Process Optimization of Keratin Extraction from Chicken Feathers Using Alkaline Oxidation: A Taguchi L9 Orthogonal Array Study †
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Protein Extraction Using Alkaline Oxidation Method
2.3. Taguchi Orthogonal Array Design
3. Results and Discussion
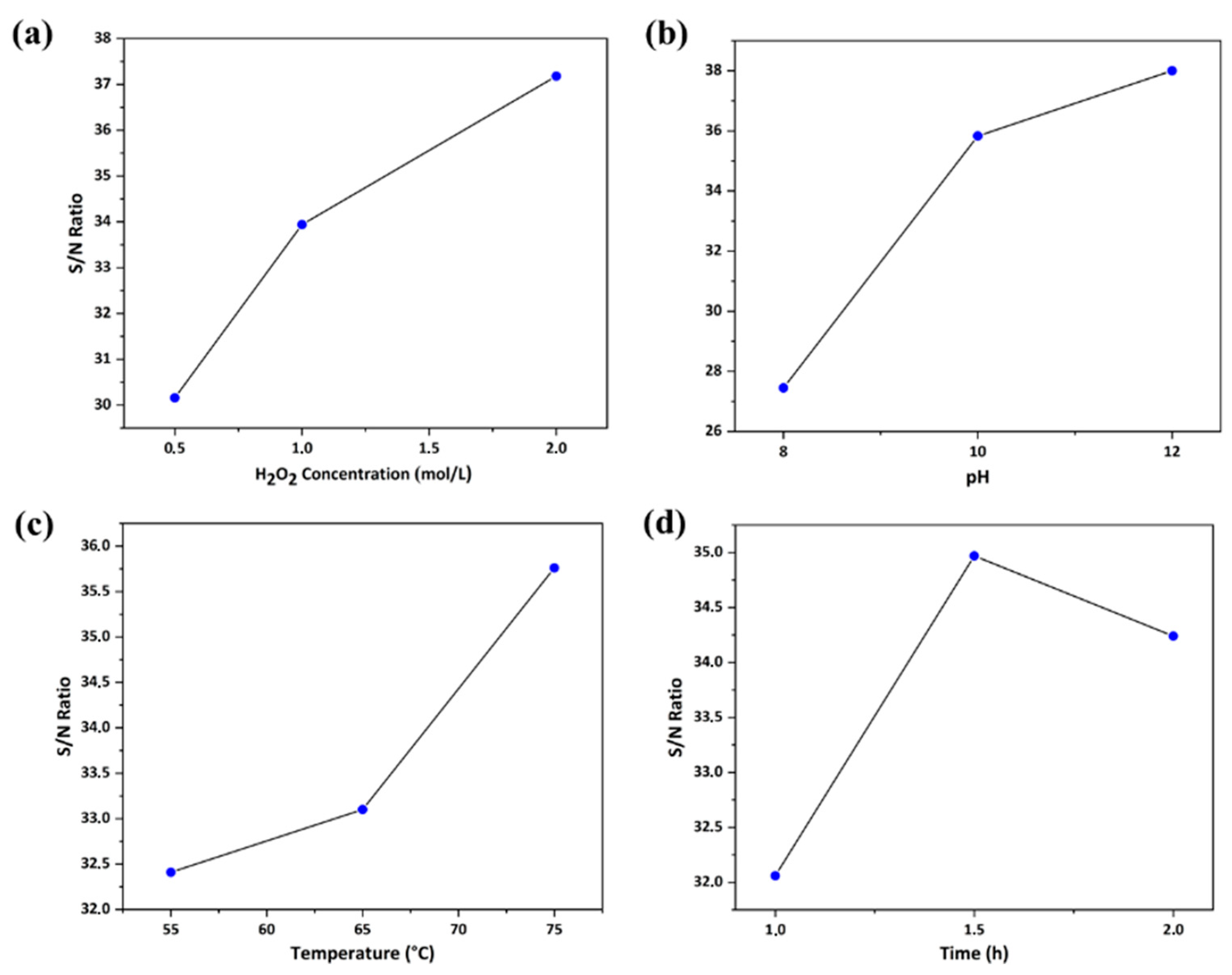
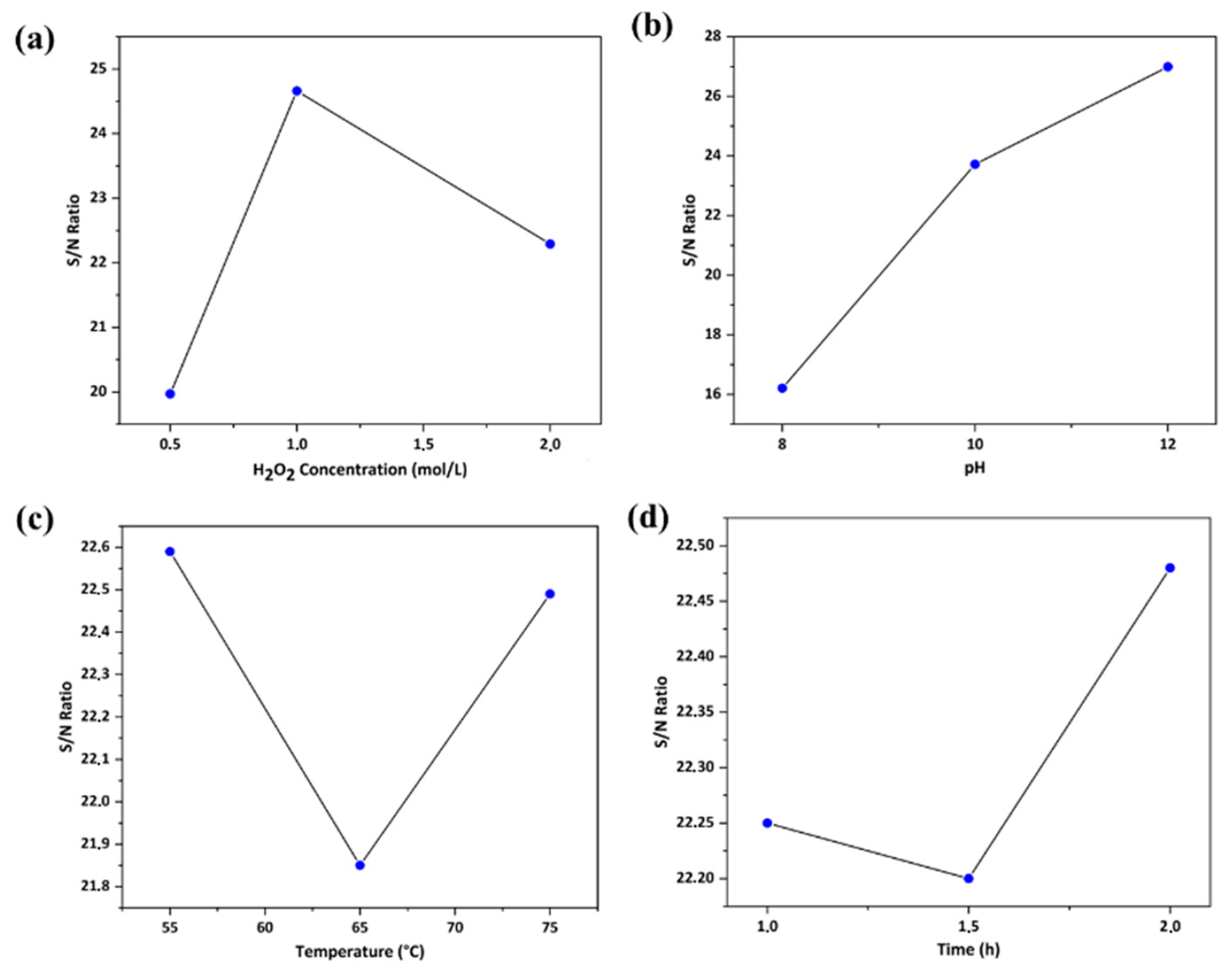
3.1. Taguchi Optimization Methodology of Operating Extraction Conditions
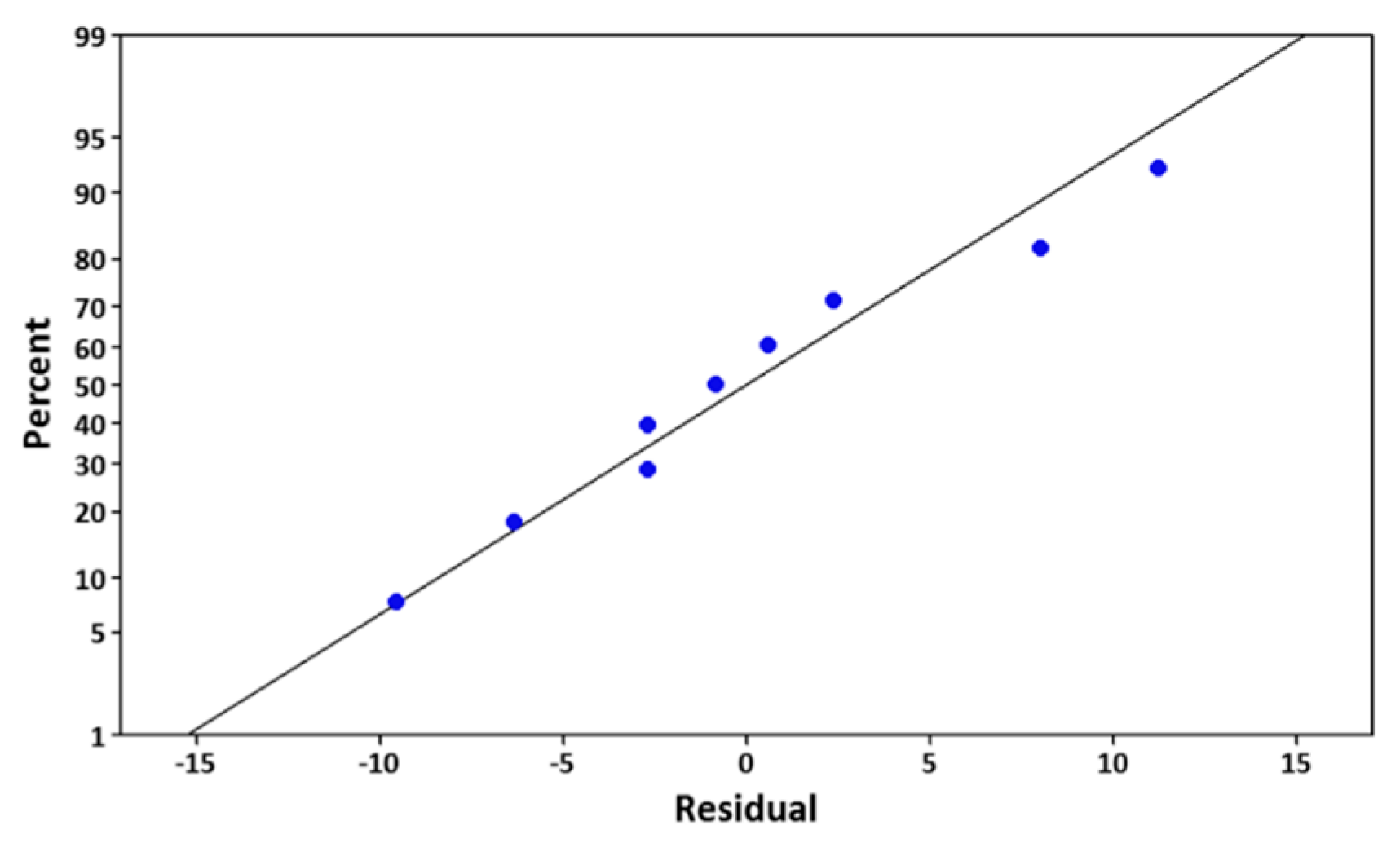
3.2. Development of Regression Models and Analysis of Variance (ANOVA) Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lazarus, B.S.; Chadha, C.; Velasco-Hogan, A.; Barbosa, J.D.V.; Jasiuk, I.; Meyers, M.A. Engineering with Keratin: A Functional Material and a Source of Bioinspiration. iScience 2021, 24, 102798. [Google Scholar] [CrossRef]
- Kamarudin, N.B.; Sharma, S.; Gupta, A.; Kee, C.G.; Chik, S.M.S.B.T.; Gupta, R. Statistical Investigation of Extraction Parameters of Keratin from Chicken Feather Using Design-Expert. 3 Biotech 2017, 7, 127. [Google Scholar] [CrossRef]
- Sinkiewicz, I.; Śliwińska, A.; Staroszczyk, H.; Kołodziejska, I. Alternative Methods of Preparation of Soluble Keratin from Chicken Feathers. Waste Biomass Valorization 2017, 8, 1043–1048. [Google Scholar] [CrossRef]
- Alahyaribeik, S.; Ullah, A. Methods of Keratin Extraction from Poultry Feathers and Their Effects on Antioxidant Activity of Extracted Keratin. Int. J. Biol. Macromol. 2020, 148, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, W.; Zhang, S. A Multifunctional Eco-Friendly Fertilizer Used Keratin-Based Superabsorbent as Coatings for Slow-Release Urea and Remediation of Contaminated Soil. Prog. Org. Coat. J. 2021, 154, 106158. [Google Scholar] [CrossRef]
- Khumalo, M.; Sithole, B.; Tesfaye, T. Valorisation of Waste Chicken Feathers: Optimisation of Keratin Extraction from Waste Chicken Feathers by Sodium Bisulphite, Sodium Dodecyl Sulphate and Urea. J. Environ. Manag. 2020, 262, 110329. [Google Scholar] [CrossRef]
- Polesca, C.; Passos, H.; Neves, B.M.; Coutinho, J.A.; Freire, M.G. Valorization of Chicken Feathers Using Aqueous Solutions of Ionic Liquids. Green Chem. 2023, 25, 1424–1434. [Google Scholar] [CrossRef]
- He, J.; Xu, D.; Li, J.; Li, L.; Li, W. Highly Efficient Extraction of Large Molecular-Weight Keratin from Wool in a Water/Ethanol Co-Solvent. Text. Res. J. 2020, 90, 1084–1093. [Google Scholar] [CrossRef]
- Shavandi, A.; Silva, T.H.; Bekhit, A.A.; Bekhit, A.E.D.A. Keratin: Dissolution, Extraction and Biomedical Application. Biomater. Sci. 2017, 5, 1699–1735. [Google Scholar] [CrossRef]
- Woodin, A.M. The Free Amino-Groups of Soluble Feather Keratin. Nature 1955, 176, 1117. [Google Scholar] [CrossRef]
- Shavandi, A.; Carne, A.; Bekhit, A.A.; Bekhit, A.E.D.A. An Improved Method for Solubilisation of Wool Keratin Using Peracetic Acid. J. Environ. Chem. Eng. 2017, 5, 1977–1984. [Google Scholar] [CrossRef]
- Pakkaner, E.; Yalç, D.; Uysal, B.; Top, A. Self-Assembly Behavior of the Keratose Proteins Extracted from Oxidized Ovis Aries Wool Fibers. Int. J. Biol. Macromol. 2019, 125, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, C.R.; Timmons, S.F.; Smith, R.A. Keratin-based hydrogel for biomedical applications and method of production. U.S. Patent 5932552, 3 August 1999. [Google Scholar]
- Fernández-d’Arlas, B. Improved Aqueous Solubility and Stability of Wool and Feather Proteins by Reactive-Extraction with H2O2 as Bisulfide (–S–S–) Splitting Agent. Eur. Polym. J. 2018, 103, 187–197. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, J.H.; Kang, Y.; Yoo, J.W.; Choi, J.; Lee, H.J. Green Chemistry Method for Hair Strengthening and Setting Using Visible Light-Mediated Protein Crosslinking. J. Clean. Prod. 2022, 363, 132535. [Google Scholar] [CrossRef]
- Thonpho, A.; Srihanam, P. Preparation and Characterization of Keratin Blended Films Using Biopolymers for Drug Controlled Release Application. Orient. J. Chem. 2016, 32, 1739–1748. [Google Scholar] [CrossRef]
- Aranberri, I.; Montes, S.; Azcune, I.; Rekondo, A.; Grande, H.J. Flexible Biocomposites with Enhanced Interfacial Compatibility Based on Keratin Fibers and Sulfur-Containing Poly(Urea-Urethane)s. Polymers 2018, 10, 1056. [Google Scholar] [CrossRef]
- Fernández-d’Arlas, B. Tough and Functional Cross-Linked Bioplastics from Sheep Wool Keratin. Sci. Rep. 2019, 9, 14810. [Google Scholar] [CrossRef]
- Maghsoodloo, S.; Ozdemir, G.; Victoria, J.; Chen-Hsiu, H. Strengths and Limitations of Taguchi ’ s Contributions to Quality, Manufacturing, and Process Engineering. J. Manuf. Syst. 2004, 23, 73–126. [Google Scholar] [CrossRef]
- Priyadarshi, D.; Karar, K. Optimisation of Biodiesel Production Using Taguchi Model. Waste Biomass Valorization 2017, 10, 1547–1559. [Google Scholar] [CrossRef]
- John, I.; Pola, J.; Thanabalan, M.; Appusamy, A. Bioethanol Production from Musambi Peel by Acid Catalyzed Steam Pretreatment and Enzymatic Saccharification: Optimization of Delignification Using Taguchi Design. Waste Biomass Valorization 2019, 11, 2631–2643. [Google Scholar] [CrossRef]
- Pădurețu, C.; Isopescu, R.D.; Gîjiu, C.L.; Rău, I.; Apetroaei, M.R.; Schröder, V. Optimization of Chitin Extraction Procedure from Shrimp Waste Using Taguchi Method and Chitosan Characterization. Mol. Cryst. Liq. Cryst. 2020, 695, 19–28. [Google Scholar] [CrossRef]
- Lymperopoulou, T.; Georgiou, P.; Tsakanika, L. Optimizing Conditions for Scandium Extraction from Bauxite Residue Using Taguchi Methodology. Minerals 2019, 9, 236. [Google Scholar] [CrossRef]
- Gao, J.; Yang, H.; Gao, J.; Yang, H.; Huang, X.; Hung, S.; Cai, W.; Jia, C. Enabling Direct H2O2 Production in Acidic Media through Rational Design of Transition Metal Single Atom Catalyst. Chem 2020, 6, 658–674. [Google Scholar] [CrossRef]
- Yamauchi, K.; Yamauchi, A.; Kusunoki, T.; Kohda, A.; Konishi, Y. Preparation of Stable Aqueous Solution of Keratins, and Physiochemical and Biodegradational Properties of Films. J. Biomed. Mater. Res. 1996, 31, 439–444. [Google Scholar] [CrossRef]
- Filomeni, G.; Society, D.C.; Desideri, E.; Ciriolo, M.R. Under the ROS: Thiol Network Is the Principal Suspect for Autophagy Commitment. Autophagy 2010, 6, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Dan, J.; Li, J.; Du, J.; Xiao, J.; Xu, J. Experimental Study on the Cutting Force during Laser-Assisted Machining of Fused Silica Based on the Taguchi Method and Response Surface Methodology. J. Manuf. Process. 2019, 38, 9–20. [Google Scholar] [CrossRef]

| Symbol | Process Parameters | Units | Levels | ||
|---|---|---|---|---|---|
| 1 | 2 | 3 | |||
| A | H2O2 Concentration | mol/L | 0.5 | 1 | 2 |
| B | pH | - | 8 | 10 | 12 |
| C | Temperature | °C | 55 | 65 | 75 |
| D | Time | h | 1 | 1.5 | 2 |
| Exp. Runs | Controllable Process Parameters | Average Experimental Results | S/N Ratios of Results | |||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | Solubilization (%) | Regeneration (%) | Solubilization | Regeneration | |
| 1 | 0.5 | 8 | 55 | 1 | 11.0900 | 5.1200 | 20.8082 | 14.0921 |
| 2 | 0.5 | 10 | 65 | 1.5 | 43.8800 | 11.0867 | 32.7885 | 20.8169 |
| 3 | 0.5 | 12 | 75 | 2 | 70.0867 | 17.8567 | 36.8928 | 25.0033 |
| 4 | 1 | 8 | 65 | 2 | 23.6033 | 8.2500 | 27.4482 | 18.2726 |
| 5 | 1 | 10 | 75 | 1 | 65.5633 | 20.5000 | 36.3149 | 26.1931 |
| 6 | 1 | 12 | 55 | 1.5 | 80.0933 | 29.9367 | 38.0473 | 29.5096 |
| 7 | 2 | 8 | 75 | 1.5 | 50.8300 | 6.5867 | 34.0797 | 16.2654 |
| 8 | 2 | 10 | 55 | 2 | 83.0933 | 16.2167 | 38.3874 | 24.1534 |
| 9 | 2 | 12 | 65 | 1 | 89.9000 | 21.1167 | 39.0692 | 26.4623 |
| Process Parameters | Mean S/N Ration | ||||
|---|---|---|---|---|---|
| Level 1 | Level 2 | Level 3 | Max–Min | Rank | |
| A | 30.16 | 33.94 | 37.18 | 7.02 | 2 |
| B | 27.45 | 35.83 | 38 | 10.56 | 1 |
| C | 32.41 | 33.1 | 35.76 | 3.35 | 3 |
| D | 32.06 | 34.97 | 34.24 | 2.91 | 4 |
| Process Parameters | Mean S/N Ration | ||||
|---|---|---|---|---|---|
| Level 1 | Level 2 | Level 3 | Max–Min | Rank | |
| A | 19.97 | 24.66 | 22.29 | 4.69 | 2 |
| B | 16.21 | 23.72 | 26.99 | 10.78 | 1 |
| C | 22.59 | 21.85 | 22.49 | 0.73 | 3 |
| D | 22.25 | 22.2 | 22.48 | 0.28 | 4 |
| Source | Degree of Freedom | Sum of Squares | Mean Squares | % Contribution | F-Value | p-Value |
|---|---|---|---|---|---|---|
| Regression | 4 | 5628.06 | 1407.01 | 94.248681 | 16.39 | 0.01 |
| A | 1 | 1604.5 | 1604.5 | 26.869296 | 18.69 | 0.012 |
| B | 1 | 3981.29 | 3981.29 | 66.671523 | 46.37 | 0.002 |
| C | 1 | 24.82 | 24.82 | 0.415641 | 0.29 | 0.619 |
| D | 1 | 17.44 | 17.44 | 0.2920539 | 0.20 | 0.676 |
| Error | 4 | 343.44 | 85.86 | 5.7513188 | ||
| Total | 8 | 5971.5 | 100 |
| Source | Degree of Freedom | Sum of Squares | Mean Squares | % Contribution | F-Value | p-Value |
|---|---|---|---|---|---|---|
| Regression | 4 | 414.156 | 103.539 | 78.23504743 | 3.59 | 0.12 |
| A | 1 | 4.826 | 4.826 | 0.911642808 | 0.17 | 0.70 |
| B | 1 | 399.405 | 399.405 | 75.44854866 | 13.87 | 0.02 |
| C | 1 | 6.678 | 6.678 | 1.261489986 | 0.23 | 0.66 |
| D | 1 | 3.246 | 3.246 | 0.613177073 | 0.11 | 0.75 |
| Error | 4 | 115.218 | 28.805 | 21.76495257 | ||
| Total | 8 | 529.374 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belhajja, M.; Cherkaoui, O.; Bougrin, K. Process Optimization of Keratin Extraction from Chicken Feathers Using Alkaline Oxidation: A Taguchi L9 Orthogonal Array Study. Chem. Proc. 2025, 18, 27. https://doi.org/10.3390/ecsoc-29-26914
Belhajja M, Cherkaoui O, Bougrin K. Process Optimization of Keratin Extraction from Chicken Feathers Using Alkaline Oxidation: A Taguchi L9 Orthogonal Array Study. Chemistry Proceedings. 2025; 18(1):27. https://doi.org/10.3390/ecsoc-29-26914
Chicago/Turabian StyleBelhajja, Mohamed, Omar Cherkaoui, and Khalid Bougrin. 2025. "Process Optimization of Keratin Extraction from Chicken Feathers Using Alkaline Oxidation: A Taguchi L9 Orthogonal Array Study" Chemistry Proceedings 18, no. 1: 27. https://doi.org/10.3390/ecsoc-29-26914
APA StyleBelhajja, M., Cherkaoui, O., & Bougrin, K. (2025). Process Optimization of Keratin Extraction from Chicken Feathers Using Alkaline Oxidation: A Taguchi L9 Orthogonal Array Study. Chemistry Proceedings, 18(1), 27. https://doi.org/10.3390/ecsoc-29-26914






