Abstract
This study investigates the application of natural chitosan as an efficient adsorbent for the removal of heavy metals from aqueous solutions. Experimental results showed that Mn(II), Co(II), and Ni(II) ions were effectively retained on the chitosan surface. Kinetic analysis revealed a preferential adsorption order of Co(II) > Mn(II) > Ni(II), following a pseudo-second-order model with rapid kinetics. Equilibrium adsorption capacities were influenced by initial concentration, temperature, and pH. Thermodynamic analysis indicated that the adsorption process was exothermic and physical in nature. Overall, chitosan proved to be a promising and cost-effective adsorbent for water decontamination.
1. Introduction
The rapid development and continuous growth of industrial activities have significantly increased the release of toxic pollutants into the environment [1]. Among them, heavy metal contamination of water resources has become one of the most pressing environmental concerns, attracting global attention due to its persistence, non-biodegradability, and harmful effects on ecosystems and human health [2]. As a result, the search for reliable and efficient techniques to treat wastewater contaminated with heavy metals has become a major research priority.
Over the past decades, various conventional treatment methods have been applied for the removal of heavy metals from aqueous solutions, including chemical precipitation, ion exchange, reverse osmosis, membrane filtration, and evaporation [3,4]. While these methods have demonstrated effectiveness under certain conditions, they are often associated with limitations such as high operational costs, energy consumption, incomplete metal removal, and the generation of secondary waste. These drawbacks have motivated researchers to explore alternative technologies that are not only efficient but also economically and environmentally sustainable.
Among the available approaches, adsorption has emerged as one of the most effective and promising techniques for wastewater removal [5,6,7,8]. Its advantages include high removal efficiency, operational simplicity, and the possibility of using low-cost and abundant natural adsorbents. In recent years, increasing attention has been directed toward the utilization of bioadsorbents derived from renewable biomass sources [9,10,11]. In particular, chitosan, a biopolymer obtained through the deacetylation of chitin, has gained significant interest due to its low cost, biodegradability, non-toxicity, and high adsorption capacity [12]. The presence of amino and hydroxyl functional groups in its structure enhances its affinity for heavy metal ions, making it a versatile and eco-friendly material for water treatment applications.
In this context, the present study was designed to evaluate the adsorption potential of natural chitosan as a low-cost and sustainable adsorbent for the removal of selected heavy metals from aqueous solutions. By employing supports derived from the recycling of fishery by-products, this work aims to contribute to the development of environmentally responsible strategies for wastewater remediation.
2. Materials and Methods
FTIR Spectroscopy
The FTIR spectrum of chitosan was recorded using a PerkinElmer Spectrum Two spectrometer over the wavenumber range 4000–400 cm−1 at the Laboratory of Inorganic and Environmental Chemistry, University of Tlemcen (Algeria). The samples were prepared in the form of KBr pellets by thoroughly mixing approximately 1 mg of finely powdered chitosan with 100 mg of dry spectroscopic-grade KBr. The mixture was then compressed into a transparent pellet using a hydraulic press. The resulting discs were placed in the sample holder for spectral acquisition. The obtained spectrum is presented in Figure 1, and the corresponding band assignments are summarized in Table 1.
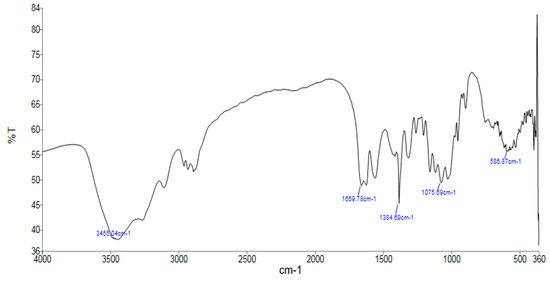
Figure 1.
FTIR spectroscopy of chitosan.

Table 1.
FTIR spectrum attribution [13].
3. Results and Discussion
3.1. Effect of Contact Time
Figure 2 illustrates the evolution of the amounts of metals adsorbed as a function of the contact time between chitosan and the aqueous solutions containing Mn(II), Ni(II), and Co(II) ions. The adsorption kinetics exhibited similar trends, characterized by a rapid uptake of metal ions during the initial minutes of contact, followed by a slower increase until equilibrium was reached. This rapid initial phase can be attributed to the large number of available active sites on the chitosan surface at the beginning of the process, which gradually become saturated over time [14].
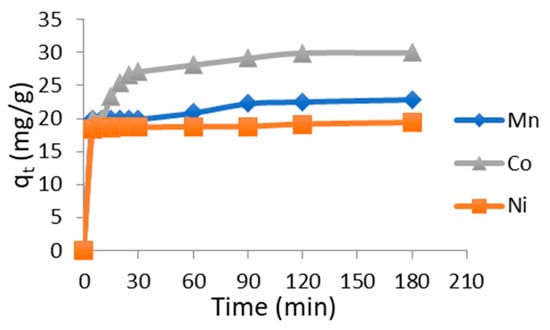
Figure 2.
Effect of contact time of the adsorption of heavy metals on chitosan Cmetal = 100 mg/L, Vsolution = 300 mL, mchitosan = 1 g, pH = neatural, T ambient, stirring speed = medium. Same results was obtained in previous investigation [15].
At equilibrium, chitosan showed a higher affinity toward Co(II), followed by Mn(II), and then Ni(II). The equilibrium contact time was the same for all three metals, approximately 180 min. The maximum adsorption capacities reached 30 mg/g for Co(II), 22.91 mg/g for Mn(II), and 19.38 mg/g for Ni(II), confirming the preferential adsorption order Co(II) > Mn(II) > Ni(II).
3.2. Kinetic Study
The adsorption rate constants of the different heavy metals onto chitosan were determined graphically using three kinetic models. The first-order model was expressed as:
- 1st order: log (qe − qt)/qe = f(t) pour la détermination de Kv (Equation (1))
- Pseudo 2nd order: t/qt = f(t) pour la détermination de K’ (Equation (2))
- 2nd order: 1/(qe − qt) = f(t) pour la détermination de K. (Equation (3))
The sorption rate constants of the different heavy metals onto chitosan were determined graphically using three kinetic models. The first-order kinetic model was evaluated based on the linear plots presented in Figure 3, with the corresponding kinetic parameters summarized in Table 2. The pseudo-second-order model was examined using the linearization shown in Figure 4, and its calculated parameters are reported in Table 3. Finally, the third kinetic model was assessed using the plots displayed in Figure 5, with the resulting rate constants and correlation coefficients compiled in Table 4.
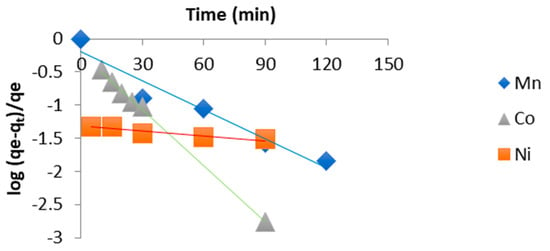
Figure 3.
First order kinetic model.

Table 2.
Results of first order rate constent Kv.
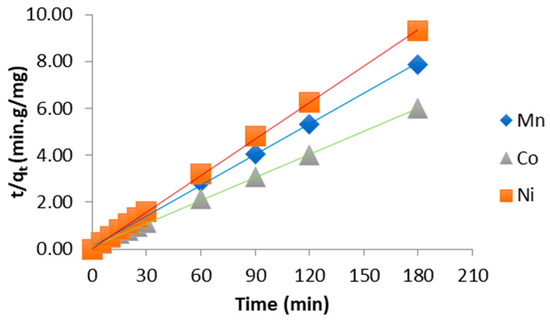
Figure 4.
Pseudo second order kinetic model.

Table 3.
Results of the pseudo second order rate model.

Figure 5.
Second order kinetic model.

Table 4.
Results of the second order rate model.
Based on the results presented in Table 1, Table 2, Table 4 and Table 5, it can be concluded that the pseudo-second-order model provides the most reliable description of the adsorption kinetics of the different heavy metals onto chitosan. Furthermore, the calculated qe values are in close agreement with the experimentally determined ones, and the correlation coefficient was found to be as high as 0.99.

Table 5.
Results of the thermodynamic parameters.
3.3. Influence of the Initial Metal Ion Concentration on the Adsorption
To investigate the influence of initial concentration on the adsorption of heavy metals by chitosan, the following concentrations were selected: 30, 50, 70, 100, 300, and 500 mg/L. The curves presented in Figure 6 exhibit similar trends, characterized by a rapid and nearly linear increase in the amount of metal adsorbed at equilibrium as the initial concentration increases [16]. It was observed that the maximum adsorption capacity rises with higher initial concentrations, which can be attributed to the progressive saturation of available adsorption sites on the chitosan surface. The adsorption curves are nearly superimposed, indicating that the equilibrium amounts of metal retained are comparable for each of the tested metals.
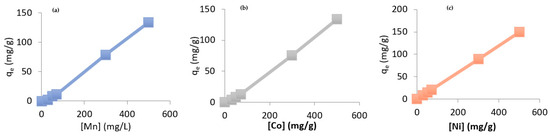
Figure 6.
Initial concentration on the adsorption of heavy metals (a) Mn, (b) Co, (c) Ni. Vsolution = 300 mL, mchitosan = 1 g, pH = neatural, T ambient, stirring speed = medium.
3.4. Influence of the Temperature of Metal Solutions on the Adsorption
To investigate the influence of temperature on the adsorption capacity of heavy metals onto chitosan, we selected the following temperatures: 10, 20, 30, 40, and 50 °C.
The results of this study, presented in Figure 7, show a slight increase in the metal binding capacity with increasing temperature.
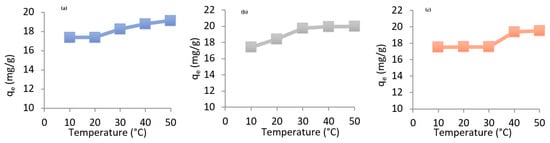
Figure 7.
The influence of temperature on the adsorption capacity of heavy metals onto chitosan (a) Mn (b) Co, (c) Ni. Csolution = 100 mg/L, Vsolution = 300 mL, mchitosan = 1 g, pH = neatural, sttirring speed = medium.
Determination of Thermodynamic Parameters
The adsorption heats of the different heavy metals onto chitosan are determined in Figure 8, which represent the plots of ln Kc versus 1/T, where the temperature is expressed in Kelvin. ΔH was obtained from the slope, while ΔS was calculated from the intercept. All the results are listed in Table 5 and Table 6.
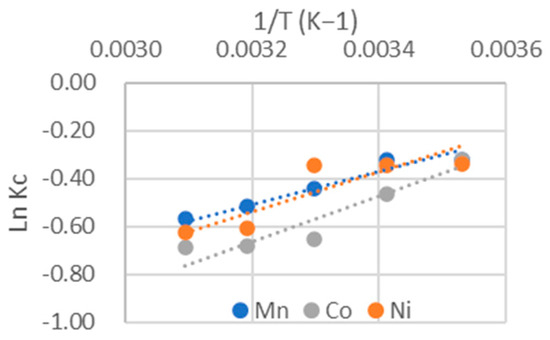
Figure 8.
Determination of thermodynamic parameters.

Table 6.
Results of free enthalpy at different temperature.
According to the results presented in Table 5, the values of ΔH are negative, indicating that the adsorption of heavy metals onto chitosan is an exothermic process. This implies that the system releases heat to the external environment. The relatively low values of ΔH suggest that the process corresponds to physical adsorption.
Furthermore, the ΔS values are also negative, which means that the adsorption of heavy metals on chitosan is accompanied by an increase in order within the system [17]. In other words, the metal ions adsorbed on the surface of the adsorbent are more organized compared to their distribution in the aqueous phase.
3.5. Influence of the Initial pH of Metal Solutions on the Adsorption
To investigate the effect of pH on the adsorption of heavy metals onto chitin and chitosan, the following pH values were selected:
- For manganese: 2, 4, 6, and 8;
- For nickel: 2, 4, 6, and 7;
- For cobalt: 2, 4, 6, and 7.
Figure 9 illustrates the amount of each metal adsorbed at equilibrium as a function of pH.
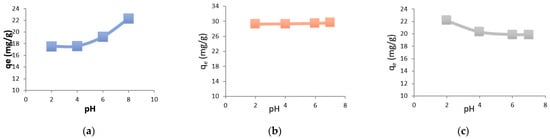
Figure 9.
The amount of metals adsorbed at equilibrium as a function of pH (a) Mn, (b) Ni, (c) Co. Csolution = 100 mg/L; Vsolution = 300 mL, mchitosan = 1 g; temperature = ambient; stirring speed = medium.
At acidic pH values, metal ions compete with H3O+ ions present in the solution. Due to their higher mobility, hydronium ions are more readily adsorbed than metal ions [18].
At slightly acidic pH (between 4 and 7), the competitive effect of H3O+ ions decreases as the pH increases, which explains the higher adsorption of heavy metals by both supports within this pH range.
4. Conclusions
This study demonstrated that chitosan is an efficient and eco-friendly adsorbent for the removal of heavy metals from aqueous solutions. The equilibrium contact time was the same for all three metals, approximately 180 min. The maximum adsorption capacities reached 30 mg/g for Co(II), 22.91 mg/g for Mn(II), and 19.38 mg/g for Ni(II), confirming the preferential adsorption order Co(II) > Mn(II) > Ni(II).
Adsorption kinetics were best described by the pseudo-second-order model, and thermodynamic analysis confirmed that the process is spontaneous, exothermic, and mainly physical in nature. The adsorption capacity increased with metal concentration and was strongly influenced by pH, with optimal removal observed under slightly acidic conditions. Overall, chitosan shows great potential as a low-cost and sustainable material for wastewater treatment applications.
Author Contributions
Conceptualization, I.L., K.T. and B.B.; methodology, I.L., K.T. and B.B.; validation, I.L., K.T. and B.B.; formal analysis, I.L., K.T. and B.B.; investigation, I.L., K.T. and B.B.; resources, I.L., K.T. and B.B.; data curation, I.L., K.T. and B.B.; writing—original draft preparation, I.L., K.T. and B.B.; writing—review and editing, I.L., K.T. and B.B. All authors contributed equally to the conception, methodology, data analysis, and writing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aziz, K.H.H.; Mustafa, F.S.; Omer, K.M.; Hama, S.; Hamarawf, R.F.; Rahman, K.O. Heavy metal pollution in the aquaticen-vironment: Efficient and low-cost removal approaches to eleminatetheir toxicity: A review. RSC Adv. 2023, 13, 17595–17610. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, P.; Saravanan, V.; Rajeshkannan, R.; Arnica, G.; Rajasimman, M.; Baskar, G.; Pugazhendhi, A. Comprehensive review on toxic heavy metals in the aquatic system: Sources, identification, treatment strategies, and health risk assessment. Environ. Res. 2024, 258, 119440. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Z.; Chao, G.; Yanliang, W.; Lifei, S.; Xiaolei, H.; Wei, R.; Qirong, S. Removal of heavy metals from aqueous solution by lipopeptides and lipopeptides modified Na-montmorillonite. Bioresour. Technol. 2013, 147, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Hayati, B.; Maleki, A.; Najafi, F.; Daraei, H.; Gharibi, F.; McKay, G. Super high removal capacities of heavy metals (Pb2+ and Cu2+) using CNT dendrimer. J. Hazard. Mater. 2017, 336, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, A.; Hollywood, J.; Lamping, D.L.; Pease, C.T.; Chakravarty, K.; Silverman, B.; Choy, E.H.S.; Scott, D.G.; Hazleman, B.L.; Bourke, B.; et al. Clinical outcomes, quality of life, and diagnostic uncertainty in the first year of polymyalgia rheumatica. Arthritis Rheum. 2007, 57, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Lansari, I.; Benguella, B.; Kruchinina, N.; Nistratov, A. Adsorption of textile dyes from aqueous solution using activated carbon from human hair. J. React. Kinet. Mech. Catal. 2022, 135, 1891–1903. [Google Scholar] [CrossRef]
- Lansari, I.; Tizaoui, K.; Benguella, B.; Bensaid, F.Z.; Chekroun, A.; Kruchinina, N.; Nistratov, A. Kinetic study of removal of reactive violet and bemacid yellow from aqueous solution by adsorption onto activated carbon derived from human hair. React. Kinet. Mech. Catal. 2025, 138, 2597–2611. [Google Scholar] [CrossRef]
- Lansari, I.; Benguella, B.; Kruchinina, N.; Nistratov, A. The removal of acid green 4G and anthraquinone orange from aqueous solution using adsorption on activated carbon from human hair. React. Kinet. Mech. Catal. 2022, 135, 987–998. [Google Scholar] [CrossRef]
- Biswal, B.K.; Balasubramanian, R. Use of biochar as a low-cost adsorbent for removal of heavy metals from water and wastewater: A review. J. Environ. Chem. Eng. 2023, 11, 110986. [Google Scholar] [CrossRef]
- Haris, M.; Amjad, Z.; Usman, M.; Saleem, A.; Dyussenova, A.; Mahmood, Z.; Dina, K.; Guo, J.; Wang, W. A review of crop residue-based biochar as an efficient adsorbent to remove trace elements from aquatic systems. Biochar 2024, 6, 47. [Google Scholar] [CrossRef]
- Shree, B.; Kumari, S.; Singh, S.; Rani, I.; Dhanda, A.; Chauhan, R. Exploring various types of biomass as adsorbents for heavy metal remediation: A review. Environ. Monit. Assess. 2025, 197, 406. [Google Scholar] [CrossRef]
- Ashraf, A.; Dutta, J.; Farooq, A.; Rafatullah, M.; Pal, K.; Kyzas, G.Z. Chitosan-based materials for heavy metal adsorption: Recent advancements, challenges and limitations. J. Mol. Struct. 2024, 1309, 138225. [Google Scholar] [CrossRef]
- Hafsa, J.; Smach, M.; Charfeddine, B.; Limem, K.; Majdoub, H.; Rouatbi, S. Antioxidant and antimicrobial proprieties of chitin and chitosan extracted from Parapenaeus Longirostris shrimp shell waste. Ann. Pharm. Fr. 2016, 74, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Fayoud, N.H.; Tounsi, L.A.; Tahiri, S.; Abderrahmene, A. Etude cinétique et thermodynamique de l’adsorption de bleu de methylene sur les cendres de bois (Kinetic and thermodynamic study of the adsorption of methylene blue on wood ashes). J. Mater. Environ. Sci. 2015, 6, 3295–3306. [Google Scholar]
- Lansari, I.; Tizaoui, K.; Benguella, B.; Bendimerad, F.A.; Bouchaour, S.M. Optimizing chitosan-based adsorbents for the removal of triso-dium ferric complex of N-methyl-1,8-naphtalimide-4-sulfonate. J. Reac. Kinet. Mech. Cat. 2025, 74, 27–33. [Google Scholar]
- Tizaoui, K.; Benguella, B.; Makhoukhi, B. Selective adsorption of heavy metals (Co2+, Ni2+, and Cr3+) from aqueous solutions onto natural marne clay. Desalination Water Treat. 2019, 142, 252–259. [Google Scholar] [CrossRef]
- Belbachir, I.; Makhoukhi, B. Adsorption of Bezathren dyes onto sodic bentonite from aqueous solutions. J. Taiwan Inst. Chem. Eng. 2017, 75, 105–111. [Google Scholar] [CrossRef]
- Roussy, J.; Van Vooren, M.; Dempsey, B.A.; Guibal, E. Influence of chitosan characteristics on the coagulation and the flocculation of bentonite suspensions. Water Res. 2005, 39, 3247–3258. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).