Synthesis and In Silico Studies of a Novel 1,4-Disubstituted-1,2,3-Triazole-1,3-Oxazole Hybrid System †
Abstract
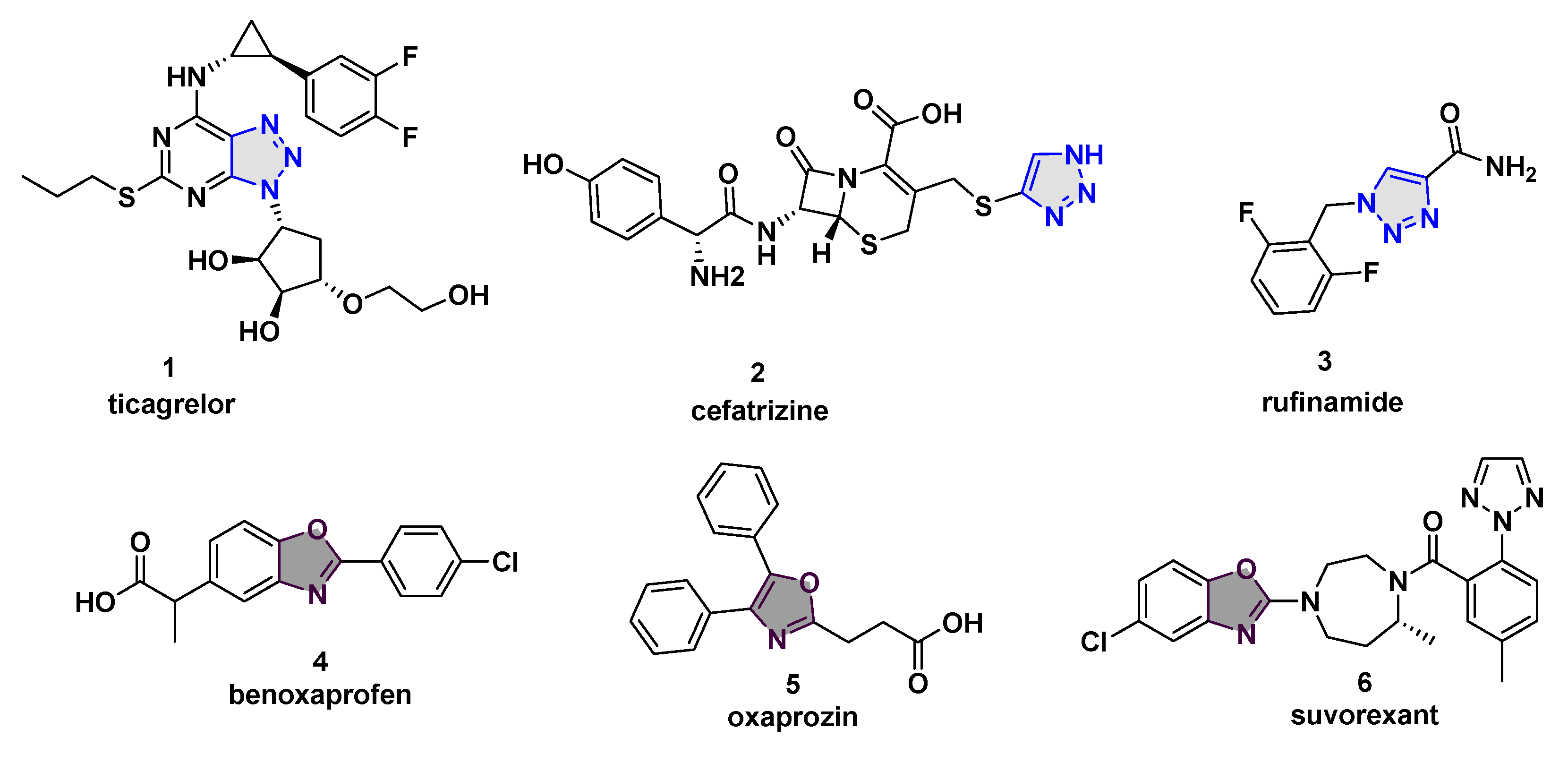
1. Introduction
2. Materials and Methods
2.1. Experimental Section
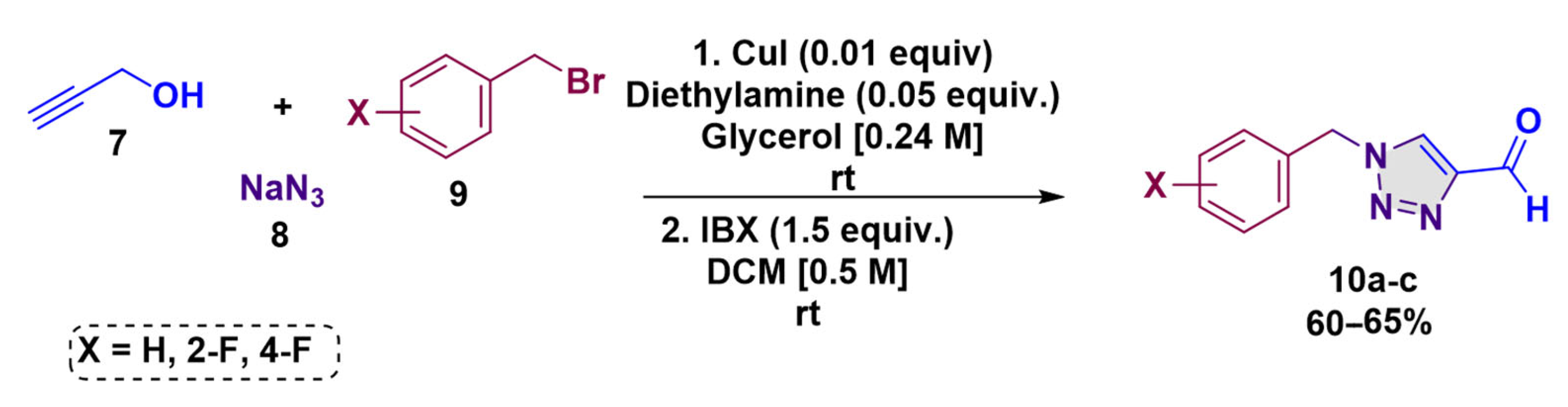
2.2. General Procedure for Aldehyde-1,2,3-Triazol 10a-c
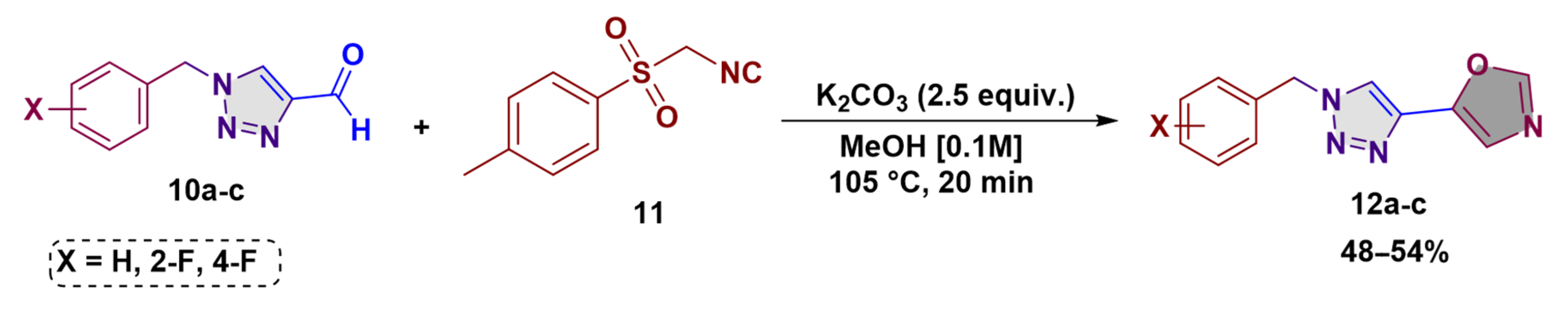
2.3. General Procedure for 1,4-Disubstituted-1,2,3-Triazole-1,3-Oxazoles 12a-c
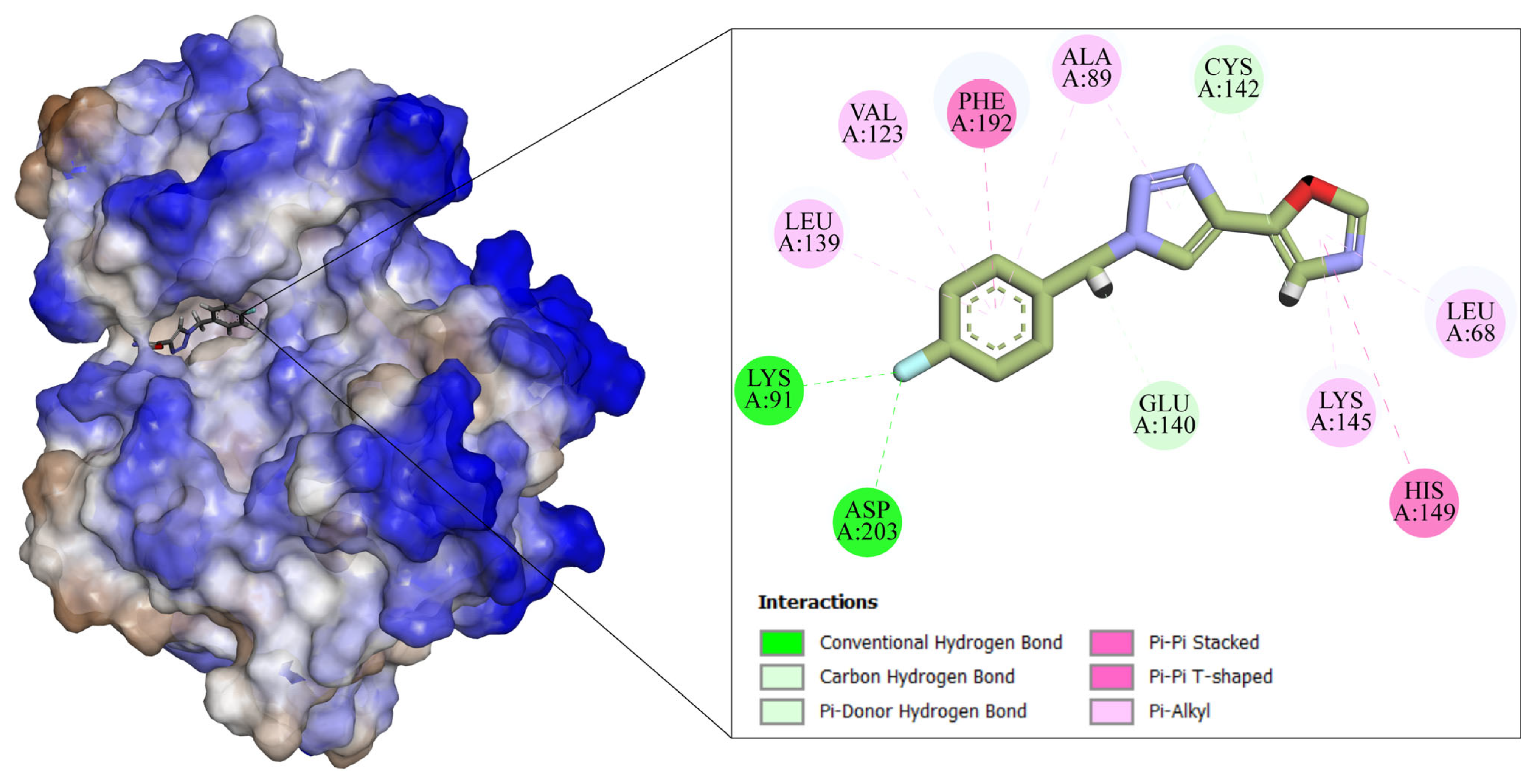
2.4. Computational Details
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kerru, N.; Gummidi, L.; Maddila, S.; Gangu, K.K.; Jonnalagadda, S.B. A Review on Recent Advances in Nitrogen-Containing Molecules and Their Biological Applications. Molecules 2020, 25, 1909. [Google Scholar] [CrossRef] [PubMed]
- Skoreński, M.; Sieńczyk, M. The Fellowship of Privileged Scaffolds—One Structure to Inhibit Them All. Pharmaceuticals 2021, 14, 1164. [Google Scholar] [CrossRef] [PubMed]
- Schneider, P. Privileged Structures Revisited. Angew. Chem. Int. Ed. 2017, 56, 9833–9839. [Google Scholar] [CrossRef] [PubMed]
- Shaveta; Mishra, S.; Singh, P. Hybrid molecules: The privileged scaffolds for various pharmaceuticals. Eur. J. Med. Chem. 2016, 124, 500–536. [Google Scholar] [CrossRef] [PubMed]
- SM Forezi, L.; Lima, C.G.; Amaral, A.A.; Ferreira, P.G.; de Souza, M.C.B.; Cunha, A.C.; de C. da Silva, F.; Ferreira, V.F. Bioactive 1,2,3-Triazoles: An Account on Their Synthesis, Structural Diversity and Biological Applications. Chem. Rec. 2021, 21, 2782–2807. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-Z.; Zhao, Z.-L.; Zhou, C.-H. Recent Advance in Oxazole-Based Medicinal Chemistry. Eur. J. Med. Chem. 2018, 144, 444–492. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Morales, C.M.; Araujo-Huitrado, J.G.; López-Hernández, Y.; Contreras-Celedón, C.; Islas-Jácome, A.; Granados-López, A.J.; Solorio-Alvarado, C.R.; López, J.A.; Chacón-García, L.; Cortés-García, C.J. A One-Pot Six-Component Reaction for the Synthesis of 1,5-Disubstituted Tetrazol-1,2,3-Triazole Hybrids and Their Cytotoxic Activity against the MCF-7 Cell Line. Molecules 2021, 26, 6104. [Google Scholar] [CrossRef] [PubMed]
- Frías-López, A.A.; Vázquez-Fuentes, S.; Garibay-Manríquez, C.; Reyes de la Cruz, H.; Islas-Jácome, A.; Chacón-García, L.; Díaz-Cervantes, E.; López-Bucio, J.S.; Cortés-García, C.J. Synthesis, in vitro activity and molecular docking of 1,5-disubstituted tetrazol-1,2,3-triazole hybrids against fungal plant pathogen Botrytis cinerea and Colletotrichum gloeosporioides. J. Agric. Food Chem. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Zhou, Y.; Dai, B.; Huo, C.; Liu, C.; Zhao, Y. CuI/Et2NH-Catalyzed One-Pot Highly Efficient Synthesis of 1,4-Disubstituted 1,2,3-Triazoles in Green Solvent Glycerol. Synthesis 2018, 50, 2191–2199. [Google Scholar] [CrossRef]
- Filimonov, D.A.; Lagunin, A.A.; Gloriozova, T.A.; Rudik, A.V.; Druzhilovskii, D.S.; Pogodin, P.V.; Poroikov, V.V. Prediction of the Biological Activity Spectra of Organic Compounds Using the PASS Online Web Resource. Chem. Heterocycl. Compd. 2014, 50, 444–457. [Google Scholar] [CrossRef]
- Zhu, T.; Zhao, C.; Gong, R.; Qian, A.; Wang, X.; Lu, F.; Huo, G.; Qiao, L.; Chen, S. Comprehensive Analysis Reveals PLK3 as a Promising Immune Target and Prognostic Indicator in Glioma. Oncol. Res. 2025, 33, 431–442. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garibay-Manríquez, C.; Caldelas-Guerrero, A.L.; Frías-López, A.A.; Chacón-García, L.; Díaz-Cervantes, E.; Cortés-García, C.J. Synthesis and In Silico Studies of a Novel 1,4-Disubstituted-1,2,3-Triazole-1,3-Oxazole Hybrid System. Chem. Proc. 2025, 18, 63. https://doi.org/10.3390/ecsoc-29-26701
Garibay-Manríquez C, Caldelas-Guerrero AL, Frías-López AA, Chacón-García L, Díaz-Cervantes E, Cortés-García CJ. Synthesis and In Silico Studies of a Novel 1,4-Disubstituted-1,2,3-Triazole-1,3-Oxazole Hybrid System. Chemistry Proceedings. 2025; 18(1):63. https://doi.org/10.3390/ecsoc-29-26701
Chicago/Turabian StyleGaribay-Manríquez, Camila, Ana L. Caldelas-Guerrero, América A. Frías-López, Luis Chacón-García, Erik Díaz-Cervantes, and Carlos J. Cortés-García. 2025. "Synthesis and In Silico Studies of a Novel 1,4-Disubstituted-1,2,3-Triazole-1,3-Oxazole Hybrid System" Chemistry Proceedings 18, no. 1: 63. https://doi.org/10.3390/ecsoc-29-26701
APA StyleGaribay-Manríquez, C., Caldelas-Guerrero, A. L., Frías-López, A. A., Chacón-García, L., Díaz-Cervantes, E., & Cortés-García, C. J. (2025). Synthesis and In Silico Studies of a Novel 1,4-Disubstituted-1,2,3-Triazole-1,3-Oxazole Hybrid System. Chemistry Proceedings, 18(1), 63. https://doi.org/10.3390/ecsoc-29-26701





