Abstract
This work reports progress in the semi-synthetic modification of cassane-type diterpenes isolated from Coulteria platyloba, a plant of ethnopharmacological relevance. The approach involves a sequence of transformations, including oxidative aromatization, ring opening, and Knoevenagel condensation, to generate key intermediates for further diversification. Preliminary studies demonstrated the feasibility of organocatalytic reactions under trienamine activation, including a successful Diels–Alder cycloaddition. The initial steps were achieved with good yields and high purity, underscoring the potential of this strategy to access novel molecular scaffolds through efficient and sustainable methods aligned with the principles of Diversity-Oriented Synthesis (DOS).
1. Introduction
Coulteria platyloba is an endemic species of Mexico belonging to the Fabaceae family, traditionally used for its anti-inflammatory, antimicrobial, and anticancer properties. Previous studies have reported that this plant contains high levels of 6β-acetoxyvouacapane (Figure 1), making it an ideal candidate for semi-synthetic modifications aimed at generating compounds with potential chemical and pharmacological value [1].
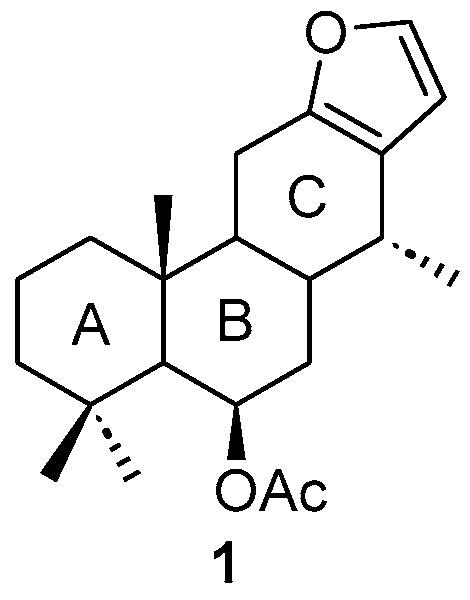
Figure 1.
Structure of 6β-acetoxyvouacapane. Rings A–C are labeled for clarity, and the stereochemistry is indicated in the structure.
This work presents progress in the semi-synthetic transformation of 6β-acetoxyvouacapane, a cassane-type diterpene isolated from C. platyloba, using organocatalytic strategies based on trienamine activation.
The proposed approach consists of first isolating 6β-acetoxyvouacapane from C. platyloba, followed by the development of semi-synthetic routes for its structural modification (Scheme 1). The aim is to generate new derivatives with potential applications in cascade organocatalytic reactions or [4 + 2] cycloadditions mediated by trienamine activation (Scheme 2). These processes allow multiple transformations in a single step, thereby reducing reaction time and minimizing the number of intermediates.
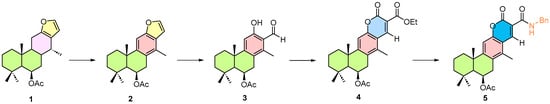
Scheme 1.
Initial semi-synthetic transformation of 6β-acetoxyvouacapane. Transformations 1–5 are numbered sequentially; colored rings indicate the regions of the vouacapane skeleton undergoing structural modification.
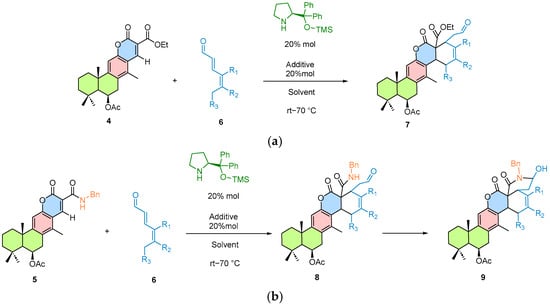
Scheme 2.
(a) Cascade organocatalytic strategy for the semi-synthesis of compound 7 from derivative 5 and dienals via trienamine activation mode. (b) Diels–Alder [4 + 2] cycloaddition between compounds 4 and 6 to afford product 8 through an organocatalytic strategy based on trienamine activation. The chiral Hayashi–Jørgensen organocatalyst is highlighted in green and the cycloaddition product is indicated in blue.
This strategy aligns with the ApDOS (Aminocatalytic Privileged Diversity-Oriented Synthesis) concept, which aims to generate structurally and stereochemically diverse compound libraries from a natural metabolite [2].
2. Materials and Methods
2.1. Isolation of 6β-Acetoxyvouacapane
Plant Material
Plant material was collected and identified as previously reported [1].
2.2. Extraction and Isolation
1 kg of dried C. platyloba leaves were macerated in dichloromethane (CH2Cl2) at room temperature for three days. The mixture was then filtered, and the solvent was evaporated under reduced pressure to yield 77 g of a dark green extract. This material was subjected to silica gel column chromatography using a hexane/CH2Cl2 (4:1) as the eluent system, affording compound 1 in fractions 34–58, although with slight impurities.
Fractions with higher impurity content were repurified by silica gel column chromatography using hexane/ethyl acetate (95:5) as the mobile phase. Finally, the purified material was recrystallized and washed with cold hexane/petroleum ether, yielding the pure compound.
2.3. Semi-Synthetic Transformations
2.3.1. Oxidation of Ring C
1 g of compound 1 was reacted with 2.5 equivalents of 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) in CH2Cl2 with concentrated HCl (10:1 ratio) for 20 min in a round-bottom flask. The reaction mixture was then neutralized and subjected to liquid–liquid extraction. The organic phase was evaporated, and the residue was purified by silica gel column chromatography [3].
2.3.2. Photooxidation Reaction
For furan ring opening, a photooxidation methodology was employed. A total of 900 mg of compound 2, dissolved in a 10:1 mixture of acetonitrile and CH2Cl2, was placed in a flask with solid methylene blue as a catalyst (5 mol%). The reaction was carried out under stirring in a dark box, using a 50 W white LED reflector as the light source, for 7 days. The resulting mixture was evaporated and purified by column chromatography [4].
2.3.3. Synthesis of Coumarins via Knoevenagel Condensation
To synthesize the coumarin core, 630 mg of compound 3 was reacted with 1.2 equivalents of diethyl malonate in the presence of piperidine (10 mol%) as a catalyst. The reaction was carried out in ethanol under reflux conditions for 18 h. Upon completion, the solvent was removed under reduced pressure, and the crude product was purified by column chromatography, yielding the desired coumarin derivative [5].
2.3.4. Synthesis for the Coumarin-3-Carboxamide Core (5)
A stirred solution of compound 3 (100 mg, 1 equiv.) in acetonitrile was treated with benzylamine (1.5 equiv.). The reaction mixture was refluxed for 18 h, after which the solvent evaporated under reduced pressure. The crude product was purified by column chromatography to afford the desired compound.
2.3.5. Organocatalytic Diels–Alder Reaction
1.5 equivalents of 9 were dissolved in toluene together with benzoic acid (20 mol%) and catalyst (20 mol%). The mixture was stirred for 5 min to activate the reagents. Then, 50 mg of 4 dissolved in toluene was added, and the reaction was maintained at 70 °C for 7 days. Upon completion, the solvent was evaporated, and the product was purified by column chromatography [6].
2.4. Characterization
The compounds synthesized to date were characterized by electrospray ionization time-of-flight mass spectrometry (ESI(+)-TOF-MS) and by nuclear magnetic resonance (NMR) spectroscopy using a 400 MHz instrument.
3. Results
3.1. Extraction of 6β-Acetoxyvouacapane
As a result of this study, 6β-acetoxyvouacapane was successfully isolated from the dichloromethane extract of C. platyloba via fractionation on a silica gel column. This process afforded 7.12 g of slightly yellow crystals, corresponding to a 9.3% yield based on the weight of the dichloromethane extract. The isolated material was characterized by mass spectrometry, nuclear magnetic resonance spectroscopy, and X-ray diffraction (Figure 2), showing full agreement with previously reported data [1].
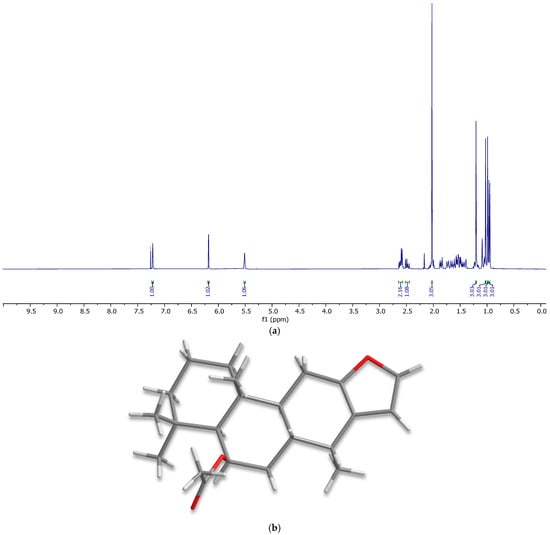
Figure 2.
(a) 1H NMR spectrum of 6β-acetoxyvouacapane. (b) X-ray structure of 6β-acetoxyvouacapane.
3.2. Semi-Synthetic Pathway
The semisynthetic pathway afforded moderate to excellent results (Scheme 3), with the initial compounds successfully obtained as anticipated according to the proposed strategy.
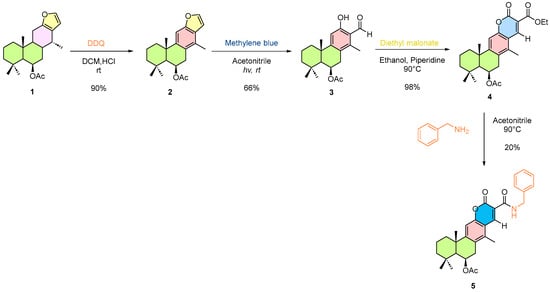
Scheme 3.
Semi-synthetic pathway of 6β-acetoxyvouacapane. Compounds 1–5 are numbered sequentially; colored rings indicate the regions of the vouacapane skeleton undergoing modification. Reaction conditions are shown above the arrows.
The oxidation of ring C was achieved in good yield, affording compound 2 as a colorless, sticky oil. Subsequent oxidative transformation of the furan ring proceeded with good conversion, although partial recovery of unreacted material was observed, yielding compound 3 as a white solid.
It is worth noting that compound 3 was obtained during silica gel column chromatography through the degradation of an unknown intermediate.
In the final steps, formation of the coumarin core (4) was achieved in excellent yield, producing a glassy, amorphous solid that readily crumbled into a fine powder upon solvent removal under vacuum. Compound 5 was obtained in low yield, due to the formation of byproducts, exhibiting similar physical characteristics to those of 4.
All synthesized compounds to date were fully characterized by 1H and 13C NMR spectroscopy, as well as mass spectrometry.
3.3. Cycloadditions and Cascade Reactions
To evaluate the feasibility of the cascade reaction, a Diels–Alder [4 + 2] cycloaddition catalyzed via trienamine activation was performed. In this process, compound 4 reacted with an activated diene, as described in the methodology (Scheme 4). This reaction afforded a 40% yield of a light brown foam-like product. A diastereomeric ratio (dr) of 65:35 was determined by 1H NMR analysis of the crude reaction mixture.
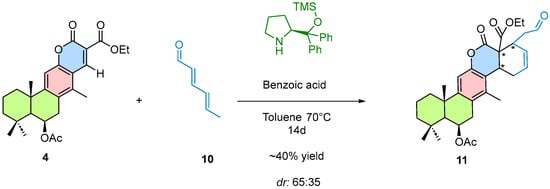
Scheme 4.
Reaction conditions for organocatalytic cycloaddition 4 + 2. the newly formed C–C bonds in the cycloaddition product are highlighted in blue. The reaction was performed using the chiral Hayashi–Jørgensen organocatalyst under the conditions indicated above the arrows.
Preliminary reaction trials for the cascade cycloaddition are currently in progress. Based on the cycloaddition [4 + 2], cascade cycloaddition studies were initiated using a different dienal; however, further optimization is still required to improve the reaction outcome and to determine the diastereomeric ratio (Scheme 5).
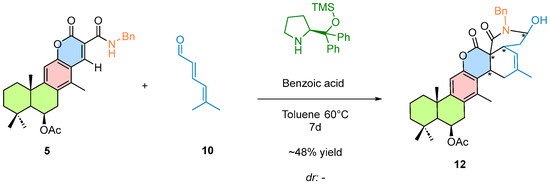
Scheme 5.
Initial Reaction Conditions for the Organocatalytic Cascade Cycloaddition. the newly formed C–C bonds in the cycloaddition product are highlighted in blue. The reaction was performed using the chiral Hayashi–Jørgensen organocatalyst under the conditions indicated above the arrows.
4. Discussion
The semi-synthetic strategy developed in this study demonstrated the successful isolation and transformation of 6β-acetoxyvouacapane from Coulteria platyloba. In the initial steps, a clear and reproducible pathway was established, beginning with the oxidative aromatization of ring C, followed by the selective photooxidative opening of the furan ring. These reactions proceeded in good to excellent yields, and the structural integrity of the intermediates was confirmed by 1H and 13C NMR spectroscopy as well as mass spectrometry. The isolation of these intermediates strongly supports the feasibility of this approach for generating structurally diverse derivatives.
The subsequent Knoevenagel condensation efficiently afforded the coumarin scaffold (4) in excellent yield, producing a glassy, amorphous solid that readily fragmented after solvent removal. This step represents a key advance, as it establishes a stable intermediate suitable for further structural elaboration. However, the following step, involving nucleophilic substitution with benzylamine to generate 5, proceeded in low yield, suggesting that the reaction conditions require significant optimization. Parameters such as solvent system, base concentration, and stoichiometry should be carefully evaluated to improve conversion and selectivity.
To assess the viability of the proposed cascade sequence, a Diels–Alder [4 + 2] cycloaddition via trienamine activation was carried out using compound 4 and an activated diene. Although the reaction produced the expected product, the yield was moderate (40%), and the product was obtained as a light brown foam, indicating possible side reactions or incomplete conversion. This result validates the conceptual framework of the cascade but also highlights the need for optimization of catalyst loading, reaction time, and substrate ratios to maximize efficiency.
Overall, the early stages of the semi-synthetic pathway were successfully established, confirming the feasibility of employing C. platyloba metabolites as starting points for diversity-oriented synthesis under the ApDOS concept. Nevertheless, the final two transformations remain suboptimal, and future efforts will focus systematically improving these steps to achieve higher yields and better stereochemical control. Such improvements will be essential to fully realize the potential of this strategy for generating novel scaffolds with structural and pharmacological relevance.
5. Conclusions
This work validates the semisynthetic strategy for modifying 6β-acetoxyvouacapane and demonstrates its potential in cascade reactions. These findings provide a solid platform for the development of new molecular scaffolds through efficient and stereocontrolled processes, paving the way for compounds of chemical and pharmacological relevance.
Author Contributions
Conceptualization, D.C.C. and C.V.G.; methodology, P.H.H.L.; validation, D.C.C. and C.V.G.; formal analysis, P.H.H.L.; investigation, P.H.H.L.; resources, D.C.C., C.V.G., A.T.A. and R.E.N.d.R.T.; data curation, P.H.H.L.; writing—original draft preparation, P.H.H.L.; writing—review and editing, D.C.C. and C.V.G.; supervision, D.C.C. and C.V.G.; project administration, D.C.C. and C.V.G.; funding acquisition, D.C.C. and C.V.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by SECIHTI, Proyecto de Ciencia Básica y de Frontera 2023–2024 (project code: CBF2023-2024-266).
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
Data available on request.
Acknowledgments
This work was supported by SECIHTI, Proyecto de Ciencia Básica y de Frontera 2023–2024 (project code: CBF2023-2024-266), the Becas Nacionales para estudios de Posgrado 2024-2 (scholarship No. 4042186), the Laboratorio Nacional de Caracterización de Propiedades Fisicoquímicas y Estructura Molecular (LANCAPFEM), Universidad de Guanajuato.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gómez, M.A.; Álvarez, F.E.; Rodríguez, G.; Martínez, M.M.; Espinoza, R.M.; Pamatz, T.; Salvador, J.L.; García, H.A.; Cerda, C.M.; Joseph, P.; et al. Cassane diterpenes from Caesalpinia platyloba. Phytochemistry 2013, 96, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Pawar, T.J.; Jiang, H.; Vázquez, M.A.; Villegas, G.C.; Cruz, C.D. Aminocatalytic privileged diversity-oriented synthesis (ApDOS): An efficient strategy to populate relevant chemical spaces. Eur. J. Org. Chem. 2018, 2018, 1835–1881. [Google Scholar] [CrossRef]
- Talavera-Alemán, A.; Gómez-Hurtado, M.A.; Rodríguez-García, G.; Ochoa-Zarzosa, A.; Thomassigny, C.; Cerda-García-Rojas, C.M.; Joseph-Nathan, P.; del Río, R.E. Preparation and cytotoxic evaluation of vouacapane oxidation products. Heterocycles 2020, 100, 207–224. [Google Scholar]
- Talavera-Alemán, A.; Gómez-Hurtado, M.A.; del Río, R.E.; Marrot, J.; Thomassigny, C.; Greck, C. Epoxy lactones by photooxidative rearrangement of 6β-acetoxyvouacapane. Tetrahedron Lett. 2017, 58, 2901–2903. [Google Scholar] [CrossRef]
- Ortíz, A.M.; Cruz, D.C.; Gómez, C.V. Organocatalytic cascade reactions for the synthesis and diversification of privileged structures. Chem. Proc. 2024, 16, 72. [Google Scholar] [CrossRef]
- Albrecht, A.; Skrzyńska, A.; Pietrzak, A.; Bojanowski, J.; Albrecht, Ł. Asymmetric aminocatalysis in the synthesis of δ-lactone derivatives. Asian J. Org. Chem. 2016, 5, 1115–1119. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).