Study and Development on the Hydroxamation of Natural Resinic Acids: Synthesis and Computational Studies †
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Equipment
2.2. General Procedure for Abietohydroxamic Acid (1a)
2.3. Computational Methods
3. Results and Discussion
3.1. Screening of Coupling Conditions
3.2. One-Pot Phosphate-Mediated Hydroxamation
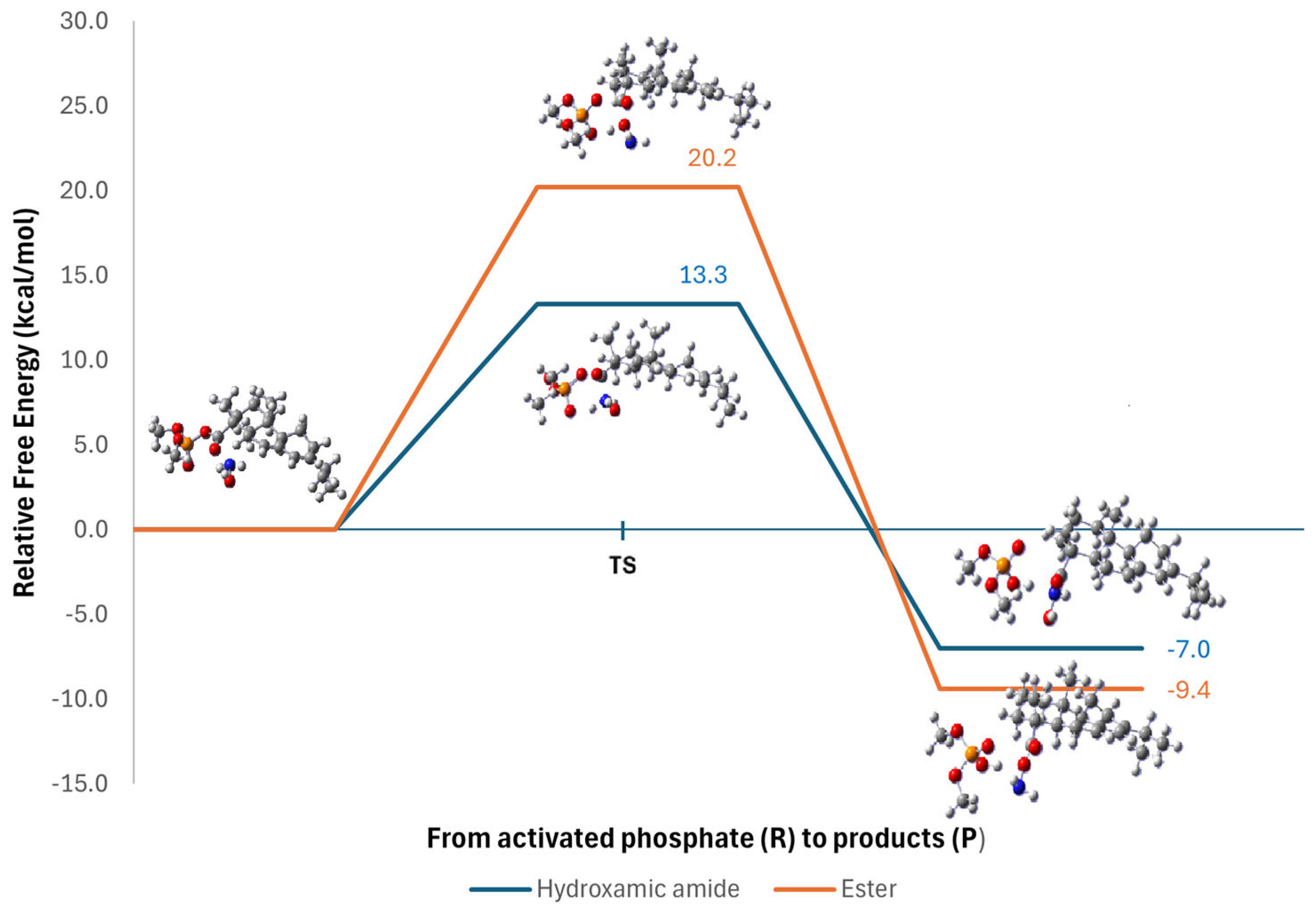
3.3. Computational Studies
3.4. General Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- González, M.A.; Correa-Royero, J.; Agudelo, L.; Mesa, A.; Betancur-Galvis, L. Synthesis and Biological Evaluation of Abietic Acid Derivatives. Eur. J. Med. Chem. 2009, 44, 2468–2472. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A. Methods for Hydroxamic Acid Synthesis. Curr. Org. Chem. 2019, 23, 978–993. [Google Scholar] [CrossRef] [PubMed]
- Bardyshev, I.I. Diterpenoid Carboxylic Acid Anhydrides of the Abietane, Pimarane, and Isopimarane Series. Russ. J. Org. Chem. 1999, 35, 41–55. [Google Scholar]


| Activation Method | Solvent | Result/Observation |
|---|---|---|
| EDC·HCl/DMAP | DCM | Mixture of 1 and 1a; only trace amounts of 1a isolated |
| DEPC | THF | Mixture of 1 and 1a; separation difficult |
| PPAA | CH3CN | No conversion; 1 fully recovered |
| SOCl2 | DCM | Unstable acyl chloride; 1 recovered after work-up |
| Oxalyl chloride | DCM/THF | Decomposition; 1 recovered after work-up |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendoza-Hernández, W.E.; Zaragozá, R.J.; González-Cardenete, M.A. Study and Development on the Hydroxamation of Natural Resinic Acids: Synthesis and Computational Studies. Chem. Proc. 2025, 18, 81. https://doi.org/10.3390/ecsoc-29-26736
Mendoza-Hernández WE, Zaragozá RJ, González-Cardenete MA. Study and Development on the Hydroxamation of Natural Resinic Acids: Synthesis and Computational Studies. Chemistry Proceedings. 2025; 18(1):81. https://doi.org/10.3390/ecsoc-29-26736
Chicago/Turabian StyleMendoza-Hernández, William E., Ramón J. Zaragozá, and Miguel A. González-Cardenete. 2025. "Study and Development on the Hydroxamation of Natural Resinic Acids: Synthesis and Computational Studies" Chemistry Proceedings 18, no. 1: 81. https://doi.org/10.3390/ecsoc-29-26736
APA StyleMendoza-Hernández, W. E., Zaragozá, R. J., & González-Cardenete, M. A. (2025). Study and Development on the Hydroxamation of Natural Resinic Acids: Synthesis and Computational Studies. Chemistry Proceedings, 18(1), 81. https://doi.org/10.3390/ecsoc-29-26736








