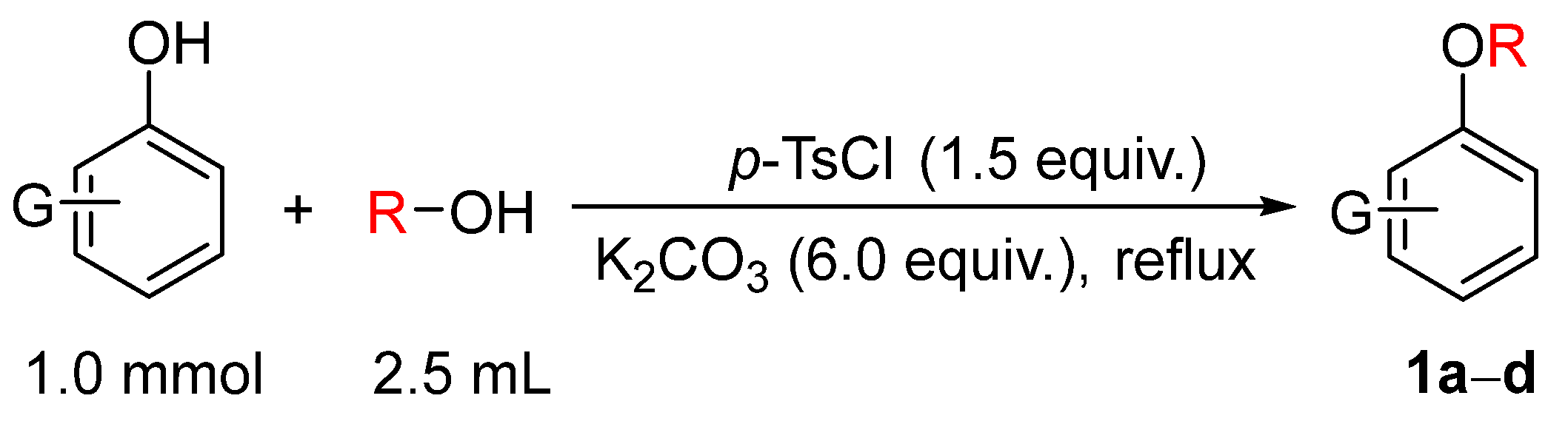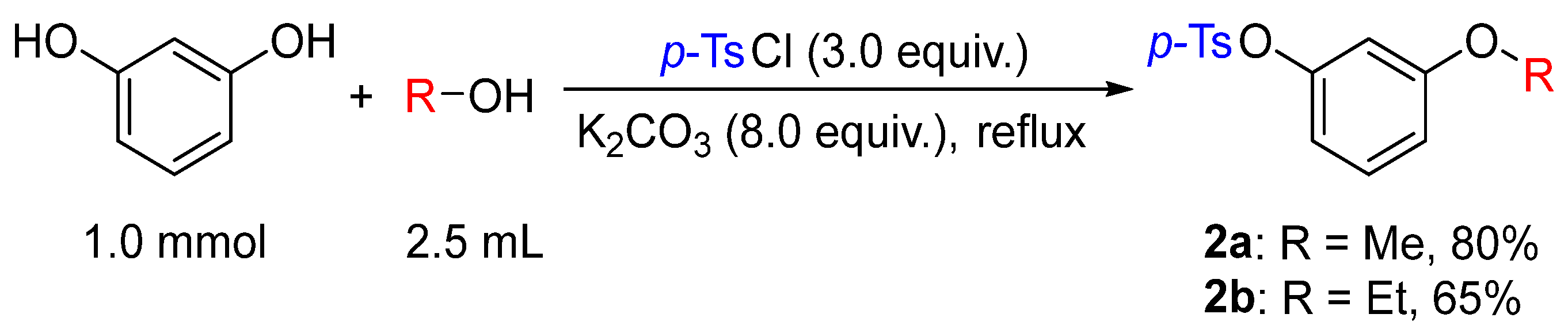Abstract
The selective derivatization of the hydroxyl group in phenols is of paramount importance in synthetic chemistry. Alcohol was employed as the alkylating agent for converting phenol to alkyl aromatic ethers mediated by sulfonyl chloride-potassium carbonate. Diphenol underwent a one-pot alkylation–sulfonylation in the alcohol–sulfonyl chloride-K2CO3 system. This process enabled the non-symmetrical derivatization of phenolic hydroxyl groups in diphenols.
1. Introduction
Phenolic hydroxyl group, occurring in various pharmaceutical and natural molecules, has been considered as an efficient skeleton or functional group for the derivation of aromatic moiety [1]. Due to the broad synthetic applications and highly sensitive reactivities, controlling the selectivity of protection and derivatization of hydroxyl groups is an indispensable challenge to achieve the desired chemical conversion [2]. Alkylation of the phenolic -OH group is not only a simple functional group transformation [3,4], but also produces alkyloxy groups that are key scaffolds dictating the properties of the final molecules [5,6]. However, the common alkylation reagents, such as halides, alkyl sulfates, and sulfonates, have been limited due to their environmental toxicity, potential danger to health [7,8].
In the previous work, alkyl sulfonyloxyphenyl ether could be synthesized selectively via a sequential procedure of monodesulfonation of diphenol bissulfonate alkylation [9]. Observation of the in situ formation of phenoxy anion indicated the feasibility of direct alkylation of the phenolic hydroxyl group. Herein, methods for the alkylation of phenolic hydroxyl groups in phenols and the alkylation–sulfonylation of diphenols are described, utilizing alcohols as alkylating agents in the presence of sulfonyl chloride.
2. Results and Discussion
2.1. Alkylation of Phenol with Alcohols
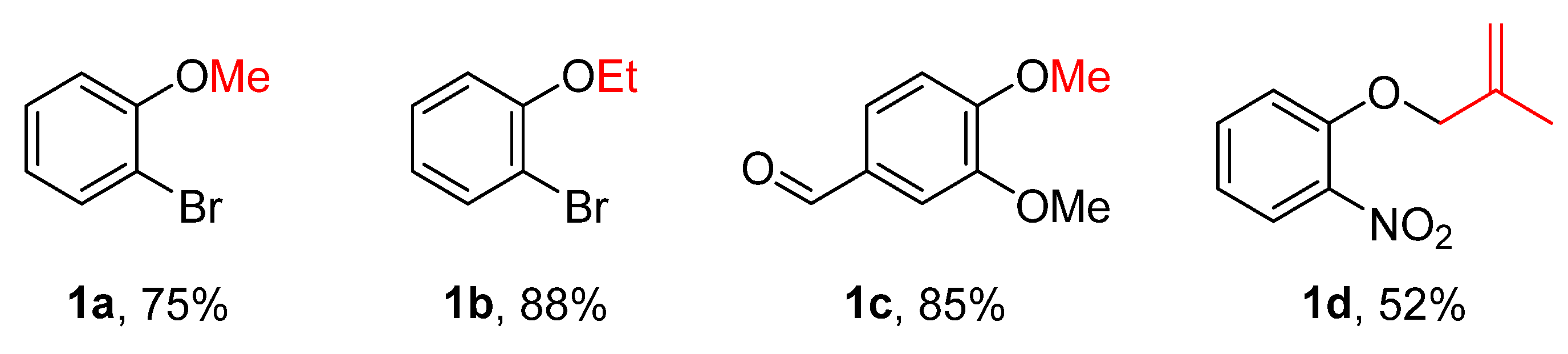
To evaluate the feasibility of the alkylation using alcohols as alkylating agents, the model substrate o-bromophenol was treated with 2.5 mL of methanol, excess potassium carbonate, and p-toluenesulfonyl chloride (p-TsCl) based on prior work [9]. The mixture was subjected to ultrasound-assisted reaction for 6 h. TLC monitoring indicated that the desired alkylated product was not obtained. Subsequently, the reaction was carried out under reflux heating for 5 h, after which TLC analysis showed complete consumption of the starting phenol. Following standard workup, the corresponding methylated product, o-bromoanisole (1a), was obtained (Figure 1). Substrate scope investigation demonstrated that this reaction condition could be applied to the alkylation of various phenols, providing an effective approach for the synthesis of a series of alkyl aromatic ethers 1a𠄲d using alcohols as alkylating agents (Figure 2).
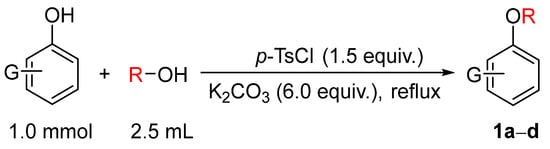
Figure 1.
Alkylation of phenol using alcohols as alkylating agents.
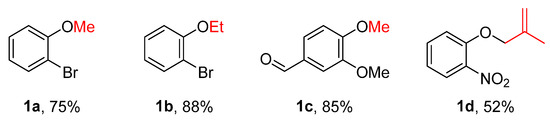
Figure 2.
Synthesis of alkyl aromatic ethers 1a–d using alcohols as alkylating agents.
As illustrated in Figure 2, the sulfonyl chloride-mediated alkylation of phenols with alcohols is a viable strategy (Figure 2). A variety of phenols bearing diverse substituents, including sensitive groups such as formyl and nitro, could be converted to alkylated products under standard conditions, highlighting the system’s broad functional group tolerance.
2.2. One-Pot Alkylation–Sulfonylation of Diphenol
Based on the results of the sulfonyl chloride-mediated alkylation of phenols with alcohols, resorcinol was employed as the model substrate to investigate the reactivity and selectivity of its two phenolic hydroxyl groups under the alcohol–TsCl–K2CO3 system. Notably, the formation of 3-methoxyphenyl sulfonate was observed in 60% yield. This indicated that, under the current conditions, the two phenolic hydroxyl groups of resorcinol underwent alkylation and sulfonylation, respectively (Figure 3).
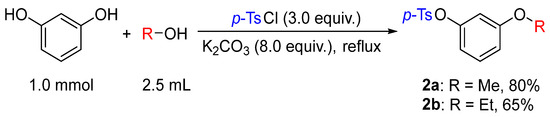
Figure 3.
Synthesis of alkyloxyphenyl sulfonate 2a–b.
In this weakly basic reaction system, the alcohol served a dual role as both the alkylating agent and the solvent.
3. Materials and Methods
1H and 13C NMR spectra were recorded on a Bruker Avance DMX500 (Bruker Corporation, Karlsruhe, Germany) in CDCl3 solutions and with tetramethylsilane as an internal standard. Melting points were recorded on the Micro melting point meter X5.
3.1. 1-Bromo-2-Methoxybenzene 1a [10]
Colorless oil, 75% isolated yield; 1H NMR (500 MHz, CDCl3) δ 7.52 (dd, J = 7.9, 1.6 Hz, 1H), 7.26 (ddd, J = 9.0, 1.6, 0.7 Hz, 1H), 6.88 (dd, J = 8.2, 1.3 Hz, 1H), 6.80 (td, J = 7.7, 1.4 Hz, 1H), 3.85 (s, 3H); 13C NMR (126 MHz, CDCl3) δ 155.9, 133.4, 128.6, 121.8, 112.0, 111.7, 56.2.
3.2. 1-Bromo-2-Ethoxybenzene 1b [11]
Colorless oil, 88% isolated yield; 1H NMR (500 MHz, CDCl3) δ 7.53 (dd, J = 7.9, 1.6 Hz, 1H), 7.27–7.25 (ddd, J = 7.4, 1.6, 0.8 Hz, 1H), 6.82 (td, J = 7.7, 1.4 Hz, 1H), 6.81 (m, 1H), 4.09 (t, J = 7.1 Hz, 2H), 1.47 (t, J = 7.0 Hz, 3H); 13C NMR (126 MHz, CDCl3) δ 155.3, 133.4, 128.4, 121.7, 113.3, 112.2, 64.8, 14.7.
3.3. 3,4-Dimethoxybenzaldehyde 1c [12]
White solid, 85% isolated yield; m.p. 41–42 °C; 1H NMR (500 MHz, CDCl3) δ 9.86 (s, 1H), 7.46 (dd, J = 8.2, 1.8 Hz, 1H), 7.42 (d, J = 1.8 Hz, 1H), 6.99 (d, J = 8.3 Hz, 1H), 3.98 (s, 3H), 3.95 (s, 3H); 13C NMR (126 MHz, CDCl3) δ 190.9, 154.5, 149.6, 130.2, 126.9, 110.4, 108.9, 56.2, 56.0.
3.4. 1-(2-Methylallyl)oxy)-2-Nitrobenzene 1d [13]
Light green oil, 65% isolated yield; 1H NMR (400 MHz, CDCl3) δ 7.86 (dd, J = 8.1 Hz, 1.7 Hz, 1H), 7.52–7.47 (m, 1H), 7.08–7.06 (m, 1H), 7.05–7.01 (m, 1H),5.14–5.01 (m, 2H), 4.56 (s, 2H), 1.82 (s, 3H); 13C NMR (126 MHz, CDCl3) δ 151.7, 139.6, 134.1, 125.8, 120.5, 114.8, 113.5, 72.6, 19.3.
3.5. 3-Methoxyphenyl 4-Methylbenzenesulfonate 2a [9]
White solid, 80% isolated yield; m.p. 54–55 °C; 1H NMR (500 MHz, CDCl3) δ 7.72 (d, J = 8.3 Hz, 2H), 7.31 (d, J = 8.3 Hz, 2H), 7.16 (t, J = 8.2 Hz, 1H), 6.78 (dd, J = 8.3, 2.2 Hz, 1H), 6.59–6.54 (m, 2H), 3.72 (s, 3H), 2.45 (s, 3H); 13C NMR (126 MHz, CDCl3) δ 160.4, 150.5, 145.3, 132.5, 129.9, 129.7, 128.9, 114.3, 113.1, 108.2, 55.5, 21.7.
3.6. 3-Ethoxyphenyl 4-Methylbenzenesulfonate 2b [9]
Colorless oil, 65% isolated yield; 1H NMR (500 MHz, CDCl3) δ 7.63 (d, J = 8.3 Hz, 2H), 7.22 (d, J = 8.0 Hz, 2H), 7.05 (t, J = 8.3 Hz, 1H), 6.67 (ddd, J = 8.4, 2.4, 0.7 Hz, 1H), 6.47 (t, J = 2.3 Hz, 1H), 6.43 (ddd, J = 8.1, 2.2, 0.8 Hz, 1H), 3.83 (q, J = 8.0 Hz, 2H), 2.35 (s, 3H), 1.27 (t, J = 8.0 Hz, 3H); 13C NMR (126 MHz, CDCl3) δ 159.8, 150.5, 145.3, 132.5, 129.8, 129.7, 128.5, 114.1, 113.6, 108.7, 63.7, 21.7, 14.6.
4. Conclusions
A green protocol for the O-alkylation of phenols was developed, utilizing alcohols as alkylating agents in the presence of a weak base (K2CO3) and a sulfonyl chloride. This system enabled highly selective alkylation–sulfonylation of diphenols, allowing the introduction of distinct groups onto different oxygen atoms. This strategy provided a novel approach for the non-symmetric derivatization of phenolic hydroxyl groups. The successful synthesis of allyl ether 1d not only offered a versatile handle for arene functionalization via Claisen rearrangement but also presented a potential synthetic route to natural products containing allyl aryl ether motifs, such as eugenol. This method employed low-toxicity, readily available alcohols and mild K2CO3, offering a green, efficient, and operationally simple alternative.
Author Contributions
Conceptualization, W.Z. and Y.L.; formal analysis, Y.L., D.Z. and C.L.; investigation, Y.L. and C.L.; resources, Y.L.; data curation, Y.L. and D.Z.; writing—original draft preparation, C.L.; writing—review and editing, W.Z. and Y.L.; supervision, W.Z.; project administration, W.Z.; funding acquisition, W.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (20972037), PCSIRT (IRT 1231), and the Excellent Young Teacher Support Program of Hangzhou Normal University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Reitzer, F.; Allais, M.; Ball, V.; Meyer, F. Polyphenols at interfaces. Adv. Colloid Interface Sci. 2018, 257, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Sartori, G.; Ballini, R.; Bigi, F.; Bosica, G.; Maggi, R.; Righi, P. Protection (and deprotection) of functional groups in organic synthesis by heterogeneous catalysis. Chem. Rev. 2004, 104, 199–250. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Gong, Q.; Huang, T.; Liu, L.; Chen, T. Practical electro-oxidative sulfonylation of phenols with sodium arenesulfinates generating arylsulfonate esters. J. Org. Chem. 2021, 86, 15914–15926. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, F.; Yan, K.; Feng, W.; Sun, X.; Yang, J.; Wen, J. Electrochemical-in-situ-oxidative sulfonylation of phenols with sulfinic acids as an access to sulfonylated hydroquinones. Adv. Synth. Catal. 2021, 363, 3485–3490. [Google Scholar] [CrossRef]
- Liargkova, T.; Eleftheriadis, N.; Dekker, F.; Voulgari, E.; Avgoustakis, C.; Sagnou, M.; Mavroidi, B.; Pelecanou, M.; Hadjipavlou-Litina, D. Small multitarget molecules incorporating the enone moiety. Molecules 2019, 24, 199. [Google Scholar] [CrossRef] [PubMed]
- Hämmerling, S.; Thiele, G.; Steinhauer, S.; Beckers, H.; Müller, C.; Riedel, S. A very strong methylation agent:[Me2Cl][Al(OTeF5)4]. Angew. Chem. Int. Ed. 2019, 58, 9807–9810. [Google Scholar] [CrossRef] [PubMed]
- Dor, Y.; Cedar, H. Principles of DNA methylation and their implications for biology and medicine. Lancet 2018, 392, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Kannan, D.; Naveen, S.; Jagadeesan, G.; Lokanath, N.K.; Thennarasu, S. Ultrasonic Cavitation Facilitates Rapid Synthesis of Trisubstituted Pyrazole Scaffolds through Michael Addition/Domino Cyclization. ChemistrySelect 2019, 4, 9807–9810. [Google Scholar] [CrossRef]
- Ma, J.; Li, Y.; Wu, P.; Zhao, J.; Zheng, W. Ultrasound-mediated monodesulfonation of polyphenol sulfonate and one-pot generation of alkyl aryl ether under solvent-free conditions. ChemistrySelect 2023, 8, e202300659. [Google Scholar] [CrossRef]
- Massah, A.R.; Mosharafian, M.; Momeni, A.R.; Aliyan, H.; Naghash, H.J.; Adibnejad, M. Solvent-free Williamson synthesis: An efficient, simple, and convenient method for chemoselective etherification of phenols and bisphenols. Synth. Commun. 2007, 37, 1807–1815. [Google Scholar] [CrossRef]
- Sadygov, O.A.; Alimardanov, K.M.; Chalabiev, C.A. Induced bromination of aromatic hydrocarbons by alkali metals bromides and sodium hypochlorite. Russ. J. Org. Chem. 2005, 41, 1631–1636. [Google Scholar] [CrossRef]
- Iioka, R.; Yorozu, K.; Sakai, Y.; Kawai, R.; Hatae, N.; Takashima, K.; Tnabe, G.; Wasada, H.; Yoshimatsu, M. Synthesis of azepino [1,2-a] indole-10-amines via [6+1] annulation of ynenitriles with Reformatsky reagent. Eur. J. Org. Chem. 2021, 2021, 1553–1558. [Google Scholar] [CrossRef]
- Liu, Z.; Luan, N.; Lu, H.; Liang, A.; Li, J.; Zou, D. Boron-promoted ether interchange reaction: Synthesis of alkyl nitroaromatic ethers from methoxynitroarenes. Eur. J. Org. Chem. 2020, 2020, 702–707. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).