Abstract
Photodynamic therapy (PDT) is a minimally invasive cancer treatment that uses light-activated photosensitizers to selectively kill cancer cells. While effective, residual photosensitizers can cause phototoxicity when exposed to sunlight. To address this, drug delivery systems (DDS) such as polymeric micelles have been explored to improve targeting and reduce side effects. In this study, photosensitizer-loaded block copolymers were synthesized and incorporated into micelles. Their phototoxicity was evaluated using HeLa and MCF−7 cells, showing significant cell death upon light exposure. HT29 cell tests are ongoing, and results will be reported at the time of presentation.
1. Introduction
Among cancer treatments, photodynamic therapy (PDT) has gained significant attention in recent years. PDT utilizes photosensitizers, specific wavelengths of light, and molecular oxygen, harnessing the photochemical reaction between the photosensitizer and light. This method offers the major advantage of being minimally invasive, with a low risk of damage to normal cells, thereby reducing the burden on patients [1].
The mechanism of PDT is as follows. After administering a photosensitizer to the patient, irradiation with a specific wavelength of light activates it. This results in the generation of singlet oxygen (1O2), a highly cytotoxic reactive oxygen species that causes necrosis of cancer cells.
Photosensitizers are compounds that absorb light of specific wavelengths to become activated. After accumulating in the target tissue, the photosensitizer absorbs photons, transitioning from the ground triplet state to an excited singlet state. Subsequently, through intersystem crossing, it reaches an excited triplet state and transfers energy to ground-state molecular oxygen, generating singlet oxygen [2,3].
In recent years, Drug Delivery Systems (DDS) have gained attention as a strategy to reduce PDT side effects. DDS is a technology that controls drug distribution within the body to maximize therapeutic effects while minimizing side effects. By leveraging the EPR effect (Enhanced Permeability and Retention), DDS can efficiently deliver drugs to tumor tissue and reduce systemic side effects.
The EPR effect is a mechanism that selectively accumulates drugs by exploiting the unique pathological characteristics of tumor tissue. Tumor blood vessels are structurally immature, with large gaps between endothelial cells, allowing macromolecular drugs and nanoparticles to pass through the vessel walls and achieve high accumulation easily. Furthermore, the lymphatic drainage function in tumor tissue is impaired, preventing rapid clearance of infiltrated drugs and allowing them to remain within the tumor for extended periods.
To achieve efficient DDS-based PDT, the use of nanocarriers capable of encapsulating photosensitizers and delivering them selectively to tumor cells is essential.
In this study, phthalocyanine, which absorbs long-wavelength light, was used as the photosensitizer. Micelles containing POEGMA chains were prepared, and photocytotoxicity evaluations were conducted using cancer cells.
2. Materials and Methods
2.1. Instruments and Regents
The synthetic reagents were purchased from Tokyo kasei. And cancer cell lines (HeLa and MCF-7 cell lines) were obtained from the RIKEN Institute.
2.2. Synthesis of Compound 1 (BuPc-OH)
See Reference [4].
2.3. Synthesis of Compound 4
Under argon atmosphere, BuPc-C6MA (8.0 mg, 8.2 μmol) and AIBN (0.08 mg, 0.49 μmol) were added to a 10 mL round-bottom flask. Additionally, under an argon atmosphere, POEGMA homopolymer (7.4 mg, 1.8 μmol) and THF (0.08 mL, 0.1 M) were added to a 10 mL round-bottom flask, stirred briefly, and then added to the above flask. The mixture was stirred at 70 °C for 24 h. The mixture was then quenched in −40 °C ethanol, precipitated with diethyl ether, and subjected to 1H NMR measurement.
2.4. DLS Measurement
DLS was dissolved in pure water to achieve concentrations of 0.1, 0.16, 0.2, 0.5, and 1.0 mg/mL. After sonication for 10 min, measurements were performed.
2.5. Singlet Oxygen Generation Test
Singlet oxygen generation capacity was evaluated using the chemical trapping method with 1,3-diphenylisobenzofuran (DPBF). A CH2Cl2 solution containing POEGMA-b-PBuPc-C6MA (0.96 mg) and DPBF (50 μM) was irradiated at 660 nm, and the decrease in DPBF absorbance (λ = 416 nm) versus irradiation time was measured by UV–Vis spectroscopy.
2.6. Photocytotoxicity Evaluation
The phototoxicity test was conducted using the MTT assay. The conditions were irradiation with light at 660 nm (2.5 W, 5 J/cm2) for 2 s at a distance of 4 cm. Under these conditions, irradiation was performed once daily for 5 days. Finally, toxicity was assessed 24 h after the last irradiation.
3. Results and Discussion
We designed the molecules with the idea that phthalocyanine, which absorbs long wavelengths, could function as a photosensitizer, and that the presence of polymer chains would enable the formation of polymeric micelles (Scheme 1).
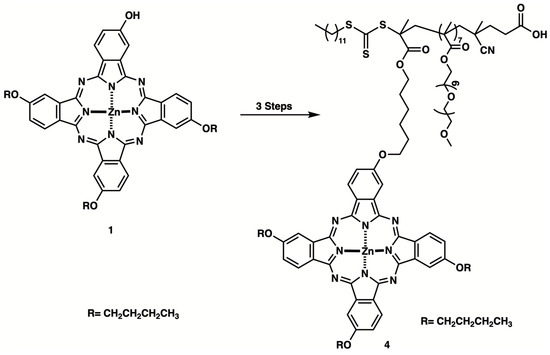
Scheme 1.
Synthetic route of photosensitizer-containing block copolymers.
We synthesized target compound 4 according to Scheme 1. NMR spectra revealed signals at δ 0.98–1.86 ppm and δ 4.08 ppm originating from the butoxy group. Aromatic signals at δ 7.17–7.59 ppm were identified as originating from the phthalocyanine moiety. Although the target compound was successfully synthesized, the yield was low (5%), primarily due to a low conversion rate, with a significant amount of starting materials remaining unreacted. The compound BuPc-C6OH 2 was synthesized by reacting BuPc-OH 1 with 6-chloro-1-hexanol. 1H NMR analysis revealed signals at δ 0.97–2.05 ppm and δ 3.62–4.09 ppm from the butoxy group and the hexanol side chain, as well as aromatic signals at δ 7.12–7.73 ppm, confirming successful synthesis. Subsequently, the obtained compound was further reacted to synthesize target compound 4, which was successfully obtained.
Block copolymers were synthesized via RAFT polymerization using POEGMA homopolymers. The successful synthesis was confirmed by 1H NMR analysis, which showed characteristic peaks originating from both the photosensitizer and the polymer backbone. Furthermore, UV–vis spectroscopy (in CH2Cl2) revealed an absorption band at 684 nm, corresponding to the characteristic wavelength of the photosensitizer (Figure 1).
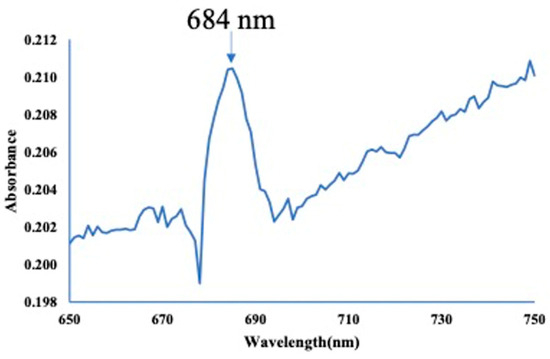
Figure 1.
Absorption spectrum of POEGMA-b-PBuPc-C6MA.
To evaluate the micelle formation, dynamic light scattering (DLS) measurements were performed. Samples at a concentration of 0.16 mg/mL were prepared in pure water, and the micelle size distribution was determined (Figure 2).
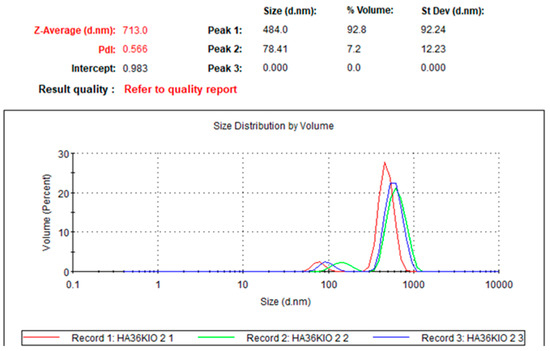
Figure 2.
DLS measurement at 0.16 mg/mL.
As a result, the average sizes of the micelles were confirmed to be 484 nm and 78 nm. The 78 nm micelles are considered to be broken micelles.
Before conducting the photocytotoxicity tests, singlet oxygen generation was evaluated. 1,3-Diphenylisobenzofuran (DPBF) efficiently reacts with singlet oxygen, resulting in a decrease in absorbance at 416 nm. Therefore, the greater the decrease in absorbance at 416 nm, the higher the singlet oxygen generation ability. Since POEGMA-b-PBuPc-C6MA exhibits a maximum absorption wavelength at 684 nm, a red LED lamp with a wavelength of 660 nm was used as the light source. The absorbance results at a fixed wavelength of 416 nm before and after light irradiation are shown in Table 1. Furthermore, the quantum yield was calculated using Equation (1) below.

Table 1.
Comparison of quantum yield.
As a result, the slight change in quantum yield upon addition of POEGMA-b-PBuPc-C6MA suggested the generation of singlet oxygen.
The phototoxicity test was evaluated using HeLa cells and MCF−7 cells. The results showed a significant decrease in survival rate in MCF−7 cells (Figure 3). In this study, photocytotoxicity was confirmed; however, it remains unclear whether this effect results from cellular uptake of the photosensitizer-loaded micelles or from their adhesion to the cell membrane. Further investigation is required to clarify the mechanism of cellular interaction.
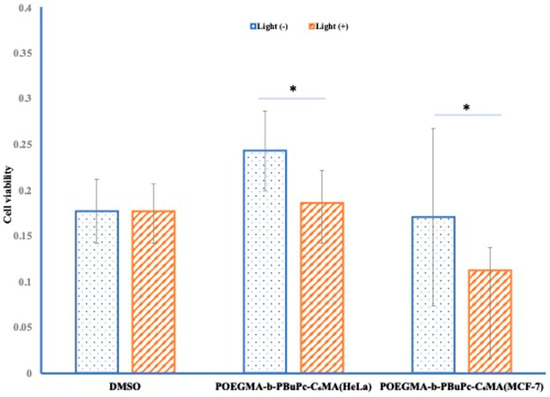
Figure 3.
Photocytotoxicity using HeLa and MCF−7.
4. Conclusions
Photosensitizer-loaded polymeric micelles were prepared, and photocytotoxicity assays revealed significant cytotoxic effects in MCF−7 cells. Ongoing toxicity tests using HT29 cells are currently underway, and the results will be presented upon completion.
Author Contributions
Conceptualization, Y.U.; methodology, Y.U.; software, H.Y.; validation, H.Y., D.M. and S.N.; formal analysis, D.M. and K.T.; investigation, Y.U. and K.T., resources, K.T. and Y.U.; data curation, Y.U.; writing—original draft preparation, H.Y. and D.M.; writing—review and editing, Y.U.; visualization, K.T.; supervision, Y.U.; project administration, Y.U.; funding acquisition, Y.U. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant-in-Aid for Scientific Research (C), grant number 24K15761.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting the findings of this study are available for non-commercial use upon request from the corresponding author. Commercial use of the data is not permitted.
Acknowledgments
We gratefully acknowledge Kunishige Onuma and Futoshi Okada for their valuable suggestions and discussions. This work was supported by Grant-in-Aid for Scientific Research (C) Grant Number 24K15761 and partly sponsored by NIT, Yonago College financial support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, L.R.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, G. Photodynamic therapy and cancer: A brief sightseeing tour. Expert Opin. Drug Deliv. 2007, 4, 131–148. [Google Scholar] [CrossRef] [PubMed]
- Iida, N.; Tokunaga, E.; Saito, N.; Shibata, N. Synthesis and property of novel phthalocyanine having a 3, 5-bis-pentafluorosulfanylphenyl group on the α-peripheral position. J. Fluor. Chem. 2014, 168, 93–98. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).