2. Materials and Methods
Chemistry. The 1H spectra were recorded on a Bruker AC-500 spectrometer (500 MHz, respectively) in DMSO-d6, and the internal standard was TMS (Agilent Technologies, Santa Clara, CA, USA). LC-MS was performed on an Agilent 1200 LC/MSD SL high-performance liquid chromatograph using DAD (215/241 nm), ELSD, Quad MS (MSD1-Pos) detectors. Elemental analysis (C, H, N, S) was made on ELEMENTAR vario EL cube (standard—sulfanilamide). The melting points are determined by the capillary method in «Stanford Research Systems Melting Point Apparatus 100» (Sunnyvale, CA, USA). Used reagents were purchased from Sigma-Aldrich (Merck, Darmstadt, Germany).
Compounds
a,
b, and
c were synthesized using the well-known method [
9,
10]
3 with constants corresponding to the literature data [
6,
7,
8].
Preparation of 6-(5-mercapto-4-methyl(phenyl)-4H-1,2,4-triazol-3-yl)pyrimidine-2,4(1H,3H)-dione 1–2 (general methods). Orotic acid hydrazide c with 10 mmol was added to 20 mL of propan-2-ol until a suspension was formed, heated, and a solution of 10 mmol of ethyl isothiocyanate in 5 mL of propan-2-ol or 10 mmol of phenyl isothiocyanate was added dropwise. Heat for 2 h, and the precipitate is filtered off, and dried. The obtained carbothioamide goes to the next stage.
A mixture of 10 mmol of 2-(2,6-dioxo-1,2,3,6-tetrahydropyrimidine-4-carbonyl)-N-methyl(phenyl)hydrazine-1-carbothioamide, 10 mmol of sodium hydroxide, and 20 mL of purified water was boiled for 2 h. After complete cooling, 2 mL of concentrated acetic acid was added to the filtrate. The resulting precipitate was filtered and washed with purified water. For analysis, the product was purified by recrystallization from DMF. It has the appearance of a light yellow powder, soluble in aqueous solutions of alkalis, DMF, and 1,4-dioxane.
6-(5-mercapto-4-ethyl-4H-1,2,4-triazol-3-yl)pyrimidine-2,4(1H,3H)-dione (1). Yield 1.72 g (72%), white powder, mp 266 °C (DMF). 1H NMR, δ, ppm. (J, Hz): 1.19 (t, 3H, J = 7.4 Hz, CH3), 4.02 (q, 2H, J = 7.3 Hz, –N–CH2–CH3), 5.94 (d, 1H, J = 5.8 Hz, H-3 pyrimidine), 11.29 (s, 1H, NH-1), 11.35 (s, 1H, NH-5), 14.11 (s, 1H, SH). Mass spectrum, m/z (Irel, %) 240 [M + H]+ (100). Anal. calcd. for C8H9N5O2S: C: 40.16%; H: 3.79%; N: 29.27%; S: 13.40%; Found C: 40.22%; H: 3.64%; N: 29.21%; S: 13.44%.
6-(5-mercapto-4-phenyl-4H-1,2,4-triazol-3-yl)pyrimidine-2,4(1H,3H)-dione (2). Yield 1.96 g (82%), light yellow powder, mp 278 °C (DMF). 1H NMR, δ, ppm. (J, Hz): 6.15 (s, 1H, H-3 pyrimidine), 7.38–7.46 (m, 5H, Ar), 7.53–7.58 (m, 1H), 8.25 (s, 1H, SH), 11.29 (s, 1H, NH-1), 11.76 (s, 1H, NH-5). Mass spectrum, m/z (Irel, %) 288 [M + H]+ (100). Anal. calcd. for C12H9N5O2S: C: 50.17%; H: 3.16%; N: 24.38%; S: 11.16%; Found C: 50.02%; H: 3.12%; N: 24.46%; S: 11.23%
Preparation of S-alkyl derivatives of 6-(5-mercapto-4-methyl(phenyl)-4H-1,2,4-triazol-3-yl)pyrimidine-2,4(1H,3H)-dione 1.1–1.5, 2.1–2.5 (general methods). A mixture of 5 mmol of 6-(5-mercapto-4-methyl-4H-1,2,4-triazol-3-yl)pyrimidine-2,4(1H,3H)-dione and 5 mmol of sodium hydroxide dissolved in 10 mL of propan-2-ol was prepared. A total of 5 mmol of the halogen derivative was added to this mixture. The mixture was heated for 2 h, then cooled, and the precipitate was filtered and washed with purified water. The product was crystallized from methanol for analysis. Crystalline or oily substances (1.1–1.5, 2.1–2.5) are yellow or brown in color, insoluble in water, and soluble in organic solvents.
6-(4-Ethyl-5-(methylthio)-4H-1,2,4-triazol-3-yl)pyrimidine-2,4(1H,3H)-dione (1.1). Yield 0.96 g (76%), brown powder, mp 135 °C (MeOH). 1H NMR, δ, ppm (J, Hz): 1.39–1.46 (m, 3H, CH3CH2–), 2.69 (s, 3H, –S–CH3), 4.29 (q, 2H, J = 6.1, –N–CH2–CH3), 6.14 (s, 1H, H-5 pyrimidine), 11.16 (s, 1H, NH-1), 11.62 (s, 1H, NH-3). Mass spectrum, m/z (Irel, %) 254 [M + H]+ (100). Anal. calcd. for C9H11N5O2S: C: 42.68%; H: 4.38%; N: 27.65%; S: 12.66%. Found C: 42.38%; H: 4.14%; N: 27.73%; S: 12.58%.
6-(4-Ethyl-5-(ethylthio)-4H-1,2,4-triazol-3-yl)pyrimidine-2,4(1H,3H)-dione (1.2). Yield 0.97 g (73%), brown powder, mp 145 °C (MeOH). 1H NMR, δ, ppm. (J, Hz): 1.36 (t, 3H, J = 6.2 Hz, CH3CH2–S), 1.39–1.46 (m, 3H, CH3CH2–N), 3.07 (q, 2H, J = 6.2 Hz, –S–CH2–CH3), 4.30 (q, 2H, J = 6.2 Hz, –N–CH2–CH3), 6.14 (s, 1H, H-5 pyrimidine), 11.16 (s, 1H, NH-1), 11.62 (s, 1H, NH-3). Mass spectrum, m/z (Irel, %) 268 [M + H]+ (100). Anal. calcd. for C10H13N5O2S: C: 44.93%; H: 4.90%; N: 26.20%; S: 11.99%. Found C: 44.85%; H: 4.78%; N: 26.35%; S: 11.79%.
6-(4-Ethyl-5-(propylthio)-4H-1,2,4-triazol-3-yl)pyrimidine-2,4(1H,3H)-dione (1.3). Yield 1.05 g (75%), brown powder, mp 148 °C (MeOH). 1H NMR, δ, ppm. (J, Hz): 1.05 (t, 3H, J = 7.1 Hz, –S–CH2–CH2–CH3), 1.39–1.46 (m, 3H, CH3CH2–N), 1.76 (qt, 2H, J = 7.0, 5.3 Hz, –S–CH2– CH2–CH3), 3.10 (t, 2H, J = 5.3 Hz, –S–CH2– CH2–CH3), 4.30 (q, 2H, J = 6.2 Hz, –N–CH2–CH3), 6.14 (s, 1H, H-5 pyrimidine), 11.16 (s, 1H, NH-1), 11.62 (s, 1H, NH-3). Mass spectrum, m/z (Irel, %) 282 [M + H]+ (100). Anal. calcd. for C11H15N5O2S: C: 46.96%; H: 5.37%; N: 24.89%; S: 11.40%. Found C: 46.91%; H: 5.45%; N: 24.81%; S: 11.49%.
6-(5-(Butylthio)-4-ethyl-4H-1,2,4-triazol-3-yl)pyrimidine-2,4(1H,3H)-dione (1.4). Yield 1.00 g (68%), brown powder, mp 151 °C (MeOH). 1H NMR, δ, ppm. (J, Hz): 0.92 (t, 3H, J = 7.1 Hz, –S–CH2–CH3), 1.34–1.46 (m, 5H, –S(N)–CH2–CH3), 1.67 (ddd, 2H, J = 12.9, 6.8, 6.1 Hz, –S–CH2–CH3), 3.15 (t, 2H, J = 6.7 Hz, –S–CH2–CH3), 4.30 (q, 2H, J = 6.2 Hz, –N–CH2–CH3), 6.14 (s, 1H, H-5 pyrimidine), 11.16 (s, 1H, NH-1), 11.62 (s, 1H, NH-3). Mass spectrum, m/z (Irel, %) 296 [M + H]+ (100). Anal. calcd. for C12H17N5O2S: C: 48.80%; H: 5.80%; N: 23.71%; S: 10.85%. Found C: 48.82%; H: 5.91%; N: 23.63%; S: 10.92%.
6-(5-(Decylthio)-4-ethyl-4H-1,2,4-triazol-3-yl)pyrimidine-2,4(1H,3H)-dione (1.5). Yield 1.57 g (83%), yellow powder, mp 198 °C (MeOH). 1H NMR, δ, ppm. (J, Hz): 0.84–0.93 (m, 3H, –S–CH2–CH3), 1.20–1.31 (m, 13H, –S–CH2–), 1.36 (ttd, 2H, J = 7.1, 6.2, 0.8 Hz, –S–CH2–), 1.42 (t, 3H, J = 6.1 Hz, –N–CH2–CH3), 1.68 (p, 2H, J = 6.5 Hz, –S–CH2–), 3.12 (t, 2H, J = 6.4 Hz, –S–CH2–), 4.30 (q, 2H, J = 6.2 Hz, –N–CH2–CH3), 6.14 (s, 1H, H-5 pyrimidine), 11.16 (s, 1H, NH-1), 11.62 (s, 1H, NH-3). Mass spectrum, m/z (Irel, %) 380 [M + H]+ (100). Anal. calcd. for C18H29N5O2S: C: 56.97%; H: 7.70%; N: 18.45%; S: 8.45%. Found C: 56.91%; H: 7.78%; N: 18.52%; S: 8.41%.
6-(5-(Methylthio)-4-phenyl-4H-1,2,4-triazol-3-yl)pyrimidine-2,4(1H,3H)-dione (2.1). Yield 1.14 g (76%), brown powder, mp 123 °C (MeOH). 1H NMR, δ, ppm. (J, Hz): 2.73 (s, 3H, –S–CH3), 6.15 (s, 1H, H-5 pyrimidine), 7.38–7.46 (m, 4H, Ar), 7.53–7.59 (m, 1H, Ar), 11.29 (s, 1H, NH-1), 11.83 (s, 1H, NH-3). Mass spectrum, m/z (Irel, %) 302 [M + H]+ (100). Anal. calcd. for C13H11N5O2S: C: 51.82%; H: 3.68%; N: 23.24%; S: 10.64%. Found C: 51.78%; H: 3.73%; N: 23.26%; S: 10.47%.
6-(5-(Ethylthio)-4-phenyl-4H-1,2,4-triazol-3-yl)pyrimidine-2,4(1H,3H)-dione (2.2). Yield 1.09 g (69%), brown powder, mp 145 °C (MeOH). 1H NMR, δ, ppm. (J, Hz): 1.36 (t, 3H, J = 6.2 Hz, –S–CH2–CH3), 3.09 (q, 2H, J = 6.2 Hz, –S–CH2–CH3), 6.15 (s, 1H, H-5 pyrimidine), 7.37–7.46 (m, 4H, Ar), 7.53–7.59 (m, 1H, Ar), 11.29 (s, 1H, NH-1), 11.83 (s, 1H, NH-3). Mass spectrum, m/z (Irel, %) 316[M + H]+ (100). Anal. calcd. for C14H13N5O2S: C: 53.32%; H: 4.16%; N: 22.21%; S: 10.17%. Found C: 53.36%; H: 4.11%; N: 22.29%; S: 10.23%.
6-(4-Phenyl-5-(propylthio)-4H-1,2,4-triazol-3-yl)pyrimidine-2,4(1H,3H)-dione (2.3). Yield 1.20 g (73%), brown powder, mp 152 °C (MeOH). 1H NMR, δ, ppm. (J, Hz): 1.05 (t, 3H, J = 7.1 Hz, –S–CH2–CH3), 1.75 (qt, 2H, J = 7.1, 5.3 Hz, –S–CH2–), 3.12 (t, 2H, J = 5.3 Hz, –S–CH2–), 6.15 (s, 1H, H-5 pyrimidine), 7.37–7.46 (m, 4H, Ar), 7.53–7.59 (m, 1H, Ar), 11.29 (s, 1H, NH-1), 11.83 (s, 1H, NH-3). Mass spectrum, m/z (Irel, %) 330 [M + H]+ (100). Anal. calcd. for C15H15N5O2S: C: 54.70%; H: 4.59%; N: 21.26%; S: 9.73%. Found C: 54.75%; H: 4.51%; N: 21.53%; S: 9.62%.
6-(5-(Butylthio)-4-phenyl-4H-1,2,4-triazol-3-yl)pyrimidine-2,4(1H,3H)-dione (2.4). Yield 1.29 g (75%), brown powder, mp 153 °C (MeOH). 1H NMR, δ, ppm. (J, Hz): 0.92 (t, 3H, J = 7.1 Hz, –S–CH2–CH3), 1.39 (dtd, 2H, J = 13.2, 7.1, 6.1 Hz, –S–CH2–), 1.62–1.71 (m, 2H, –S–CH2–), 3.15 (t, 2H, J = 6.7 Hz, –S–CH2–), 6.15 (s, 1H, H-5 pyrimidine), 7.37–7.46 (m, 4H, Ar), 7.53–7.59 (m, 1H, Ar), 11.28 (s, 1H, NH-1), 11.82 (s, 1H, NH-3). Mass spectrum, m/z (Irel, %) 344 [M + H]+ (100). Anal. calcd. for C16H17N5O2S: C: 55.96%; H: 4.99%; N: 20.39%; S: 9.34%. Found C: 55.83%; H: 4.92%; N: 20.31%; S: 9.36%.
6-(5-(Decylthio)-4-phenyl-4H-1,2,4-triazol-3-yl)pyrimidine-2,4(1H,3H)-dione (2.5). Yield 1.11g (88%), brown powder, mp 188 °C (MeOH). 1H NMR, δ, ppm. (J, Hz): 0.83–0.93 (m, 3H, –S–CH2–CH3), 1.20–1.31 (m, 12H, –S–CH2–), 1.31–1.40 (m, 2H, –S–CH2–), 1.68 (p, 2H, J = 6.4 Hz, –S–CH2–), 3.14 (t, 2H, J = 6.4 Hz), 6.15 (s, 1H, H-5 pyrimidine), 7.37–7.46 (m, 4H, Ar), 7.53–7.59 (m, 1H, Ar), 11.30 (s, 1H, NH-1), 11.83 (s, 1H, NH-3). Mass spectrum, m/z (Irel, %) 428 [M + H]+ (100). Anal. calcd. for C22H29N5O2S: C: 61.80%; H: 6.84%; N: 16.38%; S: 7.50%. Found C: 61.85%; H: 6.77%; N: 16.31%; S: 7.56%.
3. Results
The target 6-(5-mercapto-4-R-4H-1,2,4-triazol-3-yl)-pyrimidine-2,4(1
H,3
H)-diones were obtained by stepwise construction of the triazole fragment from orotic acid hydrazide (
Figure 1). In the first stage, nucleophilic addition of the terminal –NH
2 group of the hydrazide to an isothiocyanate (R–N=C=S) proceeded with high selectivity to produce the corresponding N-acylthiosemicarbazide (carbothioamide). The reaction was carried out in a low-boiling protic solvent under gentle heating and was accompanied by precipitation of the product, which allowed its isolation without chromatography.
Subsequent base-promoted cyclocondensation of the carbothioamide furnished the 1,2,4-triazole-3(2H)-thione system rigidly connected to the uracil core. After cooling, acidification of the reaction mixture with acetic acid precipitated the target mercaptotriazoles as pale-yellow solids. The products exhibit characteristic thione–thiol tautomerism; in basic media, they form soluble thiolates, which accounts for their complete solubility in aqueous alkali and polar aprotic solvents (DMF, 1,4-dioxane). The substituent at C-4 of the triazole ring (R = Me, Ph) is defined by the isothiocyanate employed.
Sulfur functionalization was achieved by selective S-alkylation: deprotonation of the SH group with sodium hydroxide in propan-2-ol generated the nucleophilic thiolate, which reacted cleanly with alkyl halides to afford the S-substituted derivatives. Under equimolar amounts of base and alkylating agent, S- over N-alkylation predominated. The products were typically crystalline or oily, insoluble in water but soluble in common organic solvents, and amenable to purification by simple recrystallization (methanol; DMF for analytical samples).
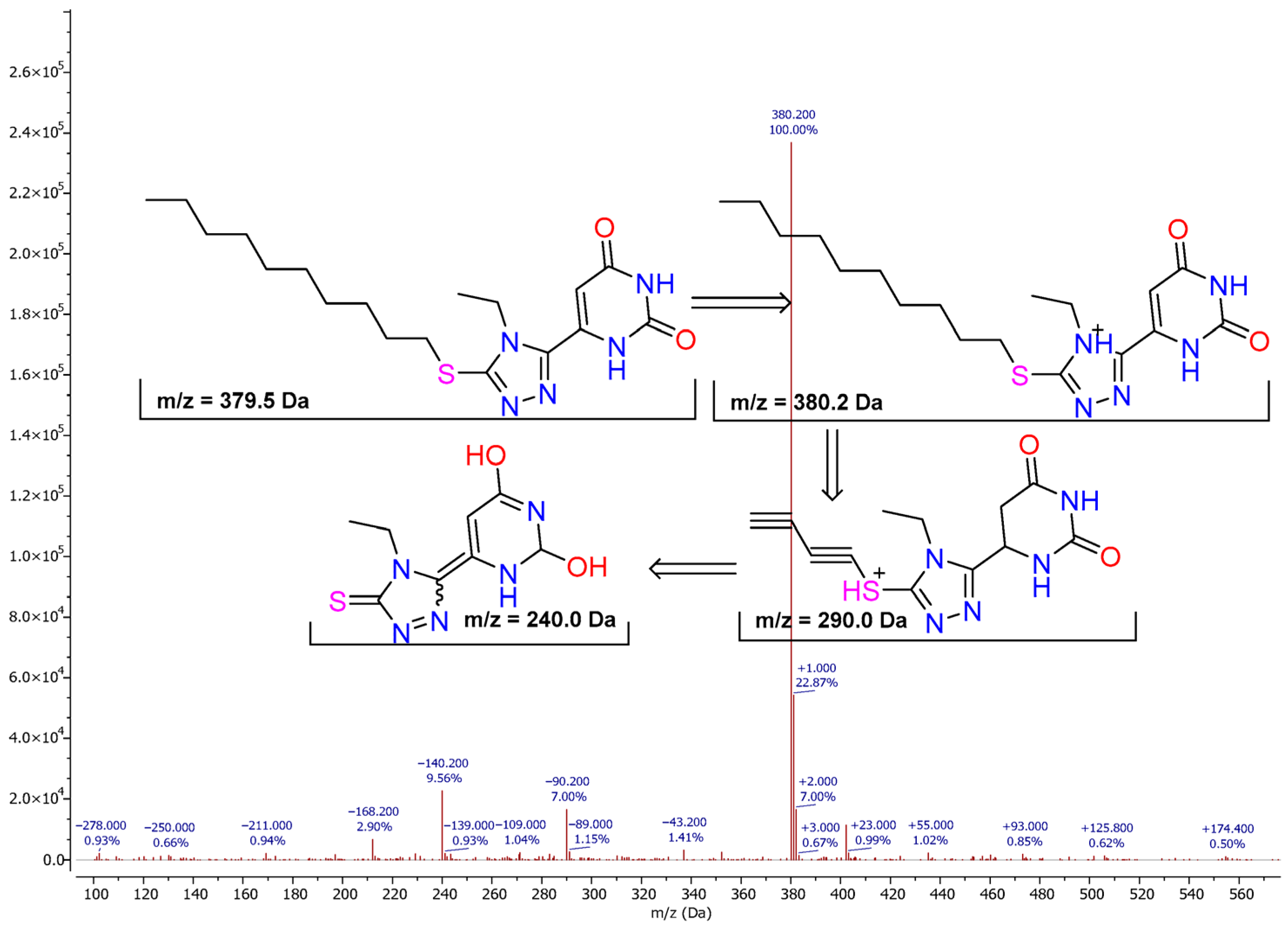
Most of the compounds produced clear protonated molecular ions, [M + H]
+, in positive-ion electrospray ionization (ESI). For example, compound
1.10 (R
1 = Et, R
2 = C
10H
21) demonstrated a well-defined fragmentation pattern under positive ESI conditions (
Figure 2). The mass spectrum revealed the protonated molecular ion at
m/
z = 380.2, which corresponds to the intact heterocyclic system comprising the uracil-2,4-dione core linked to a 1,2,4-triazole-thioether fragment bearing a decyl substituent. This signal was the dominant precursor for subsequent fragmentation processes. Importantly, the isotopic distribution exhibited an additional peak at
m/
z ≈ 382.2 with a relative intensity of about 4%, which is characteristic of the presence of the
34S isotope. This isotopic signature provides additional confirmation of the thioether moiety in the molecular structure.
The most characteristic dissociation pathway involved the cleavage of the S–C bond with the elimination of the decyl chain as a neutral alkene (C10H20), resulting in the formation of a stable fragment ion at m/z = 240.0. This behavior can be rationalized by charge-induced heterolysis at the sulfur atom, followed by β-hydrogen migration, which is a typical process for long-chain thioethers. The resulting fragment corresponds to the triazole–thione–uracil scaffold, stabilized by extensive delocalization of the positive charge across heteroatoms.
Another important pathway was observed at m/z = 290.0, which can be explained by a thiol-elimination mechanism. In this case, the loss of a neutral fragment corresponding to C4H10S occurs through intramolecular proton transfer and reorganization around the sulfur center. This leads to the generation of a fragment ion retaining the heterocyclic core, whereas the neutral thiol is expelled. Such behavior reflects the intrinsic tendency of thioethers to undergo rearrangements accompanied by small thiol eliminations.
Additional secondary fragments in the regions of m/z 313–315, 273, and 173 can be attributed to consecutive eliminations of small molecules such as H2S, COS, C2H4, or HNCO, as well as to partial cleavages within the uracil ring. These processes are consistent with the fragmentation rules of condensed heterocycles, in which the stability of the final cations is governed by conjugation within the N, O, and S heteroatomic framework.
Overall, the fragmentation study of compound 1.10 clearly confirms its proposed structure. The characteristic ions at m/z = 380.2, 240.0, and 290.0, together with the isotopic peak at m/z ≈ 382.2 corresponding to 34S, serve as reliable diagnostic markers for this class of derivatives, demonstrating the facile detachment of the long-chain alkyl substituent and the stability of the triazole–uracil nucleus. These results highlight the diagnostic value of MS analysis in the structural elucidation of novel triazole-based heterocycles.
Across the homologous S-alkyl series (R1 = Et; R2 = C2H5…C10H21), [M + H]+ values increased systematically from ~288 to ~410 m/z with chain length. For the N-aryl set (R1 = Ph; R2 = C1…C10 alkyl), [M + H]+ spanned roughly 283–410 m/z, as expected from the higher mass of the phenyl substituent.
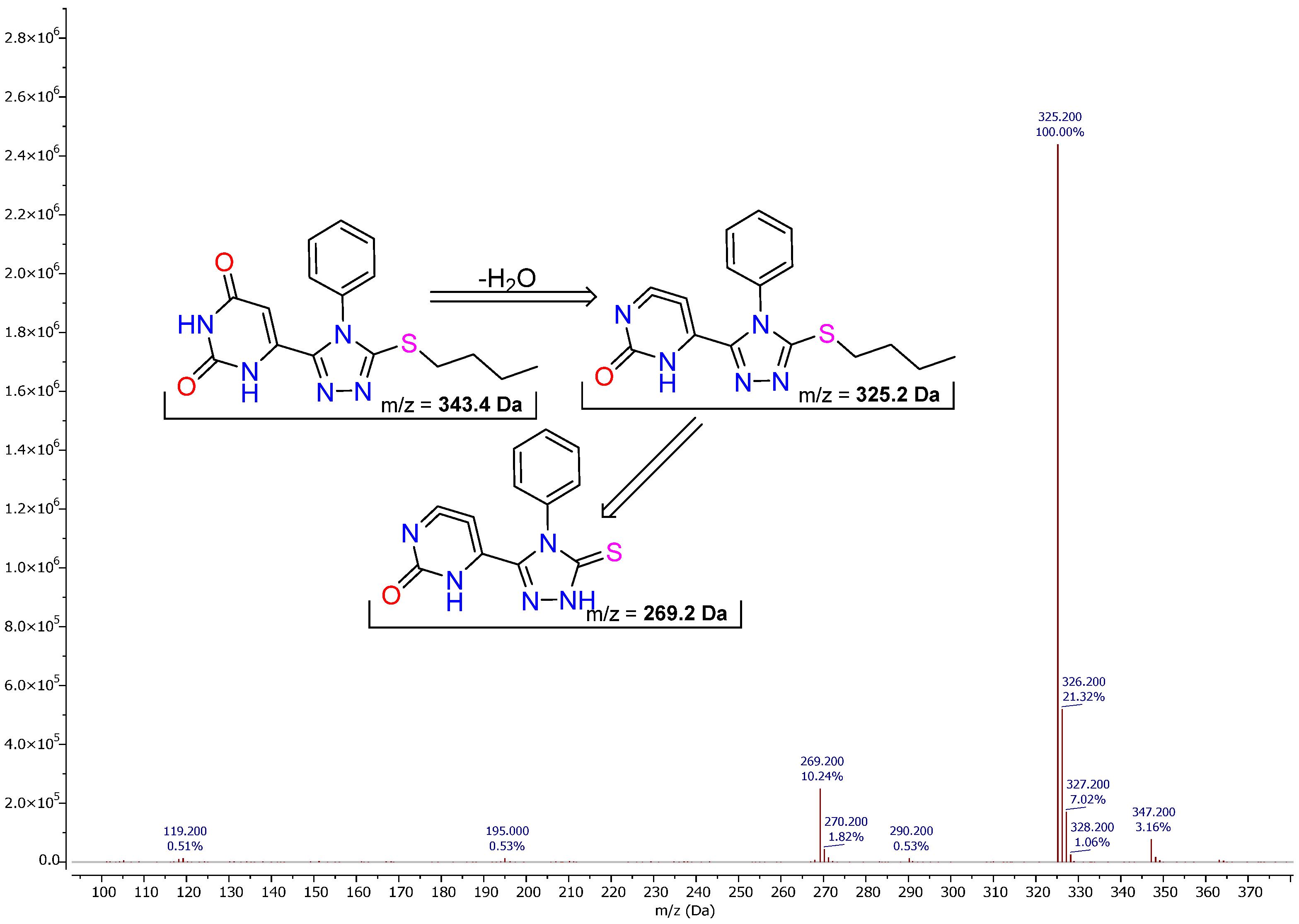
For example, compound
2.4 (R
1 = Ph, R
2 = C
4H
9) and its phenyl-bearing analogues exhibit a coherent and highly diagnostic fragmentation behavior under positive ESI conditions. In the phenyl derivatives, the mass spectra are dominated at early retention (TIC apex ~1.04 min) by a prompt dehydration channel, reflecting a specific propensity of this series to undergo loss of water (−18 Da) directly from the protonated molecular ion (
Figure 3). Thus, for the title phenylthioether, the [M + H]
+ at
m/
z = 343.4 undergoes dehydration to
m/
z = 325.2, which becomes a major, long-lived intermediate in subsequent fragmentations. The dehydrated ion
m/
z = 325.2 shows an isotopic partner at
m/
z ≈ 327.2 (≈4% relative intensity), fully consistent with the presence of a single sulfur atom (
34S, M + 2), and thereby corroborating the thioether/thione motif within the heterocycle.
From m/z = 325.2, a β-cleavage/β-elimination process along the S–(CH2)4 phenylthioalkyl segment furnishes the thione-stabilized core at m/z = 269.2. The Δm = 56.0 Da neutral loss corresponds to C4H8 (but-1-ene), which is entirely consistent with an alkylthioether undergoing charge-induced cleavage with concomitant olefin extrusion. The resulting fragment matches the triazole–thione–uracil cation where the sulfur remains embedded in a conjugated thione environment; the formula of this ion (as drawn) rationalizes both the mass and the retention of the phenyl substituent on the triazole nitrogen while the butyl portion is extruded as an olefin. The strong stability of m/z = 269.2 follows from extensive charge delocalization over the N/O/S heteroatom framework.
Mechanistically, the dehydration first → olefin loss second sequence explains the intensity order and the kinetics observed in-source: (i) protonation localizes initially on the heteroaromatic/ureide manifold; (ii) intramolecular proton relay activates a vicinal heteroatom–carbon framework, enabling neutral H2O expulsion (m/z = 343.4 → 325.2); (iii) the dehydrated cation undergoes charge-directed S–C scission with β-H migration and C4H8 elimination to yield the diagnostic thione nucleus (m/z = 325.2 → 269.2). The consistent observation of the M + 2 partner at m/z ≈ 327.2 for the dehydrated ion further substantiates sulfur retention at this stage of the cascade. Losses of small neutrals (e.g., C2H4, H2S, COS, and HNCO) from either m/z 325.2 or m/z 269.2 remain subordinate to the dominant H2O and C4H8 eliminations that define the phenylthioether signature of this class.
In summary, phenyl-bearing members of this series are readily recognized by the primary dehydration ([M + H]+ → m/z = 325.2), the ^34S isotopic feature at m/z ≈ 327.2, and the secondary C4H8 loss, giving m/z = 269.2. Together, these three markers provide a robust MS fingerprint for rapid dereplication and structural confirmation across related analogues in the uracil–1,2,4-triazole–thioether/thione family.