Abstract
Ecuador is one of the countries in the Latin American region with a high textile production. However, chemical treatment strategies in the Ambato, Tungurahua and Quito areas are inefficient and not systematically applied, and the volumes of dyes and pigment-type contaminants generate serious environmental problems. The treatments of indigo textile wastewater and related indigo derivatives are very complex. Taking these into consideration, a simple photochemical protocol in heterogeneous conditions was developed, for degrading “blue-indigo” (Ambato textile group) in solution, using TiO2 (Degussa P25, with a purity of ≈99% and BET surface area 50 ± 15 m2/g) and solar light at lab scale. The photocatalytic oxidation of “blue-indigo” in aqueous solution was assessed by solar irradiation, in the presence of TiO2 particles. The effect of indigo concentrations, pH and TiO2 loading for maximum degree of degradation were evaluated. The mineralization of “blue-indigo” was reported by measuring COD-i and COD-f of the solution that was irradiated with sunlight under optimized conditions. The results enable the re-designing of strategies for controlling contamination in textile wastewaters in eco-sustainable conditions for Ecuador.
1. Introduction
Ecuador is one of the countries in the Latin American region with a high textile production. However, chemical treatment strategies in the Ambato, Tungurahua, Esmeraldas and Quito areas are inefficient and not systematically applied, and the volumes of organic dyes and pigment-type contaminants generate serious environmental problems, considering their ecological toxicity, carcinogenicity and high resistance to biodegradation. Several physico-chemical and biotechnological methods have been reported in the past 10 years, but these processes have high operating costs and are of limited applicability in eco-sustainable conditions. Photo-catalytic oxidative degradation using solar light is the preferred alternative procedure to clean-up polluted waters due to its simplicity, functionality, potential scalability and cost effectiveness at laboratory or meso-scale in micro-textile entrepreneurships [1,2]. Various types of photo-catalysts such as perovskites, titanates, metal oxides, niobates, nanomaterials composite and semiconductors have been extensively used. TiO2 has been proven to be an excellent catalyst in the oxidative photo-degradation of organic pollutants.
The use of high-energy UV light is not only operationally expensive, it is instrumentally demanding, and it can also generate serious hazard problems. Therefore, the use of visible light, sunlight, constitutes an interesting variant for treating textile wastewater in ecologically friendly conditions. It has been demonstrated how the photo-bleaching (partial oxidative degradation) of dyes could be achieved by sunlight irradiation using TiO2 as a photo-catalyst [3,4]. Taking into consideration that not much information has been reported on the photocatalytic oxidative degradation of blue-indigo by a TiO2 (anatasa)/sunlight system, and its advantage at micro- and meso-technological scale, the main objective of the report was evaluate the capability of the proposed system (vide supra) to decolorize and degrade residual aqueous solution of indigoid dye blue-indigo using solar light with a commercial TiO2 photo-catalyst.
2. Materials and Methods
The TiO2/visible light (sunlight) photo-catalysis experiments were carried out in the facilities of the Chemical Technology laboratories of the Chemical Engineering Campus at Technical University of Esmeraldas “Luis Vargas Torres, Esmeraldas, Ecuador, (0°58′25″ N; 79°39′59″ W). TiO2 used in the experiment was Degussa P-25 (QUIMPAC supplier—Ecuador, 90% anatase) with an average particle size of 30 nm and surface area of 50 m2/g and was used as provided by the national supplier. The organic dye “blue-indigo” was obtained from the textile company REALTEC S.A., Esmeraldas, Ecuador, and was used without any purification. The sample of residual water with blue-indigo was supplied by REALTEC S.A, Ecuador (Figure 1a,b).
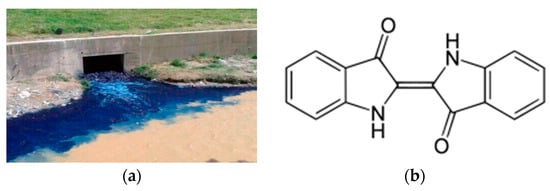
Figure 1.
(a) Textile residual water from REALTEC S.A, Esmeraldas, Ecuador, June 20, 2020, photo by the author E.F.M.Q.; (b) molecular structure of “blue-indigo”.
The photochemical reactor (cylindrical configuration) of 500 mL capacity was made up of borosilicate glass having dimensions 18 cm × 10 cm (height × diameter) with a port at the top for sampling. Solar light was used as the energy source for catalyst activation and to assess field efficiency. Experiments were performed at ambient temperature. The reactor assembly was placed on a magnetic stirring plate to further enhance the agitation, avoiding sedimentation of the catalyst. The slurry composed of the dye residual solution from REALTEC S.A. and catalyst (TiO2, 1000 mg) placed in the reactor was directly exposed to natural solar light for a specified time interval. A centrifuge (QUIMPAC-Ecuador), operated at 3600 rpm, was utilized for removing the TiO2 and to obtain the supernatant for UV/VIS (Hitachi U-2001 spectrophotometer, Tokyo, Japan) determination, in the range of λ = 400–700 nm. The percentage of degradation was calculated by measuring changes in absorbance and COD. Chemical oxygen demand (COD) was measured by the closed reflux method (APHA, 1989).
3. Results and Discussion
Time taken for the maximum degradation of the waste dye solution was around 80–110 min of irradiation. Beyond 100 min, the degradation was found to be negligible (Figure 2). From the results obtained, it can be concluded that the photo-catalytic approach promotes the decline of chromophore peaks in the dye molecule over 80–100 min. In this case, the chemical oxygen demand was used as an indicative parameter of the photo-oxidative degradation process of blue-indigo at a dose of 1000 mg of the photo-catalyst.
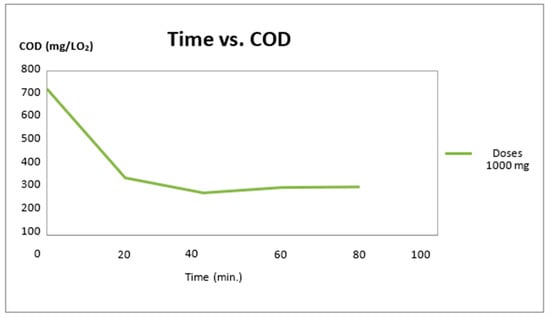
Figure 2.
Chemical oxygen demand (COD) vs. time and TiO2 doses at 1000 mg.
The results of average removal percentages of apparent color (AP) for 1000 mg of titanium dioxide at the different residence times are shown in Table 1 and Figure 3.

Table 1.
Average removal of blue-indigo color (%) vs. residence time of TiO2 in the photoreactor.
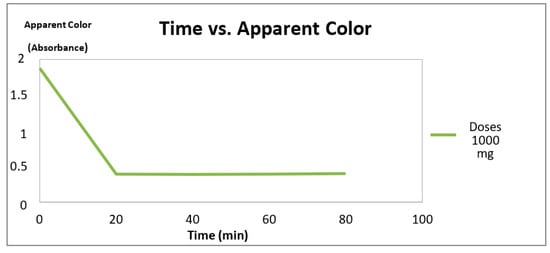
Figure 3.
Variations of apparent color vs. time and TiO2 doses at 1000 mg.
A control experiment in the absence of solar irradiation (2 h) illustrated the adsorption equilibrium of the waste dye solution onto TiO2. Another experiment of solar irradiation (2 h) of the dye solution in the absence of TiO2 showed no significant photo-oxidative degradation of the waste slurry, indicating that this phenomenon is photo-catalytic in nature.
The mechanism for photo-oxidative degradation (bleaching) in correspondence with a historical perspective could proceed as follows [5]:
This photosensitizing oxidation mechanism suggests that the electron from the excited dye molecule is transferred into the conduction band of the TiO2, and the cation radical formed at the surface of TiO2 quickly undergoes photo-degradation to intermediate products as depicted in Figure 4 [5,6].
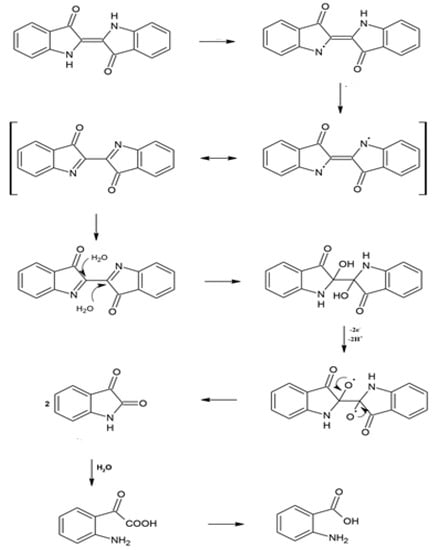
Figure 4.
Probable mechanism of initial photo-oxidative degradation of blue-indigo under TiO2/sunlight system and mechanical stirring.
4. Conclusions
A TiO2/sunlight system was developed for the photo-oxidative degradation of an organic dye “blue-indigo”. Natural sunlight can be used for the oxidative photo-degradation of this indigoid pigment particularly rapidly under these very simple and eco-sustainable conditions. Blue-indigo was degraded in the presence of a TiO2 photo-catalyst in the form of suspension by irradiation with solar light. Hence, the photo-oxidative degradation of textile dyes of an indigoid nature employing solar energy may emerge as a viable method because of its eco-sustainability, cost effective and technological simplicity.
Author Contributions
E.F.M.Q. participated in the conceptualization and investigation at the laboratory scale of the article on the basis of his master degree thesis; J.E.T.M. participated in the methodology, investigation and original draft preparation, review and editing; M.G.C.M. contributed with formal analysis, experimental data validation and project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding but was developed under the consideration of Universidad de Education a Distancia, UNED, Madrid, Spain, and Technical University of Esmeraldas, Ecuador.
Acknowledgments
The authors greatly acknowledge the technical, and administrative, support from the Technical University of Esmeraldas, Ecuador during 2019–2020.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alahiane, S.; Qourzal, M.; El Ouardi, M.; Belmouden, A.; Assabbane, Y. Ait-Ichou, Adsorption and photocatalytic degradation of indigo carmine dye in aqueous solutions using TiO2/UV/O2. J. Mater. Environ. Sci. 2013, 4, 239. [Google Scholar]
- Rauf, M.A.; Ashraf, S.S. Fundamental principles and application of heterogeneous photocatalytic degradation of dyes in solution. Chem. Eng. J. 2009, 151, 10–18. [Google Scholar] [CrossRef]
- Mondal, K.; Sharma, A. Photocatalytic Oxidation of Pollutant Dyes in Wastewater by TiO2 and ZnO nano-materials—A Mini-review. In Nanoscience & Technology for Mankind; The National Academy of Sciences India (NASI): Prayagraj, Uttar Pradesh, India, 2014; pp. 36–72. [Google Scholar]
- Jawad, A.; Shazwani, N.; Mohd, I.; Ismail, K.; Nawawi, W. Kinetics of photocatalytic decolourization of cationic dye using porous TiO2 film. J. Taibah Univ. Sci. 2016, 10, 352–362. [Google Scholar] [CrossRef]
- Bahrudin, N.N.; Nawi, M.A.; Nawawi, W.I. Enhanced photocatalytic decolorization of methyl orange dye and its mineralization pathway by immobilized TiO2/polyaniline. Res. Chem. Intermed. 2019, 45, 2771–2795. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, J.; Zang, L.; Shen, T.; Hidaka, H.; Pelizzetti, E.; Serpone, N. Photoassisted degradation of aqueous surfactant/TiO2 dispersion under visible light irradiation. J. Chem. Soc. Faraday Trans. 1997, 94, 673–676. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).