Abstract
The synthesis and application of new chiral amino acids (AAs) and peptides derived thereof is a research topic of major importance. The introduction of γ-AA as building blocks are useful for the development of original chiral small molecules and heterocycles, enabling the exploration of 3D chemical space in search of selectivity in biological properties. 4.5-Dihydro-2H-pyridazin-3-ones (DHPDOs) are 6-membered aza-heterocycles considered as masked γ-AA analogues. We herein report on the synthesis of various a-monosubstituted DHPDOs as platform-molecules using Meldrum’s acid chemistry and the a-functionalization approach upon the asymmetric Michael addition using the Phase-Transfer Catalysis (PTC).
1. Introduction
Compared to classical α-amino acids (α-Aas), the introduction of γ-AA derivatives into the corresponding peptidomimetics leads different secondary structures and improved hydrolytic stability towards peptidases, thus providing altered and sometimes better biological properties/activities [1,2].
For example, γ-aminobutyric acid (GABA) is the simplest γ-AA and the main inhibitory neurotransmitter in the mammalian central nervous system, which is involved in several brain disorders such as neuropathic pain, Alzheimer’s disease, and Parkinson’s disease. For these reasons, the signalling modulation of GABA is the basis for many pharmacologic treatments [3,4,5]. The therapeutic properties of γ-AA derivatives in enantiomerically pure form have encouraged organic chemists to develop several procedures for their enantioselective synthesis. The construction of α-disubstituted γ-AA derivatives remains challenging and furthermore the elaboration of γ-AA with a tetra-substituted stereocenter is not a trivial task [6,7]. Moreover, α-disubstituted γ-AA derivatives are also useful building blocks for the elaboration of original chiral small molecules and heterocycles allowing the exploration of the 3D-chemical space in search of selectivity in biological properties and preventing any racemization event [1,2,8,9]. 4,5-Dihydro-2H-pyridazin-3-one (DHPDO) scaffolds are important 6-membered aza-heterocycles and are widely used as key building blocks in many biologically active molecules and therapeutic agents, with a wide range of pharmacological and medicinal properties. Some of them have been used in commercial pharmaceuticals and agrochemicals [10,11,12,13,14,15].
Unsurprisingly, many research groups have developed asymmetric catalytic syntheses of DHPDO derivatives that are either mono-substituted at the C5- or the C4-position (α-position of the carbonyl functionality) [16,17,18,19,20,21,22,23]. In this respect, several of these heterocycles of potential medical interest contain one or two substituents at the C4-position [24,25]. However, to our knowledge, the asymmetric catalytic synthesis of α,α-disubstituted DHPDO derivatives containing a quaternary stereogenic center has not yet been addressed [26]. Thus, the development of reliable strategies that provide easy access to different a-functionalized DHPDOs, even asymmetrically, is a laudable goal.
In accordance with the value mentioned above for chiral 4,5-dihydro-2H-pyridazin-3-ones, we have now set ourselves the goal of establishing a robust synthetic pathway for obtaining various a-functionalized DHPDOs. We also present a strategy that involves the asymmetric quaternary ammonium salt phase-transfer catalyzed Michael addition reaction of a-monosubstituted DHPDOs.
2. Results and Discussion
Our synthetic plan was based on the elaboration of various α-functionalized DHPDOs 3 from hydrazine and Meldrum’s acid chemistry, i.e., making use of derivatives 1. The readily accessible compounds 1 were expected to enable the construction of appropriately C5-disubstituted Meldrum’s acid derivatives 2, precursors for the synthesis of DHPDOs 3–4 after treatment with hydrazine. Finally, after a suitable N-protection to modulate the reactivity of the DHPDO compound 4, we investigated the asymmetric organocatalytic a-functionalization with different Michael acceptors under chiral ammonium salt (R4N*X) phase transfer conditions for the construction of new heterocyclic derivatives 6 (Scheme 1).
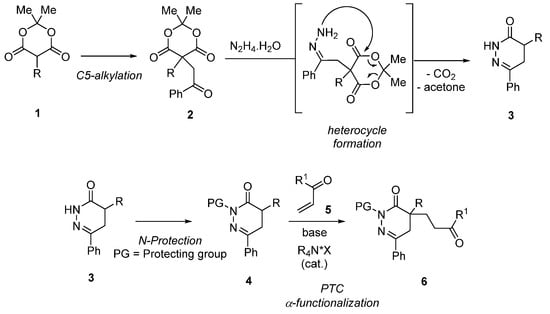
Scheme 1.
Synthetic route enabling the obtention of new α-disubstituted heterocyclic derivatives.
2.1. Synthesis of N-Boc DHPDO Derivatives 4
The synthesis of the C5-alkyl Meldrum’s acid derivatives 1a–d was easily achieved following the methodology of Ramachary and collaborators [27,28].
For the elaboration of NH-DHPDO derivatives 3, we based our methodology on the contribution of Tόth and collaborators, starting from the monosubstituted Meldrum’s acid derivatives 1 (Scheme 2A) [29]. First, the C5-disubstituted Meldrum’s acid derivatives 2 are obtained by alkylating 1 using 2-bromoacetophenone under basic conditions. Then, the NH-DHPDO derivatives 3 were obtained by the condensation of hydrazine at room temperature. Finally, the free NH-DHPDO derivatives 3 were N-protected using tert-butoxycarbonyl anhydride (Scheme 2B).
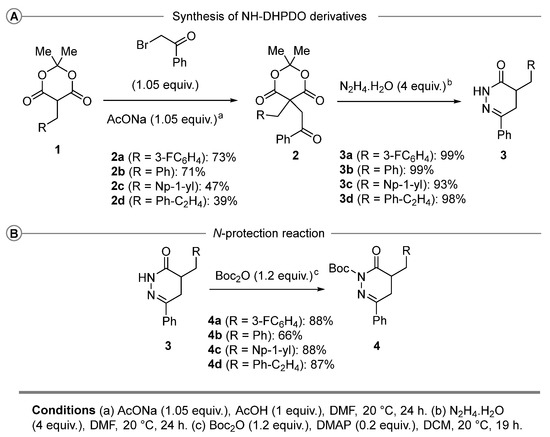
Scheme 2.
(A) NH-DHPDO derivatives. (B) Synthesis of N-Boc DHPDO derivatives.
2.2. α-Functionalization of N-Boc DHPDO Derivatives 4
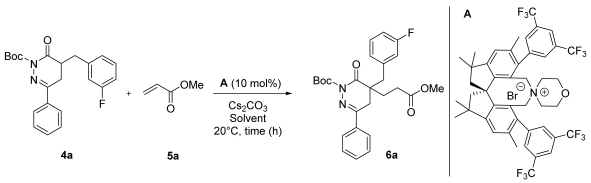
We were next interested in the asymmetric α-functionalization of N-Boc DHPDO derivatives 4 using the spirobiindane-based salt A [30,31,32]. The optimization was performed using the heterocycle 4a, methyl acrylate 5a as an acceptor and cesium carbonate as a simple inorganic base (Table 1).

Table 1.
Optimization of ammonium salt (A)-catalyzed Michael addition of N-Boc DHPDO 4a to methyl acrylate 5a 1.
The use of toluene as a solvent allowed us to achieve a good enantioselectivity (90:10 e.r.) but with a low conversion of 30% (entry 3). Preliminary investigation showed that these conditions surpassed the outcomes in THF and CH2Cl2 (entries 1–2). Then, by increasing the time of reaction to 18 and 40 h, we obtained better conversion but with a decrease in enantioselectivity as low as 18:82 e.r. (entry 4–5). This observation could be interpreted by counter-cation exchange or a catalyst degradation. Using a larger excess of base, product 5a was obtained with a high NMR yield of 95% and a moderate enantioselectivity (19:81 e.r., entry 6). It is also noteworthy that a lower isolated yield than that forecast based on the NMR yield was obtained. This can be explained by a partial retro-Michael addition after the purification by normal-phase silica gel column chromatography (a notable amount of starting material 4a was recovered).
The conditions depicted in entry 6 represent a fair compromise between conversion and enantioselectivity. Then, we tackled the exemplification of this method testing various a-substituted N-Boc DHPDOs and methyl vinylketone 5b as an acceptor (Scheme 3).
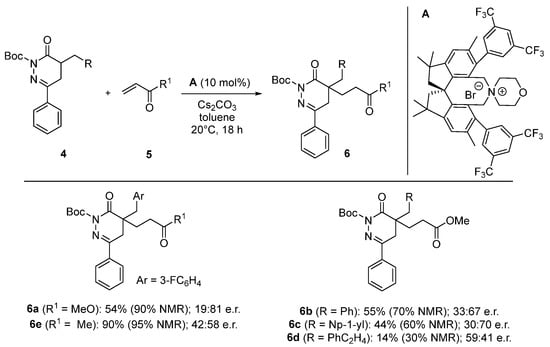
Scheme 3.
Application scope for the asymmetric 1,4-addition of N-Boc DHPDOs 4 to Michael acceptors 5.
The use of acceptor 5b (R1 = Me) gave the product 6e with a low enantioselectivity (42:58 e.r.) despite a high yield of 90%. Then, a-substituted N-Boc DHPDO derivatives were investigated using methyl acrylate 5a as acceptors giving products 6b–c, having benzylated pendants, with yields ranging from 44% to 55% and up to 30:70 e.r. Unfortunately, we obtained modest enantioselectivities (59:41 e.r.) combined with a lower yield (14%) for compound 6d having a longer alkyl chain (R = PhC2H4).
3. Conclusions
We successfully developed a methodology to access α-functionalized 4,5-dihydro-2H-pyridazin-3-ones using Meldrum’s acid chemistry. Subsequently, we developed a new methodology based on asymmetric Michael addition reaction employing Phase-Transfer Catalysis. This resulted in the formation of novel α,α-disubstituted pyridazinone derivatives, which contain a quaternary stereocenter and had not been previously addressed.
Author Contributions
Conceptualization, M.W. and J.-F.B.; methodology, M.W. and J.-F.B.; investigation, P.J.H., G.B. and W.N.; resources, M.W. and J.-F.B.; data curation, P.J.H. and W.N.; writing—original draft preparation, P.J.H. and W.N.; writing—review and editing, P.J.H., W.N., M.W. and J.-F.B.; visualization, M.W. and J.-F.B.; supervision, M.W. and J.-F.B.; project administration, M.W. and J.-F.B.; funding acquisition, M.W. and J.-F.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by AUSTRIAN SCIENCE FUNDS (FWF) through project No. P36004, and European Regional Development Fund (ERDF) and Région Normandie.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
Austrian Science Funds (FWF) is gratefully acknowledged. This work has been partially supported by Université Rouen Normandie, INSA Rouen Normandie, Centre National de la Recherche Scientifique (CNRS), European Regional Development Fund (ERDF), Région Normandie, Labex SynOrg (ANR-11-LABX-0029), Carnot Institute I2C, and the graduate school for research XL-Chem (ANR-18-EUR-0020 XL CHEM).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ordóñez, M.; Catieviela, C. Stereoselective synthesis of γ-amino acids. Tetrahedron Asymmetry 2007, 18, 3–99. [Google Scholar] [CrossRef]
- Ordóñez, M.; Catieviela, C.; Romero-Estudillo, I. An update on the stereoselective synthesis of γ-amino acids. Tetrahedron Asymmetry 2016, 27, 999–1055. [Google Scholar] [CrossRef]
- Brown, K.M.; Roy, K.K.; Hockerman, G.H.; Doerksen, R.J.; Colby, D.A. Activation of the γ-Aminobutyric Acid Type B (GABAB) Receptor by Agonists and Positive Allosteric Modulators. J. Med. Chem. 2015, 58, 6336–6347. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Halim, H.; Hanrahan, J.R.; Hibbs, D.E.; Johnston, G.A.R.; Chebib, M. A Molecular Basis for Agonist and Antagonist Actions at GABAC Receptors. Chem. Biol. Drug Des. 2008, 71, 306–327. [Google Scholar] [CrossRef] [PubMed]
- Filip, M.; Frankowska, M. GABAB receptors in drug addiction. Pharmacol. Rep. 2008, 60, 755–770. [Google Scholar]
- Marek, I.; Minko, Y.; Pasco, M.; Mejuch, T.; Gilboa, N.; Chechik, H.; Das, J.P. All-Carbon Quaternary Stereogenic Centers in Acyclic Systems through the Creation of Several C–C Bonds per Chemical Step. J. Am. Chem. Soc. 2014, 136, 2682–2694. [Google Scholar] [CrossRef]
- Quasdorf, K.W.; Overman, L.E. Catalytic enantioselective synthesis of quaternary carbon stereocentres. Nature 2014, 516, 181–191. [Google Scholar] [CrossRef]
- Lovering, F. Escape from Flatland 2: Complexity and promiscuity. MedChemComm 2013, 4, 515–519. [Google Scholar] [CrossRef]
- Seebach, D.; Abele, S.; Sifferlen, T.; Hänggi, M.; Gruner, S.; Seiler, P. Preparation and Structure of β-Peptides Consisting of Geminally Disubstituted β2,2- and β3,3-Amino Acids: A Turn Motif for β-Peptides. Helv. Chim. Acta 1998, 81, 2218–2243. [Google Scholar] [CrossRef]
- Mertens, A.; Friebe, W.-G.; Müller-Beckmann, B.; Kampe, W.; Kling, L.; von der Saal, W. Nonsteroidal cardiotonics. 3. New 4,5-dihydro-6-(1H-indol-5-yl)pyridazin-3(2H)-ones and related compounds with positive inotropic activities. J. Med. Chem. 1990, 33, 2870–2875. [Google Scholar] [CrossRef]
- Bansal, R.; Thota, S. Pyridazin-3(2H)-ones: The versatile pharmacophore of medicinal significance. Med. Chem. Res. 2013, 22, 2539–2552. [Google Scholar] [CrossRef]
- Dubey, S.; Bhosle, P.S. Pyridazinone: An important element of pharmacophore possessing broad spectrum of activity. Med. Chem. Res. 2015, 24, 3579–3598. [Google Scholar] [CrossRef]
- Kushwaha, B.; Kushwaha, N.D.; Shaik, B.B.; Chandrasekaran, B.; Obakachi, V.A.; Mokoena, S.; Mohite, S.B.; Karpoormath, R. Pyridazinone: A privileged scaffold for synthetic and biomedical applications. J. Mol. Struct. 2025, 1326, 140948. [Google Scholar] [CrossRef]
- Nieminen, M.S.; Fruhwald, S.; Heunks, L.M.A.; Suominen, P.K.; Gordon, A.C.; Kivikko, M.; Pollesello, P. Levosimendan: Current data, clinical use and future development. Heart Lung Vessels 2013, 5, 227–245. [Google Scholar]
- Ökçelik, B.; Ünlü, S.; Banoglu, E.; Küpeli, E.; Yeşilada, E.; Sahin, M.F. Investigations of New Pyridazinone Derivatives for the Synthesis of Potent Analgesic and Anti-Inflammatory Compounds with Cyclooxygenase Inhibitory Activity. Arch. Pharm. Pharm. Med. Chem. 2003, 336, 406–412. [Google Scholar] [CrossRef]
- Provencher, B.A.; Bartelson, K.J.; Liu, Y.; Foxman, B.M.; Deng, L. Structural Study-Guided Development of Versatile Phase Transfer Catalysts for Asymmetric Conjugate Additions of Cyanide. Angew. Chem. Int. Ed. 2011, 50, 10565–10569. [Google Scholar] [CrossRef]
- Shen, L.-T.; Sun, L.-H.; Ye, S. Highly Enantioselective γ-Amination of α,β-Unsaturated Acyl Chlorides with Azodicarboxylates: Efficient Synthesis of Chiral γ-Amino Acid Derivatives. J. Am. Chem. Soc. 2011, 133, 15894–15897. [Google Scholar] [CrossRef]
- Mao, J.-H.; Wang, Z.-T.; Wang, Z.-Y.; Cheng, Y. N-Heterocyclic Carbene-Catalyzed Oxidative Annulations of α,β-Unsaturated Aldehydes with Hydrazones: Selective Synthesis of Optically Active 4,5-Dihydropyridazin-3-ones and Pyridazin-3-ones. J. Org. Chem. 2015, 80, 6350–6359. [Google Scholar] [CrossRef]
- Zhang, C.-L.; Wang, D.-L.; Chen, K.-Q.; Ye, S. N-Heterocyclic carbene-catalyzed [3 + 3] cyclocondensation of bromoenals with hydrazones: Highly enantioselective synthesis of dihydropyridazones. Org. Biomol. Chem. 2015, 13, 11255–11262. [Google Scholar] [CrossRef]
- Zhang, Z.-J.; Song, J. An isothiourea-catalyzed asymmetric formal [4 + 2] cycloaddition of in situ generated azoalkenes with C1 ammonium enolates. Org. Chem. Front. 2018, 5, 2578–2582. [Google Scholar] [CrossRef]
- Mondal, B.; Maiti, R.; Yang, X.; Xu, J.; Tian, W.; Yan, J.-L.; Li, X.; Chi, Y.R. Carbene-catalyzed enantioselective annulation of dinucleophilic hydrazones and bromoenals for access to aryl-dihydropyridazinones and related drugs. Chem. Sci. 2021, 12, 8778–8783. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gai, K.; Yuan, Z.; Wu, J.; Lin, A.; Yao, H. Organocatalyzed Formal [4+2] Cycloaddition of in situ Generated Azoalkenes with Arylacetic Acids: An Efficient Approach to the Synthesis of 4,5-Dihydropyridazin-3(2H)-ones. Adv. Synth. Catal. 2015, 357, 3479–3484. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, H.; Xu, J. N-Heterocyclic Carbene-Promoted [4+2] Annulation of α-Chloro Hydrazones with α-Chloro Aliphatic Aldehydes to Access Enantioenriched Dihydropyridazinones. J. Org. Chem. 2022, 87, 3677–3685. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Sharma, D.; Bansal, R. Pyridazinone: An Attractive Lead for Anti-Inflammatory and Analgesic Drug Discovery. Future Med. Chem. 2017, 9, 95–127. [Google Scholar] [CrossRef]
- Kurose, A.; Ishida, Y.; Hirata, G.; Nishikata, T. Direct α-Tertiary Alkylations of Ketones in a Combined Copper–Organocatalyst System. Angew. Chem. Int. Ed. 2021, 60, 10620–10625. [Google Scholar] [CrossRef]
- Wayment, A.X.; Scheidt, K.A. Three-Component Synthesis of γ-Amino Esters with α-Quaternary Carbon Centers via NHC/Photoredox Dual Catalysis. Adv. Synth. Catal. 2025, 367, e70013. [Google Scholar] [CrossRef] [PubMed]
- Ramachary, D.B.; Kishor, M.; Ramakumar, K. A novel and green protocol for two-carbon homologation: A direct amino acid/K2CO3-catalyzed four-component reaction of aldehydes, active methylenes, Hantzsch esters and alkyl halides. Tetrahedron Lett. 2006, 47, 651–656. [Google Scholar] [CrossRef]
- Ramachary, D.B.; Reddy, G.B. Towards organo-click reactions: Development of pharmaceutical ingredients by using direct organocatalytic bio-mimetic reductions. Org. Biomol. Chem. 2006, 4, 4463–4468. [Google Scholar] [CrossRef]
- Tóth, G.; Molnár, S.; Tamás, T.; Borbély, I. An Efficient Synthesis of 4,5-Dihydro-3(2H)-pyridazinone Derivatives. Synth. Commun. 2006, 27, 3513–3523. [Google Scholar] [CrossRef]
- Xu, C.; Qi, Y.; Yang, X.; Li, X.; Li, Z.; Bai, L. Development of C2-Symmetric Chiral Spirocyclic Phase-Transfer Catalysts: Synthesis and Application to Asymmetric Alkylation of Glycinate Schiff Base. Org. Lett. 2021, 23, 2890–2894. [Google Scholar] [CrossRef]
- Xu, C.; Yang, X. Chiral Ammonium Salt Catalyzed Asymmetric Alkylation of Unactivated Amides. Synlett 2022, 33, 664–668. [Google Scholar] [CrossRef]
- Zebrowski, P.; Röser, K.; Chrenko, D.; Pospisil, J.; Waser, M. Enantioselective β-Selective Addition of Isoxazolidin-5-ones to Allenoates Catalyzed by Quaternary Ammonium Salts. Synthesis 2023, 55, 1706–1713. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).