Abstract
This work presents a step-by-step synthesis of a new derivative of 1,3,5-oxadiazine. In the first step, by reacting N-(1-amino-2,2,2-trichloroethyl)acetamide with 2-bromobenzoyl isothiocyanate, a previously undescribed thiourea, N-((1-acetamido-2,2,2-trichloroethyl)carbamothioyl)-2-bromobenzamide, was obtained. In the next step, this compound was treated with dicyclohexylcarbodiimide (DCC), resulting in cyclization and the formation of a new derivative of 1,3,5-oxadiazine-N-(1-(((2E,4E)-6-(2-bromophenyl)-3-cyclohexyl-2-(cyclohexylimino)-2,3-dihydro-4H-1,3,5-oxadiazin-4-ylidene)amino)-2,2,2-trichloroethyl)acetamide. The reaction was carried out in acetonitrile at reflux for one hour. Presumably, in the first step of the transformation, under the action of DCC, the elimination of a hydrogen sulfide molecule occurs with the formation of an intermediate carbodiimide derivative-N-(((1-acetamido-2,2,2-trichloroethyl)imino)methylene)-2-bromobenzamide. This intermediate then undergoes a [4+2] cycloaddition reaction with another DCC molecule to form the desired 1,3,5-oxadiazine product in 82% yield. Its structure was confirmed by NMR spectroscopy (1H and 13C).
1. Introduction
In recent decades, 1,3,5-oxadiazine derivatives have attracted growing attention across various scientific fields [1,2,3,4,5,6]. Compounds containing the 1,3,5-oxadiazine ring are considered in pharmacy and medicine as potential chemotherapeutic agents [4], as well as antibacterial [7,8,9,10,11,12] and antifungal agents [10,11]. They are also important in agriculture as insecticides (e.g., thiamethoxam [13,14] and its analogues [15,16]), herbicides [17], and larvicides [18,19,20]. Beyond biological activity, these compounds serve as valuable synthons and catalysts in organic synthesis and have been applied in the production of ionic liquids [21], polymers [22], and explosives [23]. Cucurbit[n]uryls incorporating a 1,3,5-oxadiazine ring are of particular interest in supramolecular chemistry for constructing molecular clips [24,25,26,27,28,29,30,31,32,33].
Most 1,3,5-oxadiazines are synthetic; only two natural representatives are known [34,35]. Their main synthetic routes involve [4+2]-cycloaddition [8,9,10,11,17,18,19,36,37,38,39,40], intramolecular cyclization of diols [15,16,23,41,42,43,44,45,46], bisamidials [47], or substituted thioureas [48,49,50]. Recently, their preparation via transformation of other heterocyclic systems has also been reported [23,51]. This article describes the synthesis of a new 1,3,5-oxadiazine derivative: N-(1-(((2E,4E)-6-(2-bromophenyl)-3-cyclohexyl-2-(cyclohexylimino)-2,3-dihydro-4H-1,3,5-oxadiazin-4-ylidene)amino)-2,2,2-trichloroethyl)acetamide.
2. Materials and Methods
Spectral studies, including 1H NMR (400 MHz) and 13C NMR (100 MHz), were conducted on DMSO-d6 solutions using a Varian Agilent VNMRS 400 MHz spectrometer (Agilent Technologies, Inc., Santa Clara, CA, USA). Residual solvent signals served as internal standards. Elemental analysis was performed with a LECO CHNS-900 instrument (LECO Corporation, St. Joseph, MI, USA). The reaction progress and purity of the synthesized compounds were monitored by thin-layer chromatography on Silufol UV-254 plates (Serva-Feinbiochemica GmbH & Co, Heidelberg, Germany) with a chloroform and acetone (3:1) eluent ratio. Melting points were determined in open capillaries.
Synthesis of N-((1-acetamido-2,2,2-trichloroethyl)carbamothioyl)-2-bromobenzamide (3). A solution of 10 mmol of 2-bromobenzoyl isothiocyanate (2) in 10 mL of acetonitrile was added to 10 mmol (2.05 g) of N-(1-amino-2,2,2-trichloroethyl)acetamide (1) [52] in 15 mL of MeCN. The mixture was brought to a boil and left at room temperature for 12 h. The resulting precipitate of thiourea (3) was filtered, washed with 10 mL of acetonitrile, and purified by recrystallization from methanol. Pale yellow crystals; yield 89% (3.98 g); mp 190–192 °C (MeOH); Rf = 0.74. 1H NMR: δ 12.25 (s, 1H, NH), 11.54 (d, J = 9.3 Hz, 1H, NH), 9.25 (br. s, 1H, NH), 7.71 (d, J = 7.3 Hz, 1H, Harom.), 7.57 (d, J = 7.3 Hz, 1H, Harom.), 7.51–7.44 (m, 2H, Harom.), 7.27 (dd, J = 8.8, 9.3 Hz, 1H, CH), 1.97 (s, 1H, NH). Anal. Calcd (%) for C12H11BrCl3N3O2S (447.55): C, 32.20; H, 23.76; N, 9.39; S, 7.16. Found: C, 32.13; H, 23.71; N, 9.45; S, 7.22.
Synthesis of N-(1-(((2E,4E)-6-(2-bromophenyl)-3-cyclohexyl-2-(cyclohexylimino)-2,3-dihydro-4H-1,3,5-oxadiazin-4-ylidene)amino)-2,2,2-trichloroethyl)acetamide (5). A mixture of 10 mmol of thiourea 3 and 20 mmol (4.13 g) of dicyclohexylcarbodiimide was added to 40 mL of dry acetonitrile and refluxed for 40–50 min. The reaction mixture was cooled, and the precipitated crystals were filtered and washed with 10 mL of MeCN. The final product was purified by recrystallization from methanol. White crystals; yield 82% (5.08 g); mp 169–171 °C (MeOH); Rf = 0.75. 1H NMR: δ 8.50 (br. s, 1H, NH), 7.91 (d, J = 7.0 Hz, 1H, Harom.), 7.84 (d, J = 7.0 Hz, 1H, Harom.), 7.66–7.58 (m, 2H, Harom.), 6.78 (d, J = 8.8 Hz, 1H, CH), 4.81–4.75 (m, 1H, cyclohexyl), 3.79 (br. s, 1H, cyclohexyl), 2.75–2.59 (m, 2H, cyclohexyl), 1.91 (s, 3H, CH3), 1.82–1.12 (m, 18H, cyclohexyl). 13C NMR: δ 168.7 (C=O), 157.4 (C=N), 147.5 (C=N), 135.1 (C=N), 134.3, 134.0, 132.4, 129.9, 128.0, 120.7 (Carom.), 103.8 (CCl3), 72.4 (CH), 56.0, 52.5, 33.8, 33.6, 26.9, 26.1, 26.0, 25.5, 25.2, 23.7, 22.7 (cyclohexyl). Anal. Calcd (%) for C25H31BrCl3N5O2 (619.81): C, 48.45; H, 5.04; N, 11.30. Found: C, 48.39; H, 4.98; N, 11.38.
3. Results and Discussion
The starting compound, N-(1-amino-2,2,2-trichloroethyl)acetamide (1), was synthesized according to a previously reported procedure [52]. It readily reacted with 2-bromobenzoyl isothiocyanate (2), yielding N-((1-acetamido-2,2,2-trichloroethyl)carbamothioyl)-2-bromobenzamide (3) (Scheme 1). The product was obtained in almost quantitative yield, reaching approximately 89% after recrystallization. Subsequently, thiourea 3 was treated with dicyclohexylcarbodiimide (DCC). Under the influence of DCC, hydrogen sulfide was eliminated from thiourea 3, most likely leading to the formation of carbodiimide intermediate 4. This intermediate then participated in a [4+2] cycloaddition with an additional DCC molecule, affording the target oxadiazine 5.
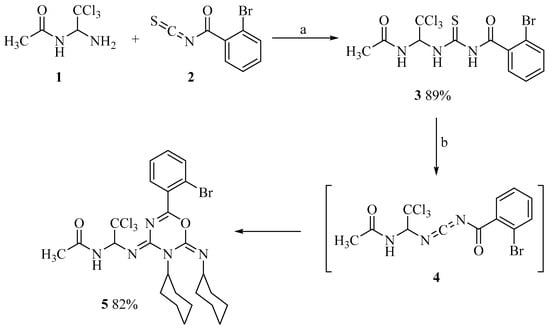
Scheme 1.
Synthesis of N-((1-acetamido-2,2,2-trichloroethyl)carbamothioyl)-2-bromobenzamide (3) and its cyclisation into 1,3,5-oxadiazine (5). Reagents and conditions: (a) MeCN, reflux 1–2 min, r.t. 12 h; (b) 2 DCC, MeCN, reflux 40–50 min, r.t. 1–2 h.
The structures of the synthesized compounds were confirmed by spectral analysis. In the 1H NMR spectrum of compound 3, signals corresponding to three NH protons were detected, along with a characteristic doublet–doublet signal from the CH proton of the alkylamide fragment. In contrast, the spectrum of oxadiazine 5 displayed a single NH proton signal and a doublet from the CH group. Additionally, the 1H NMR spectrum of compound 5 contained signals corresponding to 22 protons of two cyclohexyl rings, which is consistent with the [4+2] cycloaddition process. The 13C NMR data further corroborated the formation of oxadiazine 5, revealing the absence of the C=S signal, the presence of a single C=O signal at 168.7 ppm, and three C=N signals in the 157.4–135.1 ppm range.
4. Conclusions
In summary, we have developed a convenient and efficient method for the synthesis of a new 1,3,5-oxadiazine derivative. Our synthetic approach is based on the reaction of N-(1-amino-2,2,2-trichloroethyl)acetamide with 2-bromobenzoyl isothiocyanate, followed by DCC-mediated cyclization through a [4+2] cycloaddition mechanism. The target compound was obtained in high yield, demonstrating the effectiveness of the developed method. The structures of the intermediates and final product were confirmed by 1H and 13C NMR spectroscopy, which clearly indicated the transformation of thiourea into the oxadiazine core. The absence of the C=S signal and the presence of characteristic C=N signals supported the successful ring formation. This synthetic strategy provides a practical pathway for the preparation of structurally diverse oxadiazines. The results of our work may serve as a foundation for the further design of heterocyclic systems with potential biological activity.
Author Contributions
V.V.P.; methodology, formal analysis, investigation. P.V.Z.; conceptualization, methodology, writing—original draft, formal analysis, investigation, visualization, project administration. D.S.K.; methodology, formal analysis, investigation. O.V.O.; validation, resources, writing—review, and editing. V.V.K.; validation, resources, writing—review, and editing. A.V.K.; validation, supervision, writing—review, and editing. All authors have read and agreed to the published version of the manuscript.
Funding
The work has been carried out within the framework of the project “New principles of synthesis of fluorescent materials and biologically active N,S-containing heterocycles” (state registration number 0123U101168) funded by the Ministry of Education and Science of Ukraine.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Reported data are available from the authors via e-mail contact.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zadorozhnii, P.V.; Kiselev, V.V.; Kharchenko, A.V. 1,3,5-Oxadiazines and 1,3,5-Thiadiazines. In Comprehensive Heterocyclic Chemistry, 4th ed.; Black, D.S.C., Cossy, J., Stevens, C.V., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 9, pp. 456–506. [Google Scholar]
- Smalley, R.K. 1,3,5-Oxadiazines and 1,3,5-Thiadiazines. In Comprehensive Heterocyclic Chemistry, 2nd ed.; Katritzky, A.R., Rees, C.W., Scriven, E.F.V., Eds.; Pergamon: Oxford, UK, 1996; Volume 6, pp. 783–823. [Google Scholar]
- Shobana, N.; Farid, P. 1,3,5-Oxadiazines and 1,3,5-Thiadiazines. In Comprehensive Heterocyclic Chemistry, 3rd ed.; Katritzky, A.R., Scriven, E.F.V., Ramsden, C.A., Taylor, R.J.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; Volume 9, pp. 457–521. [Google Scholar]
- Ke, S.; Cao, X.; Liang, Y.; Wang, K.; Yang, Z. Synthesis and Biological Properties of Dihydro-Oxadiazine-Based Heterocyclic Derivatives. Mini Rev. Med. Chem. 2011, 11, 642–657. [Google Scholar] [CrossRef]
- Pasha, M.A.; Mondal, S.; Panigrahi, N. Review of Synthetic Strategies in the Development of Oxadiazine Scaffolds. Mediterr. J. Chem. 2019, 8, 338–364. [Google Scholar] [CrossRef]
- Zadorozhnii, P.V.; Pokotylo, I.O.; Kiselev, V.V.; Kharchenko, A.V.; Okhtina, O.V. In silico analysis of 6-(4-chlorophenyl)-N-aryl-4-(trichloromethyl)-4H-1, 3, 5-oxadiazin-2-amines as potential antagonists of VEGFR-1. Indo Am. J. Pharm. Sci. 2019, 6, 4196–4200. [Google Scholar]
- El-Ziaty, A.K.; Shiba, S.A. Antibacterial Activities of New (E)-2-Cyano-3-(3,4-dimethoxyphenyl)-2-propenoylamide Derivatives. Synth. Commun. 2007, 37, 4043–4057. [Google Scholar] [CrossRef]
- Patel, H.S.; Patel, K.B. Synthesis and Biological Activity of 3-[4H-(1,2,4)-Triazolyl]-2,6-diaryl-1,3,5-oxadiazine-4-thione. Phosphorus Sulfur Silicon Relat. Elem. 2009, 184, 2443–2452. [Google Scholar]
- Rambabu, N.; Viral, B.M.; Kirti, J.G. Synthesis and characterization of N-(4-(4-chlorophenyl)-6-(3,4-dimethylphenyl)pyrimidin-2-yl)-4-(2,6-diphenyl-4-thioxo-2H-1,3,5-oxadiazin-3(4H)-yl)benzenesulfonamide. Der Pharma Chem. 2012, 4, 511–516. [Google Scholar]
- Rambabu, N.; Ramachandran, D.; Viral, B.M.; Kirti, J.G. Synthesis, characterization and biological evaluation of 2,6-diphenyl-3-(4-(3-phenyl-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazol-6-yl)phenyl)-2H-1,3,5-oxadiazine-4(3H)-thione. Der Pharma Chem. 2012, 4, 639–643. [Google Scholar]
- Patel, K.H.; Mehta, A.G. Synthesis and antifungal activity of [(4-(2-naphthalenyl) thiazol-2-yl)-2- (substituted phenyl)-6-phenyl- 4-thioxo- 1,3,5-oxadiazine] derivatives. Der Chem. Sin. 2012, 3, 1410–1414. [Google Scholar]
- Modi, V.P.; Jani, D.H.; Patel, H.S. Synthesis and Antimicrobial Evaluation of Spiro Compounds Containing 1,2,4-Triazole and Isatin. Orbital Electron. J. Chem. 2011, 3, 68–79. [Google Scholar]
- Ding, J.; Li, H.; Zhang, Z.; Lin, J.; Liu, F.; Mu, W. Thiamethoxam, Clothianidin, and Imidacloprid Seed Treatments Effectively Control Thrips on Corn Under Field Conditions. J. Insect Sci. 2018, 18, 19. [Google Scholar] [CrossRef]
- Juan Valente, M.-G.; María del Refugio, C.-C.; Daniel Arturo, R.-L.; Joaquín, M.-G.; Fabiola, L.-R.; Otto Raúl, L.-O. Impact of Thiamethoxam in Papaya Cultivation (Carica papaya Linnaeus) in Rotation with Watermelon (Citrullus lanatus) Crops. Agriculture 2019, 9, 129. [Google Scholar] [CrossRef]
- Maienfisch, P. Synthesis and Properties of Thiamethoxam and Related Compounds. Z. Naturforsch. 2006, 61b, 353–359. [Google Scholar] [CrossRef]
- Yang, S.; Kang, T.; Rui, C.; Yang, X.; Sun, Y.; Cui, Z.; Ling, Y. Design, Synthesis, and Insecticidal Activity of 1,5-Diphenyl-1-pentanone Analogues. Chin. J. Chem. 2011, 29, 2394–2400. [Google Scholar] [CrossRef]
- Assy, M.G.; Haiekl, A.; Moustafa, H.Y. Behavior of terephthaloyl isothiocyanate towards carbon and nitrogen reagents. Phosphorus Sulfur Silicon Relat. Elem. 1995, 106, 179–185. [Google Scholar] [CrossRef]
- Shiba, S.A. Decomposition of 2-Propenoyl Azide Derivatives. Synthesis and Larvicidal Activity of Novel Products. Arch. Pharm. Pharm. Med. Chem. 1998, 331, 91–96. [Google Scholar] [CrossRef]
- Shiba, S.A. Synthesis and insecticidal activity of novel acrylonitrile derivatives. Phosphorus Sulfur Silicon Relat. Elem. 1996, 114, 29–37. [Google Scholar] [CrossRef]
- Chee, G.L.; Brewer, A.D.; Bell, A.R.; Aksinenko, A.Y.; Sokolov, V.B. Substituted oxadiazines useful as pesticides. U.S. Patent 6514911 B1, 4 February 2003. [Google Scholar]
- Gao, Y.; Arritt, S.W.; Twamley, B.; Shreeve, J.M. Guanidinium-Based Ionic Liquids. Inorg. Chem. 2005, 44, 1704–1712. [Google Scholar] [CrossRef] [PubMed]
- Decostanzi, M.; Auvergne, R.; Darroman, E.; Boutevin, B.; Caillol, S. Reactivity and kinetics of HDI-iminooxadiazinedione: Application to polyurethane synthesis. Eur. Polym. J. 2017, 96, 443–451. [Google Scholar] [CrossRef]
- Gao, H.; Shreeve, J.M. The Many Faces of FOX-7: A Precursor to High-Performance Energetic Materials. Angew. Chem. Int. Ed. 2015, 54, 6335–6338. [Google Scholar] [CrossRef]
- Ding, H.; Roberts, A.G.; Harran, P.G. Synthetic (±)-Axinellamines Deficient in Halogen. Angew. Chem. Int. Ed. 2012, 51, 4340–4343. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Wu, L.H.; Xiao, X.; Zhang, Y.Q.; Xue, S.F.; Tao, Z.; Day, A.I. Locating the Cyclopentano Cousins of the Cucurbit[n]uril Family. J. Org. Chem. 2012, 77, 606–611. [Google Scholar] [CrossRef]
- Limei, Z.; Jiannan, Z.; Yunqian, Z.; Qianjiang, Z.; Saifeng, X.; Zhu, T.; Jianxin, Z.; Xin, Z.; Zhanbin, W.; Lasheng, L.; et al. Opposing substitution in cucurbit[6]urils forms ellipsoid cavities: The symmetrical dicyclohexanocucurbit[6]uril is no exception highlighted by inclusion and exclusion complexes. Supramol. Chem. 2008, 20, 709–716. [Google Scholar] [CrossRef]
- Zhao, Y.; Mandadapu, V.; Iranmanesh, H.; Beves, J.E.; Day, A.I. The Inheritance Angle: A Determinant for the Number of Members in the Substituted Cucurbit[n]uril Family. Org. Lett. 2017, 19, 4034–4037. [Google Scholar] [CrossRef]
- Ma, D.; Zavalij, P.Y.; Isaacs, L. Acyclic Cucurbit[n]uril Congeners Are High Affinity Hosts. J. Org. Chem. 2010, 75, 4786–4795. [Google Scholar] [CrossRef]
- Sokolov, J.; Lizal, T.; Sindelar, V. Dimeric molecular clips based on glycoluril. New J. Chem. 2017, 41, 6105–6111. [Google Scholar] [CrossRef]
- Kikot, L.S.; Kulygina, C.Y.; Lyapunov, A.Y.; Shishkina, S.V.; Zubatyuk, R.I.; Bogashchenko, T.Y.; Kirichenko, T.I. Synthesis and complexation of molecular clips based on diphenylglycoluril and dibenzocrown ethers with alkali metal cations and paraquat. Tetrahedron 2018, 74, 5725–5732. [Google Scholar] [CrossRef]
- Hardouin-Lerouge, M.; Cotelle, Y.; Legoupy, S.; Hudhomme, P. Synthesis of glycoluril-tetrathiafulvalene molecular clips for electron-deficient neutral guests through a straightforward Diels–Alder strategy. New J. Chem. 2014, 38, 5341–5348. [Google Scholar] [CrossRef]
- Gilberg, L.; Zhang, B.; Zavalij, P.Y.; Sindelar, V.; Isaacs, L. Acyclic cucurbit[n]uril-type molecular containers: Influence of glycoluril oligomer length on their function as solubilizing agents. Org. Biomol. Chem. 2015, 13, 4041–4050. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Isaacs, L. Acyclic Cucurbit[n]uril-type Molecular Containers: Influence of Aromatic Walls on their Function as Solubilizing Excipients for Insoluble Drugs. J. Med. Chem. 2014, 57, 9554–9563. [Google Scholar] [CrossRef]
- Wu, J.B.; Cheng, Y.D.; Kuo, S.C.; Wu, T.S.; Iitaka, Y.; Ebizuka, Y.; Sankawa, U. Fissoldhimine, a novel skeleton alkaloid from Fissistigma Oldhamii. Chem. Pharm. Bull. 1994, 42, 2202–2204. [Google Scholar] [CrossRef]
- Bergmann, T.; Schories, D.; Steffan, B. Alboinon, an oxadiazinone alkaloid from the ascidian Dendrodoa grossularia. Tetrahedron 1997, 53, 2055–2060. [Google Scholar] [CrossRef]
- Onys’ko, P.P.; Sinitsa, A.A.; Pirozhenko, V.V.; Chernega, A.N. Synthesis of phosphorylated 1,3,5-oxadiazines via N-acyltrifluoroacetimidoilphosphonates. Heteroat. Chem. 2002, 13, 22–26. [Google Scholar] [CrossRef]
- Pokotylo, I.O.; Zadorozhnii, P.V.; Kiselev, V.V.; Kharchenko, A.V. Synthesis, Spectral Characteristics and Molecular Structure of 2H-1,3,5-Oxadiazine-2,4(3H)-Diimine Derivatives. J. Heterocycl. Chem. 2023, 60, 1799–1808. [Google Scholar] [CrossRef]
- Zadorozhnii, P.V.; Pokotylo, I.O.; Kiselev, V.V.; Kharchenko, A.V. Synthesis of (Z)-N,3-Dicyclohexyl-6-Substituted-4-(Trichloromethyl)-3,4-Dihydro-2H-1,3,5-Oxadiazin-2-Imines via [4+2] Hetero Diels-Alder Reaction: Their Spectral Characteristics and Molecular Structure. Chem. Data Coll. 2023, 48, 101093. [Google Scholar] [CrossRef]
- Cyrener, J.; Burger, K. Überraschende Reaktionen von 4,4-Bis(trifluormethyl)-1-oxa-3-azabuta-1,3-dienen: Tandem-Reaktion mit Acrylnitril. Monatshefte Chem. 1995, 126, 1383–1390. [Google Scholar] [CrossRef]
- Behalo, M.S.; Gad El-karim, I.A.; Issac, Y.A.; Farag, M.A. Synthesis of novel pyridazine derivatives as potential antimicrobial agents. J. Sulfur Chem. 2014, 35, 661–673. [Google Scholar] [CrossRef]
- Ni, H.; Zhang, Y.; Zhang, F.; Zhao, J.; Wu, L.; Chu, X. Synthesis, structural characterization and theoretical approach of 3-(2,6-dichlorobenzyl)-5-methyl-N-nitro-1,3,5-oxadiazinan-4-imine. Spectrochim. Acta Part A 2015, 138, 648–659. [Google Scholar] [CrossRef]
- Qu, W.Y.; She, D.M.; Zhao, J.; Lin, D.J.; Huang, Q.L.; Li, F.M. Mannich-Type Reaction for Synthesis of 3-Methyl-4-nitroimino-tetrahydro-1,3,5-oxadiazine. Synth. Commun. 2012, 42, 1950–1958. [Google Scholar] [CrossRef]
- He, J.L.; Cheng, W.H. Synthesis, Characterization and Crystal Structure of N-(3-((2-Chlorothiazol-5-yl)methyl)-5-methyl-1,3,5-oxadiazinan-4-ylidene)nitramide. Asian J. Chem. 2015, 27, 2383–2385. [Google Scholar] [CrossRef]
- Kang, T.N.; Zhang, L.; Ling, Y.; Yang, X.L. 3-[(E)-3,7-Di methyl octa-2,6-dien yl]-5-methyl-N-nitro-1,3,5-oxadiazinan-4-imine. Acta Cryst. E 2008, 64, o1154. [Google Scholar] [CrossRef]
- Vijayan, A.; Baiju, T.V.; Jijy, E.; Prakash, P.; Shimi, M.; Joseph, N.; Pihko, P.M.; Varughesed, S.; Radhakrishnan, K.V. An easy access to fused chromanones via rhodium catalyzed oxidative coupling of salicylaldehydes with heterobicyclic olefins. Tetrahedron 2016, 72, 4007–4015. [Google Scholar] [CrossRef]
- Vijayan, A.; Jumaila, C.U.; Radhakrishnan, K.V. Rhodium(III)-Catalyzed C−H Activation of O-Acetyl Ketoximes/N-Methoxybenzamides toward the Synthesis of Isoquinoline/Isoquinolone-Fused Bicycles. Asian J. Org. Chem. 2017, 6, 1561–1565. [Google Scholar] [CrossRef]
- Younis, S.K.; Ahmed, B.A. Synthesis of Some New 1,3,5-Oxadiazine Derivatives. Rafidain J. Sci. 2008, 19, 10–17. [Google Scholar]
- Zadorozhnii, P.V.; Kiselev, V.V.; Pokotylo, I.O.; Kharchenko, A.V. A new method for the synthesis of 4H-1,3,5-oxadiazine derivatives. Heterocycl. Commun. 2017, 23, 369–374. [Google Scholar] [CrossRef]
- Zadorozhnii, P.V.; Pokotylo, I.O.; Kiselev, V.V.; Kharchenko, A.V.; Okhtina, O.V. Synthesis and Spectral Characteristics of Some New 4H-1,3,5-Oxadiazine Derivatives. Res. J. Pharm. Biol. Chem. Sci. 2019, 10, 1508–1515. [Google Scholar]
- Zadorozhnii, P.V.; Kiselev, V.V.; Pokotylo, I.O.; Okhtina, O.V.; Kharchenko, A.V. Synthesis and mass spectrometric fragmentation pattern of 6-(4-chlorophenyl)-N-aryl-4-(trichloromethyl)-4H-1,3,5-oxadiazin-2-amines. Heterocycl. Commun. 2018, 24, 273–278. [Google Scholar] [CrossRef]
- Gao, Y.; Hu, Z.; Dong, J.; Liu, J.; Xu, X. Chemoselective Double Annulation of Two Different Isocyanides: Rapid Access to Trifluoromethylated Indole-Fused Heterocycles. Org. Lett. 2017, 19, 5292–5295. [Google Scholar] [CrossRef] [PubMed]
- Pokotylo, I.O.; Zadorozhnii, P.V.; Kiselev, V.V.; Kharchenko, A.V. A New Approach to the Synthesis of 4H-1,3,5-Oxadiazine Derivatives. Biointerface Res. Appl. Chem. 2023, 13, 379. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).