8c spectrum represented in Figure 5.
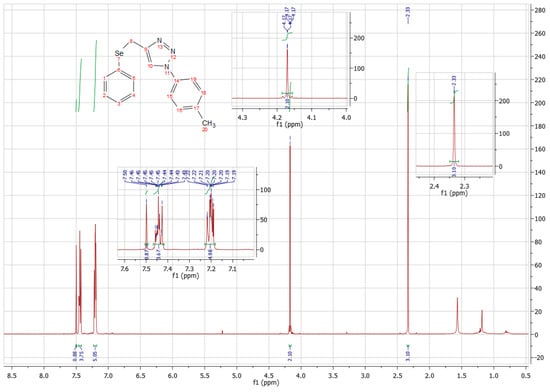
Figure 5.
H-NMR spectrum of compound 8c (300 MHz, CD3Cl, 25 °C).
It is possible to determine, by the integrations of this spectrum, the presence of 15 hydrogens, with this value being compatible with the 15 hydrogens existing in the molecule. Initially, there is a simplet at 7.50 ppm, integrating 1 H, which is relative to the H of the triazolic ring. Then, there are two multiplets centered at 7.45 and 7.20 ppm, integrating 4 and 5 H, respectively, with these being related to the aromatic hydrogens of the substance. Sequentially, a doublet is seen at 4.17 ppm, comprising two hydrogens, which refer to the 2 H of the compound’s CH2. Finally, a simplet at 2.33 pm, integrating 3 H, characteristic of the compound’s methyl hydrogens.
4. Conclusions and Perspectives
The present work showed an efficient methodology to obtain the new selenium containing 1,2,3-triazoles, which was able to unite two important nuclei in the same structure. When the activities that were interrupted due to COVID-19 resume, purification and characterization of the 7d-g products will be carried out, followed by sending all examples for pharmacological tests in partner laboratories against T. cruzi, the protozoan that causes Chagas disease.
Author Contributions
All authors contributed almost equally to this study.
Funding
This research was funded by CNPQ-PIBIC (123528/2020-8), UFF-FOPESQ and FAPERJ (E-26/202.911/2019 and E-26/200.414/2020).
Acknowledgments
This project was supported by the funding agencies: CAPES, CNPQ and FAPERJ. We thank them and the UFF chemistry institute where we are located.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Stanaway, J.A.; Roth, G. The Burden of Chagas Disease: Estimates and Challenges. Glob. Hear. 2015, 10, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Bermudez, J.; Davies, C.; Simonazzi, A.; Real, J.P.; Palma, S.D. Current drug therapy and pharmaceutical challenges for Chagas disease. Acta Trop. 2016, 156, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Suttle, N.F. Mineral Nutrition of Livestock; CABI: Oxfordshire, UK, 2010. [Google Scholar]
- Nascimento, V.; Cordeiro, P.D.S.; E Silva, R.C. Ebselen: A Brief Review of its Antioxidant Capacity and Biological Applications. Rev. Vir. Quím. 2019, 11, 1894–1907. [Google Scholar] [CrossRef]
- Santi, C. Organoselenium Chemistry: Between Synthesis and Biochemistry, 1st ed.; Bentham Science: Sharjah, UAE, 2014. [Google Scholar] [CrossRef]
- Chao, M.N.; Storey, M.; Li, C.; Rodríguez, M.G.; Di Salvo, F.; Szajnman, S.H.; Moreno, S.N.J.; Docampo, R.; Rodriguez, J.B. Selenium-containing analogues of WC-9 are extremely potent inhibitors of Trypanosoma cruzi proliferation. Bioorg. Med. Chem. 2017, 25, 6435–6449. [Google Scholar] [CrossRef] [PubMed]
- Menegatti, R.; Cunha, A.C.; Gonzaga, D.T.G.; Perreira, E.F.; El-Nabawi, A.; Eldefrawi, A.T.; Albuquerque, E.X.; Neves, G.A.; Rates, S.M.K.; Fraga, C.A.M.; et al. Design, synthesis and pharmacological profile of novel dopamine D2 receptor ligands. Bioorg. Med. Chem. 2003, 11, 4807–4813. [Google Scholar] [CrossRef]
- Ferreira, V. F.; de Souza, M. C. B. V.; Ferreira, M. L. G.; Cunha, A. C.; Melo, J. O. F.; Donnici, C. L.; Augusti, R. Heterociclos 1,2,3-triazólicos: histórico, métodos de preparação, aplicações e atividades farmacológicas. Quím. Nova 2006, 29, 569. [Google Scholar]
- Alvarez, R.; Velazquez, S.; San-Felix, A.; Aquaro, S.; De Clercq, E.; Perno, C.-F.; Karlsson, A.; Balzarini, J.; Camarasa, M.J. 1,2,3-Triazole-[2,5-Bis-O-(tert-butyldimethylsilyl)-.beta.-D-ribofuranosyl]-3′-spiro-5′’-(4′’-amino-1′’,2′’-oxathiole 2′’,2′’-dioxide) (TSAO) Analogs: Synthesis and Anti-HIV-1 Activity. J. Med. Chem. 1994, 37, 4185–4194. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.-H. Recent Researches in Triazole Compounds as Medicinal Drugs. Curr. Med. Chem. 2012, 19, 239–280. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-W.; Guo, J.-W.; Huang, M.-J.; You, Y.-Z.; Wu, Z.-H.; Liu, H.; Huang, L.-H. Design, synthesis and biological evaluation of new steroidal β-triazoly enones as potent antiproliferative agents. Steroids 2019, 150, 108431. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Koenigs, R.M. Rhodium-Catalyzed Carbene Transfer Reactions for Sigmatropic Rearrangement Reactions of Selenium Ylides. Org. Lett. 2019, 21, 3653–3657. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Zhu, Q. ’Click’ assembly of selective inhibitors for MAO-A. Bioorg. Med. Chem. Lett. 2010, 20, 6222–6225. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).