Abstract
The synthesis of Imidazo[1, 2-a]pyridine (IMP) analogs is a research field constantly growing due potential applications of Groebke–Blackburn–Bienaymé (GBB) products in several fields, focusing on the development of novel greener strategies. To date, the ultrasound assisted synthesis of IMP analogs via Groebke–Blackburn–Bienaymé reaction (GBBR) under green inexpensive catalysts such p-toluenesulfonic acid (TsOH) is practically unreported. In the present work, we describe the TsOH catalyzed GBB reaction assisted by ultrasound irradiation (USI) to access IMP analogs in excellent overall yields 77–91%.
1. Introduction
Imidazo[1, 2-a]pyridines (IMPs) are considered privileged scaffolds in medicinal chemistry due to the broad spectrum of pharmaceutical and biological applications (Figure 1). Several commercial drugs such as zolpidem, miroprofen, saripidem, zolidimide, olprinone, and minodronic acid incorporate the imidazo[1, 2-a]pyridine core in their structure (Figure 1) [1].
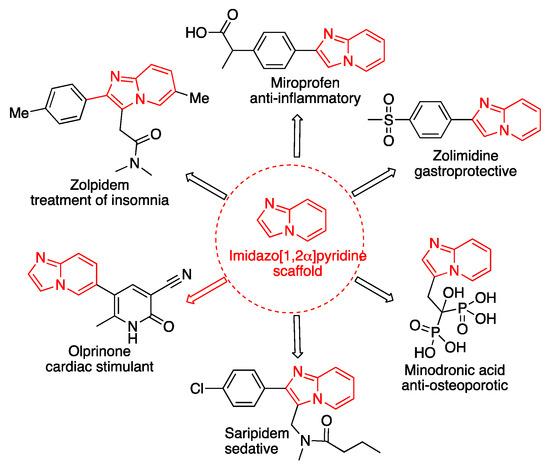
Figure 1.
Commercialized drugs containing the imidazo[1, 2–a]pyridine scaffold.
Isocyanide-based multicomponent reactions (IMCRs) have attracted significant interest in both academic and industry fields due to their efficiency in reducing the number of steps, thus minimizing waste, during the purification of intermediates [2,3,4]. In addition, ultrasound irradiation (USI) in chemistry can alter reactivity, improve yields and selectivity, reduce reaction times, energy consumption, and waste production, etc. [5].
The Groebke–Blackburn–Bienaymé reaction (GBBR) is the method of choice for the synthesis of IMPs [6,7]. In concordance with our research, line focus in the design and development of novel GBBR protocols [8,9,10] herein describes the ultrasound-assisted GBBR to the green synthesis of IMPs analogs under catalyst conditions. In 2024, Gámez-Montaño et al. (Scheme 1) developed a consecutive one-pot process by the GBBR, followed by copper-catalyzed alkyne–azide Cycloaddition (CuAAC) assisted by alternative sustainable energies (ASEs) such as ultrasonic or mechanical activation [9]. In 2025, we reported the sonochemical multicomponent synthesis of 2-(2’-hydroxyphenyl)imidazo[1,2-a]pyridine analogs (Scheme 1), which exhibited an intramolecular, hydrogen-bonded, eight-membered ring capable of excited-state intramolecular proton transfer (ESIPT) [10].
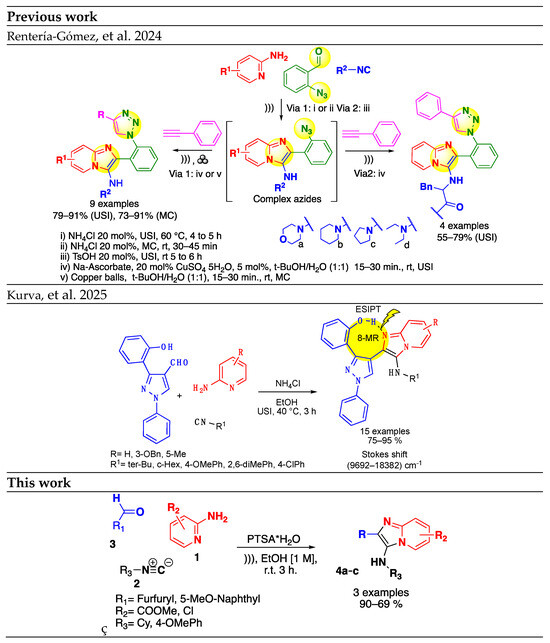
Scheme 1.
Previous reports of synthesis of imidazo[1, 2-α]pyridines [9,10].
Our research group is pioneering in the innovation and development of novel one-pot GBBR protocols using alternative energy sources. To date, the GBB synthesis of IMPs using inexpensive catalysts such as p-toluenesulfonic acid (TsOH) assisted by USI is practically unreported. In the present work, we describe the ultrasound assisted synthesis of IMP analogs catalyzed by TsOH.
2. Results and Discussion
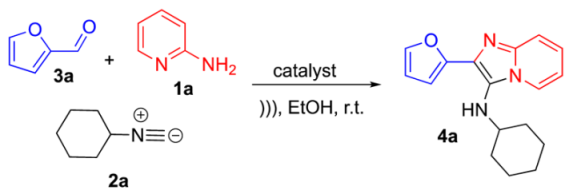
Initially, we started the synthesis of N-cyclohexyl-2-(furan-2-yl)imidazo[1,2-α]pyridin-3-amine 4a using furfural 3a (1 mmol), 2-aminopyridine 1a (1 mmol), and cyclohexyl isocyanide 2a (1 mmol) in EtOH; green catalysts such as p-toluenesulfonic acid monohydrate (PTSA*H2O) and ammonium chloride (NH4Cl) were tested under USI conditions at room temperature (Table 1). We observed better yields using PTSA*H2O, and the better yields were using 10% of catalyst PTSA*H2O. A test without catalyst was carried out, with no product formation observed.

Table 1.
Screening conditions for the synthesis 4a.
After finding optimal conditions, a series of IMPs 4a–d were successfully synthesized in good-to-excellent yields (77–91%) (Scheme 2).
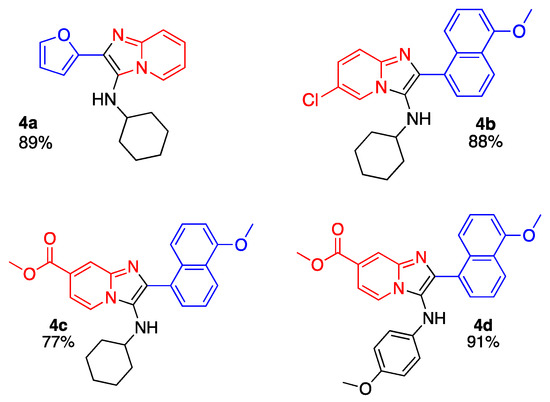
Scheme 2.
Substrate Scope.
3. Experimental Section
3.1. General Information, Instrumentation and Chemicals
General Information: Commercially available starting materials were purchased from Sigma-Aldrich and used without further purification. Solvents were distilled and dried following standard procedures. IR spectra were recorded on a Perkin Elmer 100 FT-IR spectrometer (PerkinElmer, Waltham, MA, USA) (ν in cm−1). 1H and 13C NMR spectra were acquired on a Bruker spectrometer (Bruker, Billerica, MA, USA) operating at 500 MHz. CDCl3 was used as the solvent, and chemical shifts are reported in ppm. Coupling constants are reported in Hz. For 1H NMR spectra, TMS at 0.0 ppm was used as the internal reference, and for 13C NMR spectra, the central peak of CDCl3 at 77.00 ppm served as the reference. Ultrasound-irradiated reactions were performed in sealed 10 mL tubes placed in the water bath of a Branson 1510 ultrasonic cleaner (Branson Ultrasonics, Danbury, CT, USA) operating at 42 kHz ± 6%. Reaction progress was monitored by TLC, and spots were visualized under UV light at 254 or 365 nm.
3.2. General Procedure (GP)
In a sealed tube, to a solution of aldehyde (1.0 equiv.) in ethanol [1.0 M], aminopyridine (1.0 equiv.), isocyanide (1.0 equiv.), and PSTA*H2O (10% mol) were sequentially added and the reaction mixture was sonicated at room temperature under 3 h. The solvent was dried and flash column chromatography was performed using silica gel (230–400 mesh) and mixtures of hexane and ethyl acetate were used as mobile phase.
3.3. Spectral Data
3.3.1. N-cyclohexyl-2-(furan-2-yl)imidazo[1,2-α]pyridin-3-amine (4a)
Compound 4a (89% yield) was synthetized according to GP, using 2-aminopyridine, furfural, and cyclohexyl isocyanide as compounds in GBBR. 1H NMR (500 MHz, CDCl3) δ 8.39 (s, 1H), 7.46 (t, J = 4.3 Hz, 2H), 7.12 (d, J = 9.2 Hz, 1H), 6.84 (d, J = 3.0 Hz, 1H), 6.48 (d, J = 3.2 Hz, 1H), 3.58 (s, 1H), 2.88 (q, J = 7.4, 4.5 Hz, 1H), 1.81 (d, J = 11.5 Hz, 2H), 1.69 (d, J = 10.8 Hz, 2H), 1.58–1.45 (m, 1H), 1.20 (dq, J = 33.2, 10.9, 10.4 Hz, 5H). 13C NMR (126 MHz, CDCl3) δ 149.28, 142.29, 140.79, 130.38, 129.03, 126.15, 123.51, 118.01, 117.05, 111.73, 107.98, 97.69, 57.31, 34.08, 25.58, 24.90 ppm. HRMS (ESI-TOF) m/z [M + H]+ Calcd for [C17H19N3O + H+] 282.1601, found 282.1616.
3.3.2. 6-Chloro-N-cyclohexyl-2-(5-methoxynaphthalen-1-yl)imidazo[1,2-α] pyridin-3-amine (4b)
Compound 4b was synthetized according to GP, using 2-amino-5-chloropyridine, 5-methoxy-1-naphthaldehyde, and cyclohexyl isocyanide. 4b was obtained as an oil in 88% yield. 1H NMR (500 MHz, CDCl3) δ 8.28–8.26 (m, 1H), 8.08–8.09 (m, 1H), 7.78–7.76 (m, 1H), 7.46–7.40 (m, 4H), 7.04 -7.02 (m, 1H), 6.82 (d, J = 8.0 Hz, 1H), 3.98 (s, 3H), 3.00 (d, J = 7.1 Hz, 1H), 2.59–2.51 (m, 1H), 1.53–1.50 (m, 2H), 1.41–1.30 (m, 3H), 0.91 (t, J = 10.8 Hz, 2H), 0.78 (q, J = 11.9 Hz, 2H) ppm. 13C NMR (126 MHz, CDCl3) δ 155.8, 139.9, 137.8, 133.1, 128.2, 127.2, 127.1, 125.9, 125.4, 125.30, 124.6, 124.0, 122.4, 120.7, 120.1, 118.0, 103.6, 56.4, 55.7, 33.9, 25.6, 24.6 ppm. HRMS (ESI-TOF) m/z [M + H+]+ Calcd for [C24H24ClN3O + H+]+ 406.1681, found 406.1696.
3.3.3. Methyl 3-(cyclohexylamino)-2-(5-methoxynaphthalen-1-yl)imidazo[1,2-α]pyridine-7-carboxylate (4c)
Compound 4c was synthetized according to GP, using Methyl 2-aminopyridine-4-carboxylate, 5-methoxy-1-naphthaldehyde, and cyclohexyl isocyanide. 4c was obtained as an oil in 77% yield. 1H NMR 500 MHz, Chloroform-d) δ 8.35 (m, 2H), 8.14 (d, J = 7.1 Hz, 1H), 7.87–7.80 (m, 1H), 7.57 (d, J = 7.9 Hz, 1H), 7.54–7.43 (m, 3H), 6.91 (d, J = 7.8 Hz, 1H), 4.07 (s, 3H), 3.97 (s, 3H), 2.69 (m, 1H), 1.64–1.56 (m, 2H), 1.47 (m, 2H), 1.44–1.38 (m, 1H), 0.99 (m, 3H), 0.89 (m, 2H). 13C NMR (126 MHz, CDCl3) δ 166.0, 155.9, 139.7, 132.8, 128.4, 128.4, 127.2, 125.7, 125.3, 125.1, 122.4, 122.1, 120.0, 119.9 111.2, 103.5, 56.1, 55.6, 52.6, 33.8, 25.4, 24.5 ppm. HRMS (ESI-TOF) m/z [M + H]+ Calcd for [C26H27N3O3 + H+]+ 430.2125, found 430.2150.
3.3.4. Methyl 2-(5-methoxynaphthalen-1-yl)-3-((4-methoxyphenyl)amino)imidazo[1,2-α]pyridine-7-carboxylate (4d)
Compound 4d was synthetized according to GP, using Methyl 2-aminopyridine-4-carboxylate, 5-methoxy-1-naphthaldehyde, and 4-metoxy-phenyl-isocyanide. 4d was obtained in 91% yield. 1H NMR (500 MHz, Chloroform-d) δ 8.34 (s, 1H), 8.30–8.19 (m, 2H), 7.68 (d, J = 7.1 Hz, 1H), 7.49–7.38 (m, 3H), 7.33 (dd, J = 7.1, 1.6 Hz, 1H), 6.77–6.66 (m, 3H), 6.44–6.37 (m, 2H), 5.69 (s, 1H), 3.96 (d, J = 2.9 Hz, 6H), 3.72 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 165.97, 155.89, 153.75, 141.47, 140.79, 137.88, 132.70, 128.20, 126.97, 125.78, 125.66, 125.26, 125.21, 122.76, 122.54, 122.37, 122.16, 120.26, 115.21, 115.15, 111.18, 103.31, 77.30, 77.04, 76.79, 72.29, 61.79, 60.38, 55.65, 55.49, 52.48, 31.92, 30.58, 29.70, 22.69, 14.19, 14.11. ppm. HRMS (ESI-TOF) m/z [M + H]+ Calcd for [C27H23N3O4 + H+] 454.1736, found 454.1761.
4. Conclusions
The contributions of this work fall mainly in the synthetic and pharmacological fields. GBB products could have applications in medicinal chemistry. This protocol offers several advantages, including excellent overall yields, the use of an alternative green energy source, short reaction times, eco-friendly solvents, inexpensive green catalysts, one-pot synthesis, and operational simplicity. The scope of the developed strategy reported here corresponds to the current progress of the project, which will be further expanded and published in due course.
Author Contributions
All authors contributed equally to this work. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by DAIP (132/2023 and 066/2024) and SECIHTI (CB-2016-28562). A.C.-D. (490344/2907767), D.C.-R. (666925/2782364), and D.G.-G. (1233507/4039492) thanks SECIHTI for scholarships.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All relevant information is contained within the article. Data sharing is not applicable to this article.
Acknowledgments
All authors acknowledge the Laboratorio Nacional de Caracterización de Propiedades Fisicoquímicas y Estructura Molecular UG-UAA (SECIHTI) for the provided instrumentation time.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kumar, A.; Sharma, V.; Behl, T.; Ganesan, S.; Nathiya, D.; Gulati, M.; Khalid, M.; Elossaily, G.M.; Chigurupati, S.; Sachdeva, M. Insights into Medicinal Attributes of Imidazo[1,2-a]Pyridine Derivatives as Anticancer Agents. Archiv. Pharm. 2024, 357, e2400402. [Google Scholar] [CrossRef] [PubMed]
- Nasiriani, T.; Javanbakht, S.; Nazeri, M.T.; Farhid, H.; Khodkari, V.; Shaabani, A. Isocyanide-Based Multicomponent Reactions in Water: Advanced Green Tools for the Synthesis of Heterocyclic Compounds. Top. Curr. Chem. (Z) 2022, 380, 50. [Google Scholar] [CrossRef] [PubMed]
- Rudick, J.G.; Dömling, A.; Shaabani, S. Isocyanide-Based Multicomponent Reactions; Frontiers Research Topics; Frontiers Media SA: Lausanne, Switzerland, 2020; ISBN 978-2-88963-484-2. [Google Scholar]
- Zhu, J.; Wang, Q.; Wang, M. Multicomponent Reactions in Organic Synthesis; John Wiley & Sons: Hoboken, NJ, USA, 2015; ISBN 3-527-33237-5. [Google Scholar]
- Banerjee, B. Recent Developments on Ultrasound-Assisted One-Pot Multicomponent Synthesis of Biologically Relevant Heterocycles. Ultrason. Sonochemistry 2017, 35, 15–35. [Google Scholar] [CrossRef] [PubMed]
- Boltjes, A.; Dömling, A. The Groebke-Blackburn-Bienaymé Reaction. Eur. J. Org. Chem. 2019, 2019, 7007–7049. [Google Scholar] [CrossRef] [PubMed]
- Devi, N.; Rawal, R.K.; Singh, V. Diversity-Oriented Synthesis of Fused-Imidazole Derivatives via Groebke–Blackburn–Bienayme Reaction: A Review. Tetrahedron 2015, 71, 183–232. [Google Scholar] [CrossRef]
- Kurva, M.; Pharande, S.G.; Quezada-Soto, A.; Gámez-Montaño, R. Ultrasound Assisted Green Synthesis of Bound Type Bis-Heterocyclic Carbazolyl Imidazo[1, 2-a]Pyridines via Groebke-Blackburn-Bienayme Reaction. Tetrahedron Lett. 2018, 59, 1596–1599. [Google Scholar] [CrossRef]
- Rentería-Gómez, M.A.; Calderón-Rangel, D.; Corona-Díaz, A.; Gámez-Montaño, R. A Sonochemical and Mechanochemical One-Pot Multicomponent/Click Coupling Strategy for the Sustainable Synthesis of Bis-Heterocyclic Drug Scaffolds. ChemPlusChem 2025, 90, e202400455. [Google Scholar] [CrossRef] [PubMed]
- Kurva, M.; Calderón-Rangel, D.; Corona-Díaz, A.; Kishore, K.G.; Rentería-Gómez, Á.; Ramos-Ortíz, G.; Gámez-Montaño, R. Green Multicomponent One-Pot Synthesis of 2-(2′-Hydroxyphenyl)Imidazo[1,2-a]Pyridine Analogs with an Intramolecular Hydrogen-Bonded Eight-Membered Ring Exhibiting Excited-State Intramolecular Proton Transfer Luminescence. Eur. J. Org. Chem 2025, 28, e202500630. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).