Catalysis-Free Microwave-Assisted Synthesis of Biscoumarins with Chromone Group by a Multicomponent Process †
Abstract
1. Introduction
2. Results and Discussion
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anastas, P.T.; Heine, L.G.; Williamson, T.C. Green Chemical Syntheses and Processes; Chapters 1–6; American Chemical Society: Washington, DC, USA, 2000. [Google Scholar]
- Arico, F.; Reiser, O. Green Synthesis of Heterocycles; Frontiers Media SA: Lausanne, Switzerland, 2020. [Google Scholar]
- Messire, G.; Caillet, E.; Raboin, S.B. Green Catalysts and/or Green Solvents for Sustainable Multi-Component Reactions. Catalysts 2024, 14, 593. [Google Scholar] [CrossRef]
- Hussain, M.K.; Mohammad, S.K.; Khan, F.; Akhtar, M.S.; Ahamad, S.; Saquib, M. Coumarins as Versatile Therapeutic Phytomolecules: A Systematic Review. Phytomedicine 2024, 134, 155972. [Google Scholar] [CrossRef] [PubMed]
- Karatoprak, G.Ş.; Dumlupınar, B.; Celep, E.; Celep, I.K.; Akkol, E.K.; Sobarzo-Sánchez, E. A comprehensive review on the potential of coumarin and related derivatives as multi-target therapeutic agents in the management of gynecological cancers. Front. Pharmacol. 2024, 15, 1423480. [Google Scholar] [CrossRef]
- Gadamsetti, S.; Kamala, G.; Degala, R.P.; Prasanna, V.; Yaswini, K.; Srilaya, A. A Review of Synthesis and Therapeutic Applications of Coumarin Derivatives: Review Article. J. Pharma Insights Res. 2024, 2, 171–183. [Google Scholar] [CrossRef]
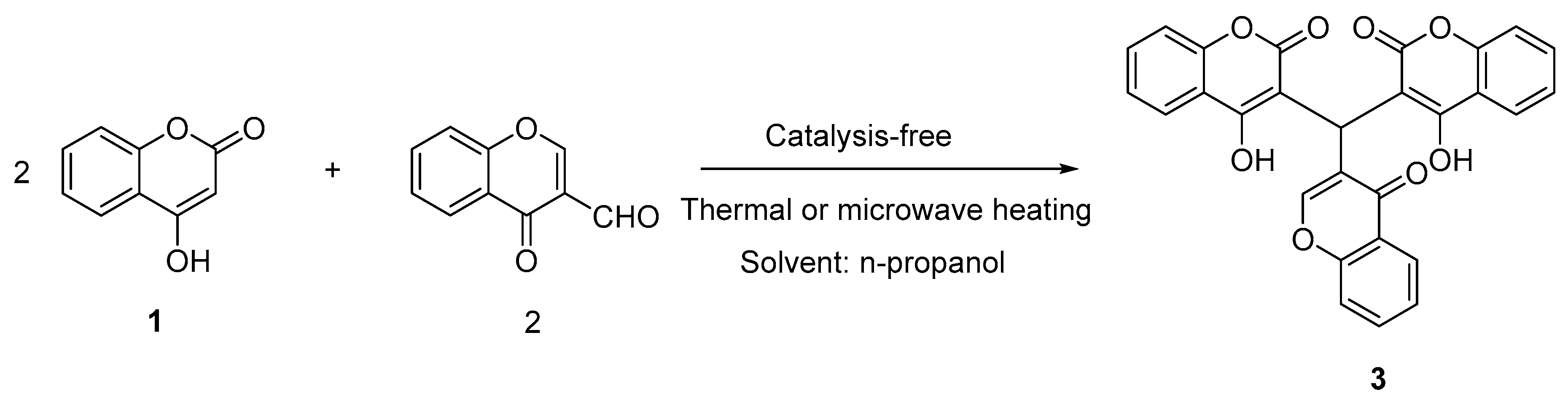
| Entry | Formylchromone | Product | Time (h) | Yield (%) |
|---|---|---|---|---|
| 1 | 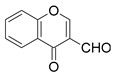 | 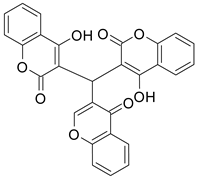 | 4 | 86 |
| 1 | 74 | |||
| 2 | 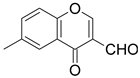 | 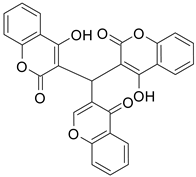 | 4 | 67 |
| 1 | 59 | |||
| 3 | 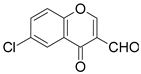 | 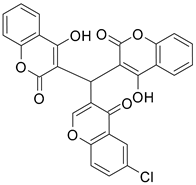 | 4 | 75 |
| 4 | 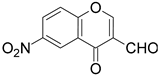 | 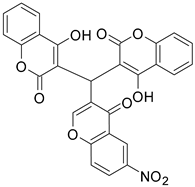 | 4 | 58 |
| 5 | 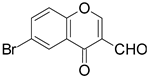 | 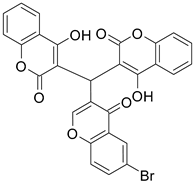 | 4 | 70 |
| 1 | 68 | |||
| 6 | 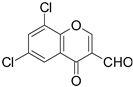 | 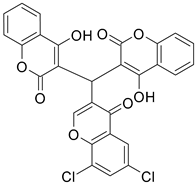 | 4 | 68 |
| 1 | 67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palacios, E.X.A.; Pasquale, G.A.; Palermo, V.; Murguía, M.C.; Sathicq, Á.G.; Romanelli, G.P. Catalysis-Free Microwave-Assisted Synthesis of Biscoumarins with Chromone Group by a Multicomponent Process. Chem. Proc. 2025, 18, 40. https://doi.org/10.3390/ecsoc-29-26844
Palacios EXA, Pasquale GA, Palermo V, Murguía MC, Sathicq ÁG, Romanelli GP. Catalysis-Free Microwave-Assisted Synthesis of Biscoumarins with Chromone Group by a Multicomponent Process. Chemistry Proceedings. 2025; 18(1):40. https://doi.org/10.3390/ecsoc-29-26844
Chicago/Turabian StylePalacios, Edna Ximena Aguilera, Gustavo Antonio Pasquale, Valeria Palermo, Marcelo César Murguía, Ángel Gabriel Sathicq, and Gustavo Pablo Romanelli. 2025. "Catalysis-Free Microwave-Assisted Synthesis of Biscoumarins with Chromone Group by a Multicomponent Process" Chemistry Proceedings 18, no. 1: 40. https://doi.org/10.3390/ecsoc-29-26844
APA StylePalacios, E. X. A., Pasquale, G. A., Palermo, V., Murguía, M. C., Sathicq, Á. G., & Romanelli, G. P. (2025). Catalysis-Free Microwave-Assisted Synthesis of Biscoumarins with Chromone Group by a Multicomponent Process. Chemistry Proceedings, 18(1), 40. https://doi.org/10.3390/ecsoc-29-26844






