Abstract
This study investigates thienyl-substituted diketopyrrolopyrrole (DPP) derivatives with tailored alkyl chain modifications on the DPP core to tune molecular packing, solubility, and optoelectronic properties critical for device applications. A key advancement is the use of microwave-assisted synthesis, which dramatically reduces reaction times (40 min vs. 12 h), improves yields (up to 80% for long chains), and lowers energy consumption, supporting green chemistry principles. The combined strategy of molecular engineering and efficient synthesis enables sustainable production of high-performance DPP materials.
1. Introduction
Diketopyrrolopyrroles (DPPs) are a class of organic dyes first discovered in the mid-1970s by Farnum [1] and co-workers. Initially developed as high-performance red pigments [2,3], DPPs found widespread use in inks, paints, and plastics due to their vibrant color and durability. Over time, these molecules have garnered significant attention from chemists and physicists owing to their exceptional optical, electronic, and structural properties, coupled with good thermal and photo-stability [4,5]. As a result, DPPs have emerged as key players in the thriving field of organic electronics [6,7]. This versatile class of organic compounds has been widely explored for applications in diverse electronic devices such as organic photovoltaics (OPVs) [8,9,10,11,12], organic field-effect transistors (OFETs) [13,14], organic light-emitting diodes (OLEDs), sensors [15,16,17], two-photon absorption materials [18], and even bioimaging [16,19,20,21] and photothermal agents for cancer treatment [10,22,23]. Recent theoretical and experimental studies have also highlighted the potential of DPP derivatives as singlet fission (SF) materials [24,25,26], attributed to their photostability, inherent diradical character, and chemically tunable structure [27,28,29].
The rapid evolution of organic electronics is not only fueled by the development of increasingly high-performance materials, such as DPP-based dyes with their π-conjugated backbone and outstanding optoelectronic properties, but also by the growing demand for environmentally sustainable processes. In line with current sustainability goals, organic optoelectronics now requires the implementation of greener synthetic methodologies. One of the most critical steps in the development of DPP derivatives is N-alkylation, which in conventional protocols often involves long reaction times, high energy consumption, and hazardous reagents.
These challenges underscore the urgent need for greener and more sustainable synthetic approaches.
In our work, we present a series of thienyl-DPP-based molecules featuring varying substituents on the lactam nitrogens. Specifically, we have synthesized derivatives with N-alkyl chains of different lengths and branching patterns, exploiting microwave-assisted organic synthesis (MAOS) to streamline this functionalization process (Figure 1).
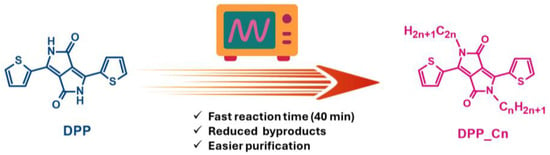
Figure 1.
Microwave synthesis method.
Microwave-assisted (MWA) synthesis has emerged as a transformative tool in organic chemistry [30], enabling rapid and uniform heating that enhances reaction rates, yields, and product purity while reducing energy consumption and environmental impact [31,32]. This method aligns with the principles of green chemistry by offering a sustainable and efficient alternative to traditional heating techniques [33,34].
By adopting MAOS for the N-alkylation of DPP derivatives, we aim to achieve efficient functionalization under milder and more sustainable conditions, reducing the environmental footprint of the synthesis process.
N-alkylation is a critical step in tailoring the solubility and electronic properties of DPP derivatives, which directly influence their performance in device applications [35]. Introducing a variety of alkyl groups allows fine-tuning of molecular packing, charge transport, and light absorption properties, essential for optimizing their functionality in organic electronic devices [36,37,38]. Despite its importance, traditional N-alkylation methods often pose challenges related to harsh conditions, hindering their scalability and environmental viability.
Our investigation focuses on employing microwave-assisted techniques to enhance the efficiency and sustainability of DPP N-alkylation, testing the use of alkyl chains of varying lengths—nominally C4, C6, and C12—as well as branched chains such as 2-ethylhexyl. By optimizing reaction conditions, we demonstrate a novel approach to achieving products with reduced reaction times and energy consumption. This methodology not only addresses the limitations of traditional N-alkylation processes but also advances the principles of green chemistry in the synthesis of high-performance materials.
2. Materials and Methods
2.1. General Information for Synthesis
All reagents were purchased from commercial sources and used without further purification. All solvents were distilled prior to use. All reactions were carried out in an inert atmosphere. The microwave reactions were conducted using CEM Discover 2.0 (CEM Corporation, Charlotte, NC, USA). The 1H- NMR spectra were recorded with a Bruker ARX 400 MHz spectrometer (Bruker, Karlsruhe, Germany). The molecules synthetized are reported in Scheme 1.
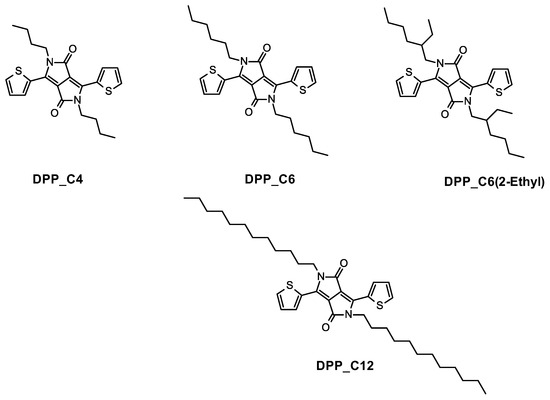
Scheme 1.
N-alkylated DPP derivatives with alkyl chains of varying lengths—nominally C4, C6, and C12 and branched chain 2-ethylhexyl, synthetized in this paper.
2.2. Conventional Synthesis
Conventional alkylation reactions in presence of a base and alkylbromide were performed using a nitrogen-filled Schlenk tube. The conventional synthesis for DPP-C4 and DPP-C6 molecules followed the procedure reported in the literature [39].
Synthesis of DPP-C4: 3,6-Di(thiophen-2-yl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione (1 eq, 3.33 mmol), K2CO3 (3.3 eq, 10.99 mmol) and a catalytic amount of 18-crown-6 (0.009 eq, 0.03 mmol) were added to a Schlenk tube and suspended with 12 mL of DMF. The reaction was stirred at 120 °C for 20 min and then 1-bromobutane (3.3 eq., 10.99 mmol) was added dropwise. The reaction mixture was left to reflux overnight. The next day the reaction was allowed to cool to room temperature; the reaction mixture was then recovered using CHCl3. The organic phase was washed with brine two times then dried over Na2SO4 and filtered, and the solvent was evaporated by vacuum. The crude mixture was purified by flash chromatography on silica gel using 9:1 petroleum ether (PE)/ethyl acetate (EtOAc) as the eluent. The clean fraction was dried in vacuum and then analyzed by NMR (1H-NMR). The analysis confirms that the desired product was obtained with 34% yield. 1H NMR (CDCl3, 400 MHz): δ (ppm) 8.92 (d, 2H,Ar-H), 7.63 (d, 2H,Ar-H), 7.27 (t, 2H,Ar-H), 4.08 (t, 4H,-NCH2-), 1.72 (m, 4H), 1.45 (m, 4H), 0.96 (t, 6H,-CH3).
Synthesis of DPP-C6: 3,6-Di(thiophen-2-yl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione (1 eq, 3.33 mmol), K2CO3 (3.3 eq,10.99 mmol) and a catalytic amount of 18-crown-6 (0.009 eq, 0.03 mmol) were added to a Schlenk tube and suspended with 12 mL of DMF. The reaction was stirred at 120 °C for 20 min and then 1-bromohexane (3.3 eq., 10.99 mmol) was added dropwise. The reaction mixture was left to reflux overnight. The next day the reaction was allowed to cool to room temperature; the reaction mixture was then recovered using CHCl3. The organic phase was washed with brine two times then dried over Na2SO4 and filtered, and the solvent was evaporated by vacuum. The crude mixture was purified by flash chromatography on silica gel using 9:1 PE/EtOAc as the eluent. The clean fraction was dried in vacuum and then analyzed by NMR (1H-NMR). The analysis confirms that the desired product was obtained with 40% yield. 1H-NMR (400 MHz, CDCl3): δ 8.85 (d, 2H, Ar-H), 7.55 (d, 2H, Ar-H), 7.21–7.18 (m, 2H, Ar-H), 3.98 (t, 4H, -NCH2-), 1.68 (quintet, 4H), 1.37–1.21 (m, 12H), 0.81 (t, 6H, -CH3).
2.3. Microwave Alkylation Reaction
Microwave-assisted alkylation reactions were performed in nitrogen-filled 10 mL pressure vials from CEM for Discover 2.0.
General procedure for Synthesis of DPP-Cn: 3,6-Di(thiophen-2-yl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione (1 eq, 0.1665mmol), K2CO3 (3.3 eq, 0.5494 mmol) and bromoalcane (3.3 eq.) as well as a catalytic amount of 18-crown-6 were added to a 10 mL microwave vessel and suspended with 1.5 mL of DMF. The reactions were conducted using the fixed power method; the condition used was 170 °C, 150 MW for 40 min. The workup procedure was the same as the conventional synthesis.
DPP-C4: Alcane: 1-bromobutane purified by column chromatography, eluent PE/EtOAc 9:1, 1H NMR (CDCl3, 400 MHz): δ (ppm) 8.92–8.91 (d, 2H,Ar-H), 7.63–7.62 (d, 2H,Ar-H), 7.28–7.26 (t, 2H,Ar-H), 4.09–4.06 (t, 4H,-NCH2-), 1.76–1.69 (m, 4H), 1.49–1.39 (m, 4H), 0.96 (t, 6H,-CH3), 59% yield.
DPP-C6: Alcane: 1-bromohexane purified by column chromatography, eluent PE/EtOAc 9:1, 1H-NMR (400 MHz, CDCl3): δ 8.84–8.83 (d, 2H, Ar-H), 7.56–7.54 (d, 2H, Ar-H), 7.21–7.19 (m, 2H, Ar-H), 4.00–3.97 (t, 4H, -NCH2-), 1.69–163 (quintet, 4H), 1.37–1.31 (m, 4H), 1.26–1.21 (m, 8H), 0.81 (t, 6H, -CH3), 61.5% yield.
DPP-C12: Alcane: 1-bromododecane purified by column chromatography, eluent Hexane/EtOAc 8:2, 1H-NMR (400 MHz, CDCl3): δ 8.92–91 (dd,2H, Ar-H), 7.63–7.62 (dd, 2H, Ar-H), 7.28–7.26 (m, 2H, Ar-H), 4.07–4.04 (t, 4H, -NCH2-), 1.76–1.70 (quintet, 4H), 1.43–1.37 (m, 4H), 1.34–1.20 (m, 32H), 0.86 (t, 6H,-CH3), 82.5% yield.
DPP-C6(2-Ethyl): Alcane: 2-Ethylhexyl bromide purified by column chromatography, eluent PE/EtOAc 9:1, 1H NMR (400 MHz, CDCl3): δ 8.88–8.87 (dd, 2H, Ar-H), 7.62–7.61 (dd, 2H, Ar-H), 7.26–7.25 (d, 2H, Ar-H), 4.06–3.97 (m, 4H -NCH2-), 1.87–1.82 (bs, 2H), 1.36–1.19 (m, 16H), 0.88–0.82 (m, 12H), 45% yield.
3. Results and Discussion
MWA N-alkylation can dramatically shorten reaction times for a range of DPP derivatives while maintaining competitive yields and improving downstream purification efficiency. Switching from conventional bulk heating (24 h) to microwave irradiation (40 min) led to a drastic reduction in reaction time without compromising yield—an effect consistent with numerous reports showing that microwave heating accelerates organic transformations by enabling rapid, uniform heating and frequently improving rates, selectivity and energy efficiency.
Microwave conditions also simplified isolation of the desired disubstituted products: the reactions run under microwave irradiation produced the disubstituted DPP as the dominant product in nearly all tested cases, easing chromatographic separation from monosubstituted impurities that typically complicate purifications in bulk procedures. This practical advantage contributes to the overall sustainability and scalability of the protocol by reducing solvent and silica usage in purification steps—an increasingly important consideration for synthetic routes aimed at organic electronic applications. MWA N-alkylation of DPP with C6 and C4 alkyl chains was first employed to optimize the reaction conditions by varying reaction time, temperature, and the presence or absence of 18-crown-6 [40]. 18-Crown-6 is commonly employed in organic synthesis to enhance the solubility and reactivity of potassium salts, as the K2CO3 base employed in our reactions, in nonpolar solvents by selectively complexing K+ ions, thereby increasing the availability of free anionic species and facilitating nucleophilic substitution or elimination reactions. The optimized protocol was subsequently applied to DPP derivatives bearing alkyl chains of varying lengths—nominally C4, C6, and C12—as well as branched chains such as 2-ethylhexyl (Table 1).

Table 1.
Reaction conditions and yields of DPP derivatives.
As expected, the yield increases significantly with longer alkyl chains, indicating a solubility limitation when shorter alkyl chains are employed, consistent with previous reports on DPP [41].
4. Conclusions
Microwave-assisted N-alkylation has been demonstrated as a highly efficient and practical method for the functionalization of diketopyrrolopyrrole (DPP) cores. Compared with conventional bulk heating, which typically requires 24 h at elevated temperatures, microwave irradiation enables full conversion in only 40 min, reflecting the substantial kinetic advantage conferred by dielectric heating. Beyond the acceleration of reaction rates, MWA promotes the selective formation of N-disubstituted DPP products, thereby minimizing the formation of monosubstituted intermediates and N,O-dialkylated by-products that complicate purification. This selectivity not only simplifies chromatographic workup but also reduces solvent and silica consumption, contributing to the sustainability, scalability, and cost-effectiveness of the synthetic process.
The effectiveness of MWA across a range of alkyl chains further underscores its versatility, with longer chains providing improved solubility and higher yields, while shorter chains highlight the influence of solubility limitations on reaction efficiency. Such insights are particularly valuable for the design and synthesis of DPP derivatives tailored for organic electronic applications, where precise control over alkyl substitution can directly impact film formation, molecular packing, and charge transport properties.
Author Contributions
Conceptualization, B.M.S. and M.P.; methodology, B.M.S. and M.P.; validation, B.M.S. and M.P.; synthesis F.T. and S.M.; investigation, F.T. and S.M.,; resources, B.M.S. and M.P.; data curation, B.M.S. and M.P.; writing—original draft preparation, B.M.S. and M.P.; writing—review and editing, B.M.S. and M.P.; visualization, B.M.S.; supervision, B.M.S. and M.P.; project administration, M.P.; funding acquisition, B.M.S. and M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by European Union—NextGeneration EU from Italian Research Projects of National Relevance—PRIN2022, “Singlet exCItoN fission in crysTallIne moLecuLAr thin films for enhanced silicon photovoltaics (SCINTILLA)”, prot. 2022SCWMT, CUP B53D23004490006 and by the European Union—NextGeneration EU from the Italian Ministry of Environment and Energy Security POR H2 AdP MMES/ENEA with involvement of CNR and RSE, PNRR—Mission 2, Component 2, Investment 3.5 “Ricerca e sviluppo sull’idrogeno” N PRR.AP015.017.002 AdC ENEA-CNR (CUP B93C22000630006).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article. Further details are available upon request from the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Farnum, D.G.; Mehta, G.; Moore, G.G.I.; Siegal, F.P. Attempted Reformatskii Reaction of Benzonitrile, 1,4-Diketo-3,6-Diphenylpyrrolo[3,4-C]Pyrrole. A Lactam Analogue of Pentalene. Tetrahedron Lett. 1974, 15, 2549–2552. [Google Scholar] [CrossRef]
- Kim, T.H.; Lee, B.J.; An, S.O.; Lee, J.H.; Choi, J.H. The Synthesis of Red Dyes Based on Diketo-Pyrrolo-Pyrrole Chromophore to Improve Heat Stability and Solubility for Colour Filter Fabrication. Dye. Pigment. 2020, 174, 108053. [Google Scholar] [CrossRef]
- Christie, R.; Abel, A. Diketopyrrolopyrrole (Dpp) Pigments. Phys. Sci. Rev. 2021, 6, 281–289. [Google Scholar] [CrossRef]
- Bao, W.W.; Li, R.; Dai, Z.C.; Tang, J.; Shi, X.; Geng, J.T.; Deng, Z.F.; Hua, J. Diketopyrrolopyrrole (DPP)-Based Materials and Its Applications: A Review. Front. Chem. 2020, 8, 567625. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lai, B.; Ran, X.; Tang, H.; Cao, D. Recent Advances of Diketopyrrolopyrrole Derivatives in Cancer Therapy and Imaging Applications. Molecules 2023, 28, 4097. [Google Scholar] [CrossRef]
- Liu, Q.; Bottle, S.E.; Sonar, P. Developments of Diketopyrrolopyrrole-Dye-Based Organic Semiconductors for a Wide Range of Applications in Electronics. Adv. Mater. 2020, 32, 1903882. [Google Scholar] [CrossRef]
- Luo, N.; Zhang, G.; Liu, Z. Keep Glowing and Going: Recent Progress in Diketopyrrolopyrrole Synthesis towards Organic Optoelectronic Materials. Org. Chem. Front. 2021, 8, 4560–4581. [Google Scholar] [CrossRef]
- Chandran, D.; Lee, K.S. Diketopyrrolopyrrole: A Versatile Building Block for Organic Photovoltaic Materials. Macromol. Res. 2013, 21, 272–283. [Google Scholar] [CrossRef]
- Li, W.; Hendriks, K.H.; Wienk, M.M.; Janssen, R.A.J. Diketopyrrolopyrrole Polymers for Organic Solar Cells. Acc. Chem. Res. 2016, 49, 78–85. [Google Scholar] [CrossRef]
- Patil, Y.; Misra, R. Rational Molecular Design towards NIR Absorption: Efficient Diketopyrrolopyrrole Derivatives for Organic Solar Cells and Photothermal Therapy. J. Mater. Chem. C Mater. 2019, 7, 13020–13031. [Google Scholar] [CrossRef]
- Zhao, C.; Guo, Y.; Zhang, Y.; Yan, N.; You, S.; Li, W. Diketopyrrolopyrrole-Based Conjugated Materials for Non-Fullerene Organic Solar Cells. J. Mater. Chem. A Mater. 2019, 7, 10174–10199. [Google Scholar] [CrossRef]
- Chochos, C.L.; Katsouras, A.; Drakopoulou, S.; Miskaki, C.; Krassas, M.; Tzourmpakis, P.; Kakavelakis, G.; Sprau, C.; Colsmann, A.; Squeo, B.M.; et al. Effects of Alkyl Side Chains Positioning and Presence of Fused Aromatic Units in the Backbone of Low-Bandgap Diketopyrrolopyrrole Copolymers on the Optoelectronic Properties of Organic Solar Cells. J. Polym. Sci. A Polym. Chem. 2018, 56, 138–146. [Google Scholar] [CrossRef]
- Cheon, H.J.; An, T.K.; Kim, Y.H. Diketopyrrolopyrrole (DPP)-Based Polymers and Their Organic Field-Effect Transistor Applications: A Review. Macromol. Res. 2022, 30, 71–84. [Google Scholar] [CrossRef]
- Liu, K.Q.; Gu, Y.H.; Yi, Z.R.; Liu, Y.Q. Diketopyrrolopyrrole-Based Conjugated Polymers as Representative Semiconductors for High-Performance Organic Thin-Film Transistors and Circuits. Chin. J. Polym. Sci. 2023, 41, 671–682. [Google Scholar] [CrossRef]
- Wang, B.; Sonar, P.; Manzhos, S.; Haick, H. Diketopyrrolopyrrole Copolymers Based Chemical Sensors for the Detection and Discrimination of Volatile Organic Compounds. Sens. Actuators B Chem. 2017, 251, 49–56. [Google Scholar] [CrossRef]
- Yang, Z.; Fan, X.; Li, H.; Li, X.; Li, S.; Zhang, Z.; Lin, H.; Qian, J.; Hua, J. A Small-Molecule Diketopyrrolopyrrole-Based Dye for in Vivo NIR-IIa Fluorescence Bioimaging. Chem.–A Eur. J. 2021, 27, 14240–14249. [Google Scholar] [CrossRef]
- Magnasco, L.; Lanfranchi, A.; Martusciello, M.; Megahd, H.; Manfredi, G.; Lova, P.; Koszarna, B.; Gryko, D.T.; Comoretto, D. Fluorimetric Detection of Vapor Pollutants with Diketopyrrolopyrrole Polymer Microcavities. ACS Omega 2024, 9, 42375–42385. [Google Scholar] [CrossRef]
- Kovalenko, A.; Vala, M.; Ciganek, M.; Weiter, M.; Krajcovic, J. Design Rules for the Large Two-Photon Absorption Diketopyrrolopyrrole-Based Quadrupolar Symmetrical Chromophores. Chem. Pap. 2018, 72, 3033–3042. [Google Scholar] [CrossRef]
- Li, W.; Wang, L.; Tang, H.; Cao, D. Diketopyrrolopyrrole-Based Fluorescent Probes for Detection and Bioimaging: Current Progresses and Perspectives. Dye. Pigment. 2019, 162, 934–950. [Google Scholar] [CrossRef]
- Auwalu, M.A.; Cheng, S. Diketopyrrolopyrrole Fluorescent Probes, Photophysical and Biological Applications. Chemosensors 2021, 9, 44. [Google Scholar] [CrossRef]
- Cao, M.; Ma, X.; Wang, C.; Zou, W.; Wang, F.; Yu, B.; Cong, H.; Shen, Y. Design of Donor–Acceptor Conjugated Polymers Based on Diketopyrrolopyrrole for NIR-II Multifunctional Imaging. J. Mater. Chem. B 2024, 12, 2294–2303. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, L.; Tang, H.; Cao, D.; Chen, W. Diketopyrrolopyrrole: An Emerging Phototherapy Agent in Fighting Cancer. Dye. Pigment. 2020, 181, 108599. [Google Scholar] [CrossRef]
- Ma, Q.; Sun, X.; Wang, W.; Yang, D.; Yang, C.; Shen, Q.; Shao, J. Diketopyrrolopyrrole-derived Organic Small Molecular Dyes for Tumor Phototheranostics. Chin. Chem. Lett. 2022, 33, 1681–1692. [Google Scholar] [CrossRef]
- Rais, D.; Toman, P.; Pfleger, J.; Acharya, U.; Panthi, Y.R.; Menšík, M.; Zhigunov, A.; Thottappali, M.A.; Vala, M.; Marková, A.; et al. Singlet Fission in Thin Solid Films of Bis(Thienyl)Diketopyrrolopyrroles. Chempluschem 2020, 85, 2689–2703. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.M.; He, G.; Bu, G.; Ramos, P.; Wu, F.; Soliman, A.; Serrano, J.; Pietraru, D.; Chan, C.; Batteas, J.D.; et al. Efficient Free Triplet Generation Follows Singlet Fission in Diketopyrrolopyrrole Polymorphs with Goldilocks Coupling. J. Phys. Chem. C 2021, 125, 12207–12213. [Google Scholar] [CrossRef]
- Kratochvíl, M.; Thottappali, M.A.; Luňák, S.; Pauk, K.; Rais, D.; Marková, A.; Pfleger, J.; Imramovský, A.; Vala, M. Solid-State Absorption, Luminescence, and Singlet Fission of Furanyl-Substituted Diketopyrrolopyrroles with Different π-Stacking Arrangements. ChemPhotoChem 2023, 7, e202300201. [Google Scholar] [CrossRef]
- Ray, S.; Sharma, S.; Salzner, U.; Patil, S. Synthesis and Characterization of Quinoidal Diketopyrrolopyrrole Derivatives with Exceptionally High Electron Affinities. J. Phys. Chem. C 2017, 121, 16088–16097. [Google Scholar] [CrossRef]
- Shen, L.; Tang, Z.; Wang, X.; Liu, H.; Chen, Y.; Li, X. Effects of Aromatic Substituents on the Electronic Structure and Excited State Energy Levels of Diketopyrrolopyrrole Derivatives for Singlet Fission. Phys. Chem. Chem. Phys. 2018, 20, 22997–23006. [Google Scholar] [CrossRef]
- Wang, W.; Ge, L.; Xue, G.; Miao, F.; Chen, P.; Chen, H.; Lin, Y.; Ni, Y.; Xiong, J.; Hu, Y.; et al. Fine-Tuning the Diradical Character of Molecular Systems via the Heteroatom Effect. Chem. Commun. 2020, 56, 1405–1408. [Google Scholar] [CrossRef]
- Gawande, M.B.; Shelke, S.N.; Zboril, R.; Varma, R.S. Microwave-Assisted Chemistry: Synthetic Applications for Rapid Assembly of Nanomaterials and Organics. Acc. Chem. Res. 2014, 47, 1338–1348. [Google Scholar] [CrossRef]
- Kappe, C.O.; Stadler, A. Microwaves in Organic and Medicinal Chemistry. Microw. Org. Med. Chem. 2006, 25, 1–409. [Google Scholar] [CrossRef]
- Martina, K.; Cravotto, G.; Varma, R.S. Impact of Microwaves on Organic Synthesis and Strategies toward Flow Processes and Scaling Up. J. Org. Chem. 2021, 86, 13857–13872. [Google Scholar] [CrossRef] [PubMed]
- Razzaq, T.; Kappe, C.O. On the Energy Efficiency of Microwave-Assisted Organic Reactions. ChemSusChem 2008, 1, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Suguro, T.; Kishimoto, F.; Movick, W.J.; Takanabe, K. Coherent Evaluation of Energy Efficiency for Microwave Catalytic Reactors Based on Reaction Equilibrium. ChemCatChem 2024, 16, e202301598. [Google Scholar] [CrossRef]
- David, J.; Weiter, M.; Vala, M.; Vyňuchal, J.; Kučerík, J. Stability and Structural Aspects of Diketopyrrolopyrrole Pigment and Its N-Alkyl Derivatives. Dye. Pigment. 2011, 89, 137–143. [Google Scholar] [CrossRef]
- Lim, B.; Sun, H.; Lee, J.; Noh, Y.Y. High Performance Solution Processed Organic Field Effect Transistors with Novel Diketopyrrolopyrrole-Containing Small Molecules. Sci. Rep. 2017, 7, 164. [Google Scholar] [CrossRef]
- Li, M.; Li, J.; Di Carlo Rasi, D.; Colberts, F.J.M.; Wang, J.; Heintges, G.H.L.; Lin, B.; Li, W.; Ma, W.; Wienk, M.M.; et al. The Impact of Device Polarity on the Performance of Polymer–Fullerene Solar Cells. Adv. Energy Mater. 2018, 8, 1800550. [Google Scholar] [CrossRef]
- Diao, R.; Ye, H.; Yang, Z.; Zhang, S.; Kong, K.; Hua, J. Significant Improvement of Photocatalytic Hydrogen Evolution of Diketopyrrolopyrrole-Based Donor–Acceptor Conjugated Polymers through Side-Chain Engineering. Polym. Chem. 2019, 10, 6473–6480. [Google Scholar] [CrossRef]
- Kirkus, M.; Wang, L.; Mothy, S.; Beljonne, D.; Cornil, J.; Janssen, R.A.J.; Meskers, S.C.J. Optical Properties of Oligothiophene Substituted Diketopyrrolopyrrole Derivatives in the Solid Phase: Joint J- and H-Type Aggregation. J. Phys. Chem. A 2012, 116, 7927–7936. [Google Scholar] [CrossRef]
- Pop, F.; Humphreys, J.; Schwarz, J.; Brown, L.; Van Den Berg, A.; Amabilino, D.B. Towards More Sustainable Synthesis of Diketopyrrolopyrroles. New J. Chem. 2019, 43, 5783–5790. [Google Scholar] [CrossRef]
- Grzybowski, M.; Gryko, D.T. Diketopyrrolopyrroles: Synthesis, Reactivity, and Optical Properties. Adv. Opt. Mater. 2015, 3, 280–320. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).