Abstract
The global rise in New Psychoactive Substances (NPS), particularly synthetic cathinones, poses significant public health concerns due to their association with toxicity and fatalities. With oral intake a common route of consumption, understanding their effects on the human intestinal epithelium is essential but remains poorly explored. This study investigates the impact of four synthetic cathinones—3-CIC (3-chloro-N-isopropylcathinone), 4-CIC (4-chloro-N-isopropylcathinone), 3-Cl-TBC (3-chloro-terc-butylcathinone, bupropion), and 4-Cl-TBC (4-chloro-terc-butylcathinone)—using Caco-2 cells, focusing on protein expression changes. The results revealed a reduced protein content, with 3-CIC producing the most significant effects, including the up-regulation of 40–50 kDa proteins. These findings suggest pathway disruptions requiring further mechanistic investigation.
1. Introduction
The global use of New Psychoactive Substances (NPS) has risen markedly in recent years. By the end of 2024, the European Union Drugs Agency (EUDA) had identified around 1000 NPS, including 47 newly detected compounds, 7 of which were synthetic cathinones [1]. Representing the second-largest category of NPS, synthetic cathinones exhibit psychostimulant properties such as cocaine [2], amphetamine, and MDMA [3], influencing monoamine neurotransmitter systems and causing various toxicological effects [4,5,6].
It has been reported in the literature that exposure to synthetic cathinones affects multiple organs and systems. In muscle tissue, compounds such as 3-methylmethcathinone (3-MMC) and mephedrone promote myotoxicity, contributing to the muscular damage frequently associated with abuse [7]. In the liver, substances like methylenedioxypyrovalerone (MDPV) and mephedrone deplete ATP, triggering mitochondrial dysfunction, oxidative stress, and protein changes as cells attempt to restore energy balance [8]. Similar disruptions are observed in the kidney, where cathinones disturb the regulation of autophagy and apoptosis, leading to endoplasmic reticulum stress, mitochondrial damage, and the activation of pro-apoptotic pathways [9]. Extensive research in neuronal models has shown that cathinones trigger early gene expression, alter synaptic plasticity, and cause neurotoxicity influenced by metabolism [6,10]. They also disrupt protein modifications [11,12], increase oxidative stress [13], and activate neuroinflammatory responses [14,15]. Additionally, the inhibition of monoamine transporters disturbs neurotransmitter balance, driving compensatory protein changes that worsen cognitive deficits and neuronal damage [16,17].
However, their effects on intestinal cells remain largely unexplored, even though the intestinal epithelium is the first physiological barrier after cathinone oral intake. This barrier not only regulates nutrient absorption but also determines the uptake and systemic distribution of xenobiotics, including drugs of abuse [18]. Alterations at the level of intestinal epithelial function may therefore alter the bioavailability of cathinones, compromise epithelial integrity, and ultimately influence the toxicological burden and systemic impact of these substances.
In this context, the present preliminary study investigates protein alterations in human intestinal epithelial Caco-2 cells following exposure to four synthetic cathinones, 3-CIC (3-chloro-N-isopropylcathinone), 4-CIC (4-chloro-N-isopropylcathinone), 3-Cl-TBC (3-chloro-terc-butylcathinone, bupropion), and 4-Cl-TBC (4-chloro-terc-butylcathinone), to characterize their impact on intestinal lining cells, assess potential consequences for bioavailability and systemic implications, and provide insights that can guide future mechanistic studies and risk assessments related to their abuse.
2. Materials and Methods
2.1. Chemicals and Biochemicals
Roswell Park Memorial Institute (RPMI) culture medium, bovine serum albumin (BSA), chloroform, Bradford reagent, Coomassie Brilliant Blue R-250, and glacial acetic acid (99.9%) were obtained from VWR (Solon, OH, USA). Dulbecco’s Modified Eagle Medium (DMEM) and heat-inactivated Fetal Bovine Serum (FBS) were purchased from Biowest (Nuaillé, France). L-glutamine (100×) was obtained from Lonza (Antwerp, Belgium), while penicillin–streptomycin solution (50×, Pen-Strep) and Phosphate-Buffered Saline (1×, PBS) were acquired from Corning (Manassas, VA, USA). Copper sulfate (>99%) was purchased from Sigma (Saint Louis, MO, USA), methanol from Honeywell (Morris Plains, NJ, USA), 96% ethanol from Labchem (Zelienople, PA, USA), ultrapure water from Merck (Irvine, UK), and sodium hydroxide (0.66 N) from Fisher Chemical (Zelienople, PA, USA). Bolt™ MOPS SDS Running Buffer (20×) and Bolt™ LDS Sample Buffer (4×) were supplied by Novex Life Technologies (Carlsbad, CA, USA).
The hydrochloride salt standards of the chlorinated cathinones used in this study have been previously synthesized and characterized [19].
2.2. Protein Extraction
For protein extraction [20], five 25 cm2 T-flasks of Caco-2 cells were cultured in RPMI medium supplemented with 10% FBS, 1% L-glutamine (100×), and 1% penicillin–streptomycin (50×). Cells were maintained under standard incubation conditions (37 °C, 5% CO2, humidified atmosphere). Humidity was achieved by placing a solution of 1 g copper sulfate in 500 mL ultrapure water inside the incubator. The culture medium was refreshed every 2–3 days until cell differentiation. Cells were then exposed for 24 h to the following concentrations: 6.2 mM (3-CIC), 4.6 mM (4-CIC), 4.3 mM (3-Cl-TBC), and 2.5 mM (4-Cl-TBC). One flask was used as a control for CIC compounds and another as a control for Cl-TBC compounds. After exposure, cells were washed twice with PBS and stored at −80 °C. Protein extraction was performed by adding 1200 μL of a solution containing ultrapure water, chloroform, and methanol (10:3:27) to promote cell lysis. Cells were detached from the surface using a cell scraper, and the suspension was sonicated on ice for 10 min.
2.3. Protein Quantification by Bradford Method
For protein quantification, 100 μL of a solution of NaOH (0.66 N) and ultrapure water (0.2 g in 10 mL) was added to 70 μL of the cell suspension to ensure complete cell lysis and protein denaturation, making them accessible for quantification. Protein concentrations were determined using a calibration curve prepared with bovine serum albumin (BSA) standards ranging from 0 to 0.5 mg/mL. For the blank, 795 μL of ultrapure water, 5 μL of ultrapure water, and 200 μL of Bradford reagent were mixed. For the calibration standards, 795 μL of ultrapure water, 5 μL of each BSA concentration, and 200 μL of Bradford reagent were used. The same procedure was applied to the samples, except that 5 μL of the cell suspension from each cathinone exposure condition replaced the BSA solution. Absorbance was measured at 595 nm.
2.4. SDS-PAGE
For SDS-PAGE electrophoresis using 4–12% Bis-Tris Plus polyacrylamide gels (1.0 mm × 12 wells), 90 μL of each previously prepared sample solution was mixed with 30 μL of Bolt™ LDS Sample Buffer (4×). Samples were boiled in a 100 °C water bath and subsequently placed on ice. One liter of Bolt™ MOPS SDS Running Buffer (20×) was prepared with ultrapure water. Gels were rinsed and assembled in a mini tank according to the manufacturer’s instructions. Five microliters of Prestained Protein MW Marker and 30 μL of each sample were loaded into the wells. Electrophoresis was performed at 120 V for approximately 1 h. After the run, gels were incubated for 2 h in Coomassie Brilliant Blue R-250 staining solution, followed by overnight incubation in a destaining solution prepared with 10% absolute ethanol (99.8%), 7.5% glacial acetic acid (99%), and 82.5% ultrapure water. Gel images were captured using the ImageQuant LAS 500 system and analyzed with ImageJ (1.54p) software. Relative changes in protein band areas were assessed by calculating the Fold Change (FC) (Equation (1)), providing a measure to compare the relative protein expression between control and exposed cells.
2.5. Statistical Analysis
One-way ANOVA was applied to analyze the variance of the results. p-values lower than 0.05 (p < 0.05) were determined as unrevealed statistical differences.
3. Results
To assess the effects on intestinal Caco-2 cell protein expression, cells were exposed to four structurally related synthetic cathinones, including positional isomers: 3-CIC (3-chloro-N-isopropylcathinone), 4-CIC (4-chloro-N-isopropylcathinone), 3-Cl-TBC (3-chloro-terc-butylcathinone, bupropion), and 4-Cl-TBC (4-chloro-terc-butylcathinone). Protein content and electrophoretic profiles were subsequently evaluated.
3.1. Protein Quantification in Caco-2 Cells
Total protein concentrations were determined using the Bradford assay. A calibration curve (R2 = 0.9963) was generated with bovine serum albumin (BSA) as standard as described in Section 2.3 (Figure 1).
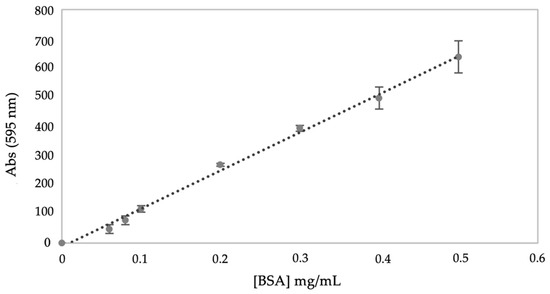
Figure 1.
Calibration curve of bovine serum albumin (BSA) obtained by Bradford method. The linear equation, Abs (595 nm) = 0.745 [BSA] (mg/mL) + 0.012 (R2 = 0.998), was applied to convert absorbance at 595 nm to protein concentration.
The calibration curve was applied to determine the content of proteins extracted from Caco-2 cells. The results shown in Figure 2 were obtained for Caco-2 cells exposed to the four cathinones under study and compared with unexposed control cells.
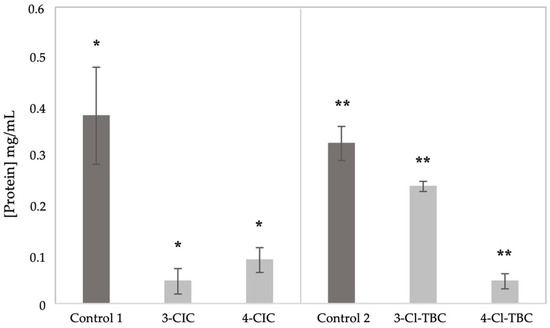
Figure 2.
Protein content of Caco-2 cells extracted after 24 h exposure to 3-CIC, 4-CIC, 3-Cl-TBC, and 4-Cl-TBC compared with unexposed controls. Control 1: unexposed cells for CIC assays; control 2: unexposed cells for TBC assays. (*) and (**) indicate statistically significant differences compared with the controls, for p < 0.05).
As shown in Figure 2, exposure to all four cathinones resulted in a general reduction in total protein content compared with their respective controls. Among the compounds tested, 3-CIC, 4-CIC, and 4-Cl-TBC caused the most pronounced decrease, suggesting a stronger impact on cellular protein homeostasis. These findings indicate that for Cl-TBC, positional isomers exert distinct effects on protein levels in Caco-2 cells.
3.2. Electrophoretic Profiling and Quantitative Analysis of Caco-2 Effects on Proteins
To further investigate which proteins were affected by cathinones exposure, protein extracts from Caco-2 cells were analyzed using SDS-PAGE to examine changes in band patterns and relative abundance (Section 2.4).
The protein profiles obtained using SDS-PAGE of the extracted proteins from Caco-2 cells exposed to 3-Cl-TBC and 4-Cl-TBC and an unexposed control (Control 2) and from Caco-2 cells and cells exposed to 3-CIC and 4-CIC and an unexposed control (Control 1) are shown in Figure 3.
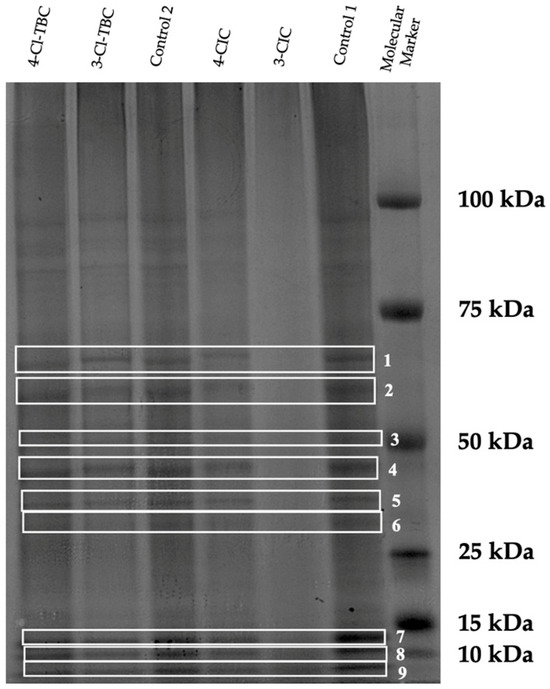
Figure 3.
SDS-PAGE 4–12% (Bis-Tris) electrophoretograms of proteins extracted from Caco-2 cells exposed or unexposed to cathinones for 24 h. Control 1: unexposed cells from CIC assay; control 2: unexposed cells from TBC assay. Molecular weight marker is shown on the left.
The electrophoretograms revealed alterations in protein profiles in cathinones-exposed cells compared with their respective controls, with structural isomers producing distinct effects on specific bands, especially for bands 1 to 8 identified in Figure 3. In order to evaluate those differences, band areas were quantified by densitometry. Band areas were normalized and expressed as Fold Change (FC) which is defined as the ratio of the normalized areas of the exposed cells to the normalized areas of the corresponding control cells. The average molecular weight (MW) was also determined for bands 1 to 8 (Table 1).

Table 1.
Fold Change (FC) and average molecular weight (MW) values for bands 1–8 identified in Figure 3 which showed Caco-2 protein profiles after 24 h exposure to 3-CIC, 4-CIC, 3-Cl-TBC, and 4-Cl-TBC.
As shown in Table 1, cathinone exposure induced both the up- and down-regulation of specific protein bands compared with unexposed controls. 3-CIC produced the most striking effects, with Fold Changes exceeding 14- and 20-fold for proteins in the 50 and 41 kDa range (bands 2 and 3, respectively) and 3-Cl-TBC a 2-fold increase also for the latter. 4-CIC and the TBC derivatives induced more moderate increases or decreases in the selected bands, suggesting a milder compound-specific modulation of protein expression.
Taken together, these findings demonstrate that cathinones not only reduce the overall protein content in Caco-2 cells but also alter the expression of specific proteins in a manner dependent on their structural features. Among the tested compounds, 3-CIC consistently caused the strongest alterations, while its positional isomer, 4-CIC, and the TBC derivatives elicited weaker or variable responses. These results provide a first insight into the ability of synthetic cathinones to differentially modulate intestinal epithelial protein profiles, potentially impacting intestinal barrier function and bioavailability.
4. Discussion
Overall, the exposure of Caco-2 cells to synthetic cathinones resulted in a reduction in total protein content, indicating that these compounds broadly affect protein homeostasis in intestinal lining cells. This decrease may be associated with cathinone-induced cytotoxicity, as cathinones have already been reported to interact and impact Caco-2 cells [21], possibly through mechanisms which interfere with protein synthesis and modulate protein expression in this cell model [22]. This general effect was accompanied by changes in specific proteins, with some proteins showing decreased abundance while others were up-regulated.
Among the cathinones tested, 3-CIC exposure was seen to produce the strongest alterations in the protein profile of Caco-2 cells, with increased expression of proteins in the 50 and 41 kDa range. Although their identity remains to be determined, such up-regulation may indicate the activation of cellular defense pathways, possibly linked to stress or detoxification responses [23]. As several cathinones have been associated with oxidative stress and mitochondrial dysfunction, the observed increases could correspond to protective mechanisms against reactive oxygen species [24]. Previous studies have also reported 3-CIC as a potent synthetic cathinone in terms of cytotoxicity and functional effects in neuronal models [19]. The present findings extend these observations to the intestinal epithelium, suggesting that 3-CIC may exert significant effects beyond neuronal systems.
The findings also suggest that even closely related isomers can differentially affect protein expression in intestinal epithelial cells. Although 3-CIC and 4-CIC share the same molecular formula and only differ in the position of the chlorine substituent, their effects on the Caco-2 protein profile were clearly distinct, with 3-CIC producing stronger and more consistent alterations. A similar pattern was observed for the terc-butylcathinone derivatives, where 3-Cl-TBC and 4-Cl-TBC induced more moderate and variable changes compared with the isopropylcathinone analogs. These results indicate that subtle structural differences among cathinones can lead to divergent cellular responses, a phenomenon that may be critical for understanding their toxicological properties.
Given that many synthetic cathinones are designed by making small structural modifications to circumvent regulation, these observations highlight the importance of studying positional isomers in greater detail. Clarifying how such differences in structure translate into differences in protein expression and cellular impact will be essential for anticipating the biological activity of new derivatives as they continue to emerge on the illicit market.
5. Conclusions
Protein quantification in Caco-2 cells exposed to cathinones revealed an overall reduction in protein content compared with unexposed cells, particularly following exposure to 3-CIC and 4-CIC. Regarding the effects of cathinones on cellular protein expression, the most pronounced changes were observed by exposure to 3-CIC, which induced an increase in the expression of certain proteins with molecular weights between 40 and 50 kDa.
Further studies will be needed to identify the proteins involved and to validate the observed changes with complementary approaches. Despite being only preliminary, the present results provide novel evidence that synthetic cathinones alter protein expression in intestinal epithelial cells. Importantly, the observation that positional isomers can produce distinct effects underlines the need to clarify how subtle structural differences shape cellular responses. These findings highlight the value of extending this line of investigation to better understand the potential impact of cathinone exposure at the intestinal barrier, which may influence bioavailability and, ultimately, the systemic toxicity and health risks associated with their abuse.
Author Contributions
Conceptualization, R.P. and H.G.; Formal analysis, M.M.; Funding acquisition, R.P. and H.G.; Methodology, R.P. and H.G.; Project administration, R.P. and H.G.; Supervision, R.P. and H.G.; Writing—original draft, M.M.; Writing—review and editing, M.M., R.P. and H.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financed by the Centro de Química Estrutural funded by the Portuguese Foundation for Science and Technology (FCT) through projects UIDB/00100/2023 and UIDP/00100/2023. The Institute of Molecular Sciences is an Associate Laboratory funded by the FCT through project LA/P/0056/2020. The work was supported by UID/04046/2025—Instituto de Biosistemas & Ciências Integrativas Centre grant from the FCT, Portugal.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is available from the corresponding authors upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- European Drug Report 2025: Trends and Developments. Available online: https://www.euda.europa.eu/publications/european-drug-report/2025_en (accessed on 9 September 2025).
- Daziani, G.; Lo Faro, A.F.; Montana, V.; Goteri, G.; Pesaresi, M.; Bambagiotti, G.; Montanari, E.; Giorgetti, R.; Montana, A. Synthetic Cathinones and Neurotoxicity Risks: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 6230. [Google Scholar] [CrossRef] [PubMed]
- Poyatos, L.; Papaseit, E.; Olesti, E.; Pérez-Mañá, C.; Ventura, M.; Carbón, X.; Grifell, M.; Fonseca, F.; Torrens, M.; de la Torre, R.; et al. A comparison of acute pharmacological effects of methylone and mdma administration in humans and oral fluid concentrations as biomarkers of exposure. Biology 2021, 10, 788. [Google Scholar] [CrossRef] [PubMed]
- Gannon, B.M.; Sulima, A.; Rice, K.C.; Baumann, M.H.; Collins, G.T. Reinforcing Potency and Effectiveness of Synthetic Cathinones: Potency versus Selectivity for DAT. FASEB J. 2018, 32, 681.7. [Google Scholar] [CrossRef]
- Nadal-Gratacós, N.; Alberto-Silva, A.S.; Rodríguez-Soler, M.; Urquizu, E.; Espinosa-Velasco, M.; Jäntsch, K.; Holy, M.; Batllori, X.; Berzosa, X.; Pubill, D.; et al. Structure–Activity Relationship of Novel Second-Generation Synthetic Cathinones: Mechanism of Action, Locomotion, Reward, and Immediate-Early Genes. Front. Pharmacol. 2021, 12, 749429. [Google Scholar] [CrossRef]
- Soares, J.; Costa, V.M.; Gaspar, H.; Santos, S.; de Lourdes Bastos, M.; Carvalho, F.; Capela, J.P. Structure-cytotoxicity relationship profile of 13 synthetic cathinones in differentiated human SH-SY5Y neuronal cells. Neurotoxicology 2019, 75, 158–173. [Google Scholar] [CrossRef]
- Zhou, X.; Luethi, D.; Sanvee, G.M.; Bouitbir, J.; Liechti, M.E.; Krähenbühl, S. Molecular Toxicological Mechanisms of Synthetic Cathinones on C2C12 Myoblasts. Int. J. Mol. Sci. 2019, 20, 1561. [Google Scholar] [CrossRef]
- Luethi, D.; Liechti, M.E.; Krähenbühl, S. Mechanisms of hepatocellular toxicity associated with new psychoactive synthetic cathinones. Toxicology 2017, 387, 57–66. [Google Scholar] [CrossRef]
- Vaz, I.; Carvalho, T.; Valente, M.J.; Castro, A.; Araújo, A.M.; Bastos, M.L.; Carvalho, M. The interplay between autophagy and apoptosis mediates toxicity triggered by synthetic cathinones in human kidney cells. Toxicol. Lett. 2020, 331, 42–52. [Google Scholar] [CrossRef]
- Lopes, R.P.; Miranda, C.C.; Fernandes, T.G.; Gaspar, H.; Antunes, A.M.M. The cytotoxicity of synthetic cathinones on dopaminergic-differentiated SH-SY5Y neuroblastoma cell line: Exploring the role of β-keto metabolic reduction. Food Chem. Toxicol. 2025, 204, 115658. [Google Scholar] [CrossRef]
- Chen, L.; Kashina, A. Post-translational Modifications of the Protein Termini. Front. Cell Dev. Biol. 2021, 9, 719590. [Google Scholar] [CrossRef]
- Walsh, G. Post-translational modifications of protein biopharmaceuticals. Drug Discov. Today 2010, 15, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Gianazza, E.; Crawford, J.; Miller, I. Detecting oxidative post-translational modifications in proteins. Amino Acids 2006, 33, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Leyrer-Jackson, J.M.; Nagy, E.K.; Olive, M.F. Cognitive deficits and neurotoxicity induced by synthetic cathinones: Is there a role for neuroinflammation? Psychopharmacology 2018, 236, 1079–1095. [Google Scholar] [CrossRef] [PubMed]
- Marusich, J.A.; Gay, E.A.; Stewart, D.A.; Blough, B.E. Sex differences in inflammatory cytokine levels following synthetic cathinone self-administration in rats. Neurotoxicology 2022, 88, 65–78. [Google Scholar] [CrossRef]
- Marusich, J.A.; Gay, E.A.; Blough, B.E. Analysis of neurotransmitter levels in addiction-related brain regions during synthetic cathinone self-administration in male Sprague-Dawley rats. Psychopharmacology 2018, 236, 903–914. [Google Scholar] [CrossRef]
- Baumann, M.H.; Walters, H.M.; Niello, M.; Sitte, H.H. Neuropharmacology of Synthetic Cathinones. In Handbook of Experimental Pharmacology; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 113–142. [Google Scholar]
- Okumura, R.; Takeda, K. Roles of intestinal epithelial cells in the maintenance of gut homeostasis. Exp. Mol. Med. 2017, 49, e338. [Google Scholar] [CrossRef]
- Gomes, A.P.; Ferro, R.; Pinto, D.; Silva, J.; Alves, C.; Pacheco, R.; Gaspar, H. Synthesis, Characterization, and Biological Effects of Chloro-Cathinones: Toxicity and Potential Neurological Impact. Int. J. Mol. Sci. 2025, 26, 3540. [Google Scholar] [CrossRef]
- García-Caóaveras, J.C.; Castell, J.v.; Donato, M.T.; Lahoz, A. A metabolomics cell-based approach for anticipating and investigating drug-induced liver injury. Sci. Rep. 2016, 6, 27239. [Google Scholar] [CrossRef]
- Silva, B.; Silva, R.; Fernandes, C.; Guedes de Pinho, P.; Remião, F. Enantioselectivity on the absorption of methylone and pentedrone using Caco-2 cell line: Development and validation of an UHPLC method for cathinones quantification. Toxicol. Appl. Pharmacol. 2020, 395, 114970. [Google Scholar] [CrossRef]
- Lin, W.Y.; Song, C.Y.; Pan, T.M. Proteomic analysis of Caco-2 cells treated with Monacolin K. J. Agric. Food Chem. 2006, 54, 6192–6200. [Google Scholar] [CrossRef]
- Lim, S.Y.M.; Loo, J.S.E.; Alshagga, M.; Alshawsh, M.A.; Ong, C.E.; Pan, Y. Protein-Ligand Identification and In Vitro Inhibitory Effects of Cathine on 11 Major Human Drug Metabolizing Cytochrome P450s. Int. J. Toxicol. 2022, 41, 355–366. [Google Scholar] [CrossRef]
- Zhu, W.; Cremonini, E.; Oteiza, P.I. Changes in redox network expression during Caco-2 cell differentiation into enterocytes. Redox Exp. Med. 2024, 2024, e240009. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).