Sustainable Conjugated Polymer Synthesis in OPV: A Case Study from Conventional to Flow and Microwave-Assisted Synthesis †
Abstract
1. Introduction
2. Materials and Methods
2.1. General Information for Synthesis
2.2. Structural and Molecular Characterizations
2.3. Photophysical Characterizations
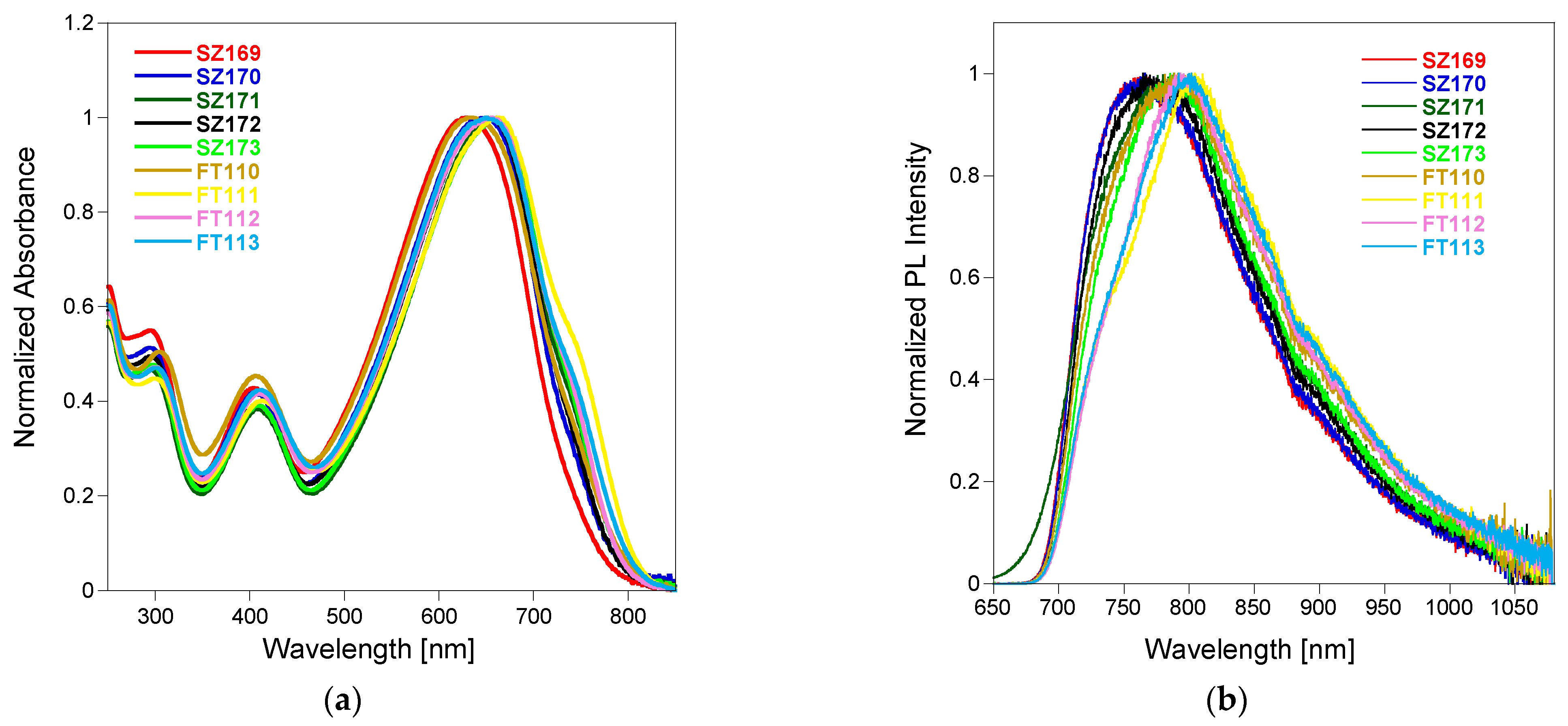
3. Results and Discussion
4. Conclusions and Outlooks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, J.; Park, S.A.; Ryu, S.U.; Chung, D.; Park, T.; Son, S.Y. Green-Solvent-Processable Organic Semiconductors and Future Directions for Advanced Organic Electronics. J. Mater. Chem. A Mater. 2020, 8, 21455–21473. [Google Scholar] [CrossRef]
- Ravikumar, K.; Dangate, M.S. Transparent Photovoltaics and Environmental Impact. In Smart Grids as Cyber Physical Systems: Artificial Intelligence, Cybersecurity, and Clean Energy for Next Generation Smart Grids; Wiley: Hoboken, NJ, USA, 2024; pp. 247–274. ISBN 9781394261727. [Google Scholar]
- Zappia, S.; Veronese, L.; Forni, A.; Dattilo, S.; Samperi, F.; Dagar, J.; Brown, T.M.; Panigati, M.; Destri, S. Carbazole-Pyridazine Copolymers and Their Rhenium Complexes: Effect of the Molecular Structure on the Electronic Properties. Eur. Polym. J. 2022, 168, 111095. [Google Scholar] [CrossRef]
- Ma, B.; Shi, Q.; Ma, X.; Li, Y.; Chen, H.; Wen, K.; Zhao, R.; Zhang, F.; Lin, Y.; Wang, Z.; et al. Defect-Free Alternating Conjugated Polymers Enabled by Room- Temperature Stille Polymerization. Angew. Chem. Int. Ed. 2022, 61, e202115969. [Google Scholar] [CrossRef]
- Lombeck, F.; Komber, H.; Fazzi, D.; Nava, D.; Kuhlmann, J.; Stegerer, D.; Strassel, K.; Brandt, J.; de Zerio Mendaza, A.D.; Müller, C.; et al. On the Effect of Prevalent Carbazole Homocoupling Defects on the Photovoltaic Performance of PCDTBT:PC71BM Solar Cells. Adv. Energy Mater. 2016, 6, 1601232. [Google Scholar] [CrossRef]
- Vangerven, T.; Verstappen, P.; Drijkoningen, J.; Dierckx, W.; Himmelberger, S.; Salleo, A.; Vanderzande, D.; Maes, W.; Manca, J.V. Molar Mass versus Polymer Solar Cell Performance: Highlighting the Role of Homocouplings. Chem. Mater. 2015, 27, 3726–3732. [Google Scholar] [CrossRef]
- Vangerven, T.; Verstappen, P.; Patil, N.; D’Haen, J.; Cardinaletti, I.; Benduhn, J.; Van Den Brande, N.; Defour, M.; Lemaur, V.; Beljonne, D.; et al. Elucidating Batch-to-Batch Variation Caused by Homocoupled Side Products in Solution-Processable Organic Solar Cells. Chem. Mater. 2016, 28, 9088–9098. [Google Scholar] [CrossRef]
- Kabir, E. Application of Microwave Heating in Polymer Synthesis: A Review. Results Chem. 2023, 6, 101178. [Google Scholar] [CrossRef]
- Newman, S.G.; Jensen, K.F. The Role of Flow in Green Chemistry and Engineering. Green Chem. 2013, 15, 1456–1472. [Google Scholar] [CrossRef]
- Pirotte, G.; Kesters, J.; Verstappen, P.; Govaerts, S.; Manca, J.; Lutsen, L.; Vanderzande, D.; Maes, W. Continuous Flow Polymer Synthesis toward Reproducible Large-Scale Production for Efficient Bulk Heterojunction Organic Solar Cells. ChemSusChem 2015, 8, 3228–3233. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.; Ko, W.; Jin, S.H.; Earmme, T.; Hwang, Y.J. Reproducible and Rapid Synthesis of a Conjugated Polymer by Stille Polycondensation in Flow: Effects of Reaction Parameters on Molecular Weight. Chem. Eng. J. 2021, 412, 128572. [Google Scholar] [CrossRef]
- Gobalasingham, N.S.; Carlé, J.E.; Krebs, F.C.; Thompson, B.C.; Bundgaard, E.; Helgesen, M. Conjugated Polymers Via Direct Arylation Polymerization in Continuous Flow: Minimizing the Cost and Batch-to-Batch Variations for High-Throughput Energy Conversion. Macromol. Rapid Commun. 2017, 38, 1700526. [Google Scholar] [CrossRef]
- Grenier, F.; Aïch, B.R.; Lai, Y.Y.; Guérette, M.; Holmes, A.B.; Tao, Y.; Wong, W.W.H.; Leclerc, M. Electroactive and Photoactive Poly[Isoindigo-Alt-EDOT] Synthesized Using Direct (Hetero)Arylation Polymerization in Batch and in Continuous Flow. Chem. Mater. 2015, 27, 2137–2143. [Google Scholar] [CrossRef]
- Ebner, C.; Bodner, T.; Stelzer, F.; Wiesbrock, F. One Decade of Microwave-Assisted Polymerizations: Quo Vadis? Macromol. Rapid Commun. 2011, 32, 254–288. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, P.; Wang, Z.; Ma, Y. Exploration of Structure and Mechanism of Insoluble Gels Formed in Microwave-Assisted Suzuki Coupling for Poly(9,9-Dihexylfluorene)s. Sci. China Chem. 2012, 55, 844–849. [Google Scholar] [CrossRef]
- Nicho, M.E.; García-Escobar, C.H.; Hernández-Martínez, D.; Linzaga-Elizalde, I.; Cadenas-Pliego, G. Microwave-Assisted Synthesis of Poly(3-Hexylthiophene) via Direct Oxidation with FeCl3. Mater. Sci. Eng. B 2012, 177, 1441–1445. [Google Scholar] [CrossRef]
- Heeney, M.; Bailey, C.; Genevicius, K.; Shkunov, M.; Sparrowe, D.; Tierney, S.; McCulloch, I. Stable Polythiophene Semiconductors Incorporating Thieno[2,3-6]Thiophene. J. Am. Chem. Soc. 2005, 127, 1078–1079. [Google Scholar] [CrossRef]
- Tierney, S.; Heeney, M.; McCulloch, L. Microwave-Assisted Synthesis of Polythiophenes via the Stille Coupling. Synth. Met. 2005, 148, 195–198. [Google Scholar] [CrossRef]
- Nehls, B.S.; Asawapirom, U.; Füldner, S.; Preis, E.; Farrell, T.; Scherf, U. Semiconducting Polymers via Microwave-Assisted Suzuki and Stille Cross-Coupling Reactions. Adv. Funct. Mater. 2004, 14, 352–356. [Google Scholar] [CrossRef]
- Gasparini, N.; Jiao, X.; Heumueller, T.; Baran, D.; Matt, G.J.; Fladischer, S.; Spiecker, E.; Ade, H.; Brabec, C.J.; Ameri, T. Designing Ternary Blend Bulk Heterojunction Solar Cells with Reduced Carrier Recombination and a Fill Factor of 77%. Nat. Energy 2016, 1, 16118. [Google Scholar] [CrossRef]
- Giovanella, U.; Betti, P.; Bolognesi, A.; Destri, S.; Melucci, M.; Pasini, M.; Porzio, W.; Botta, C. Core-Type Polyfluorene-Based Copolymers for Low-Cost Light-Emitting Technologies. Org. Electron. 2010, 11, 2012–2018. [Google Scholar] [CrossRef]
- Collins, B.A.; Li, Z.; McNeill, C.R.; Ade, H. Fullerene-Dependent Miscibility in the Silole-Containing Copolymer PSBTBT-08. Macromolecules 2011, 44, 9747–9751. [Google Scholar] [CrossRef]
- Awada, H.; Bousquet, A.; Dagron-Lartigau, C.; Billon, L. Surface-Initiated Polymerization of A-A/B-B Type Conjugated Monomers by Palladium-Catalyzed Stille Polycondensation: Towards Low Band Gap Polymer Brushes. RSC Adv. 2015, 5, 78436–78440. [Google Scholar] [CrossRef]
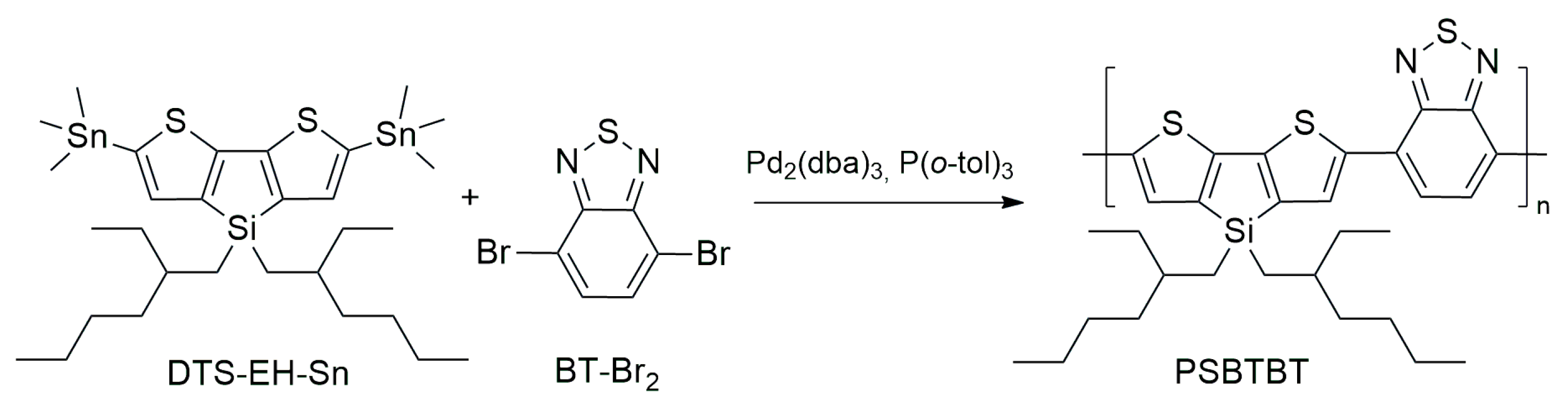
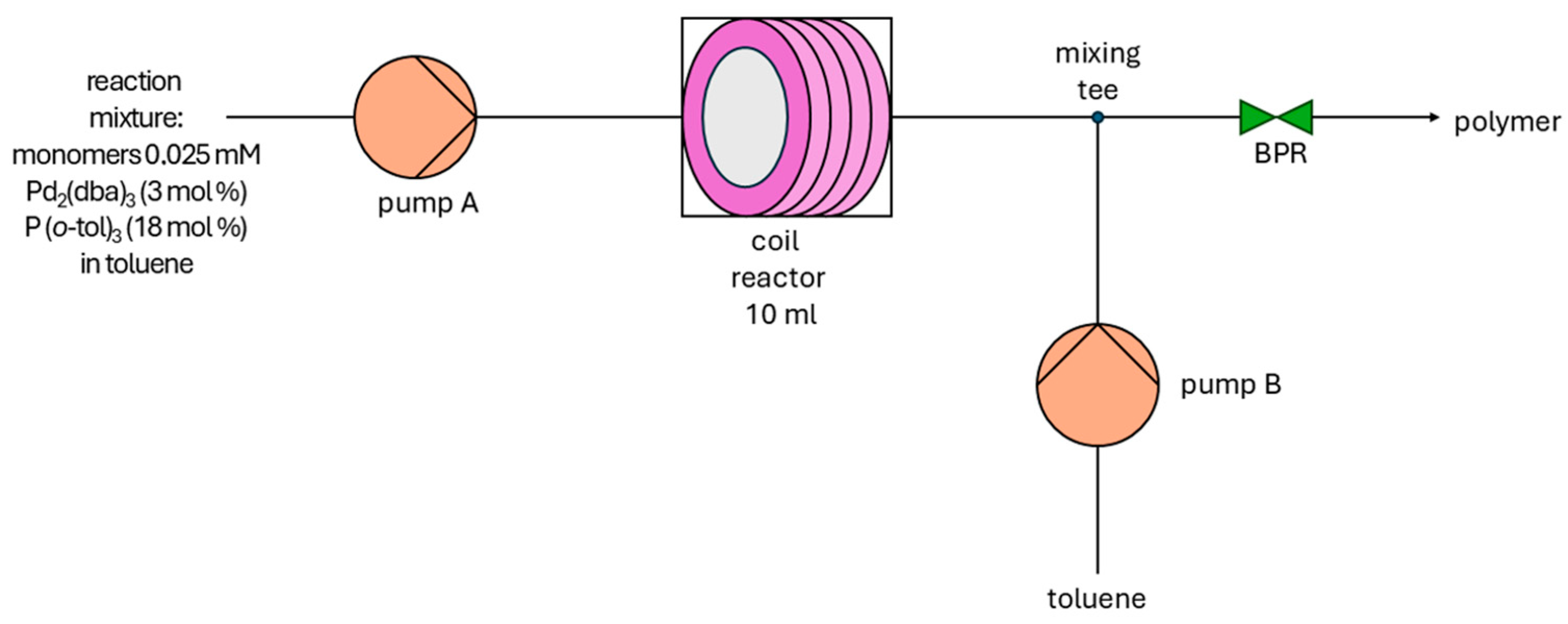
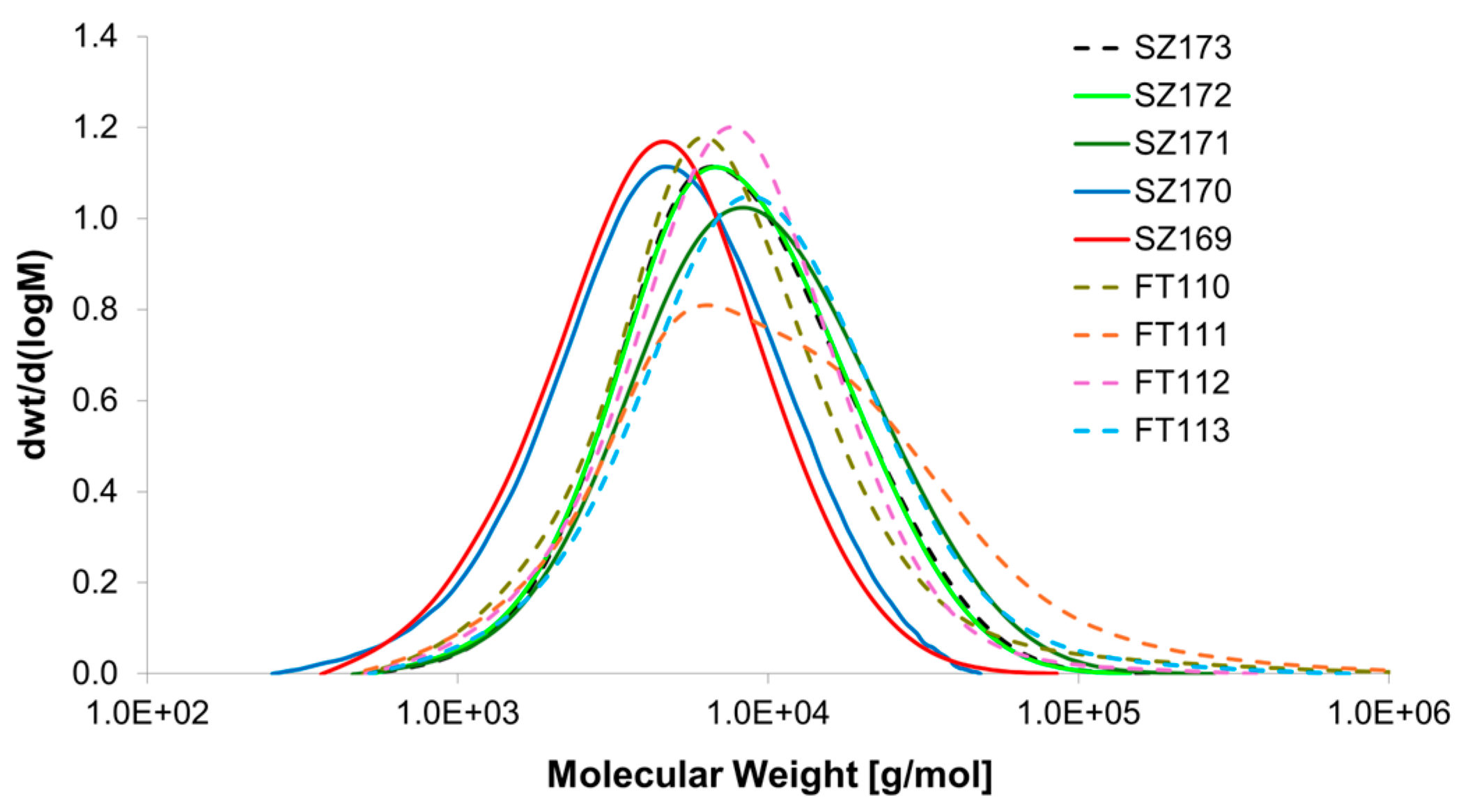
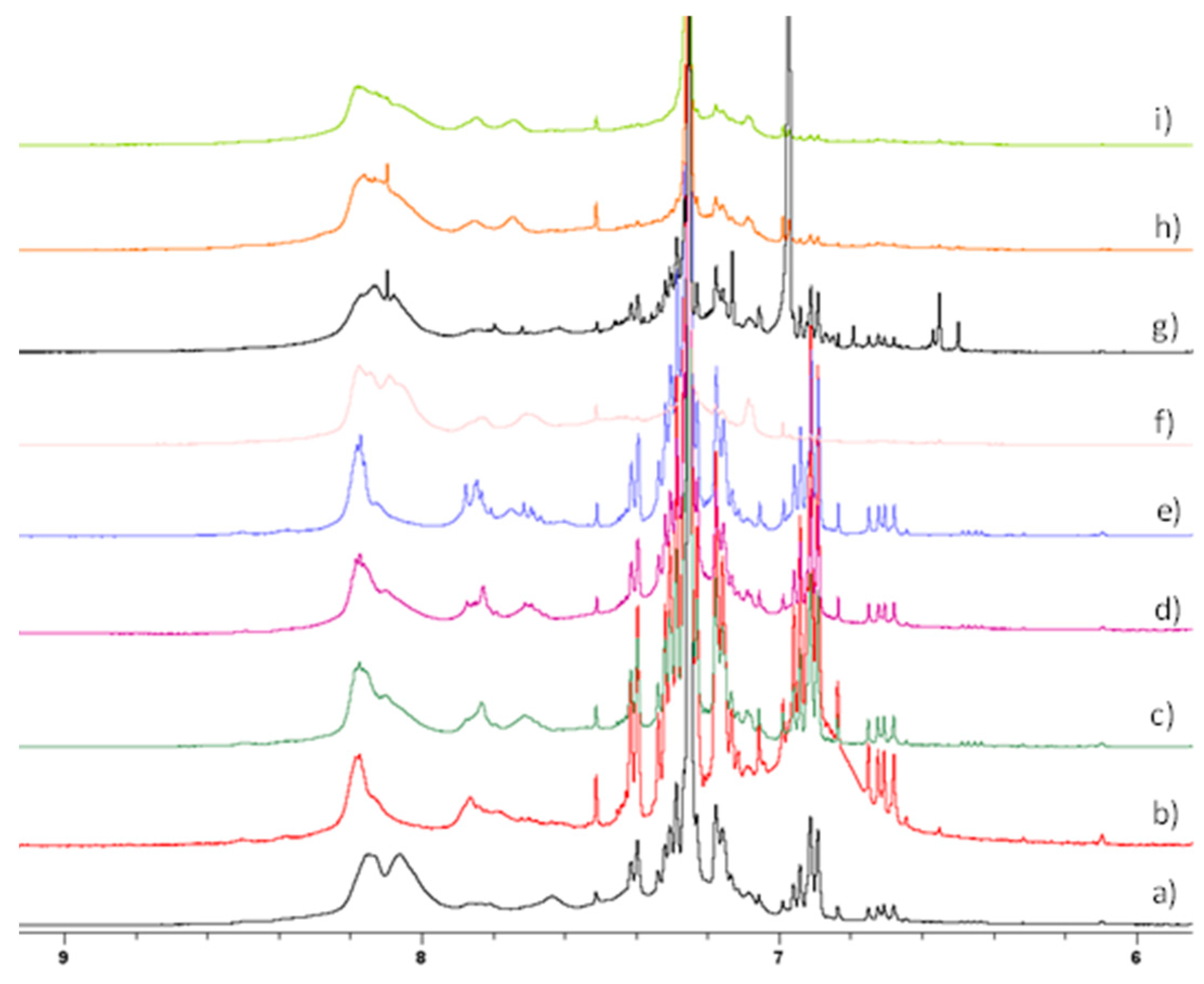
| Item | Method | Solvent | T [°C] | P [Bar] | Y [%] | Mn [g/mol] | Mw [g/mol] | Mw/Mn |
|---|---|---|---|---|---|---|---|---|
| SZ173 | Batch a | Toluene | 110 | 1 | 31 | 5656 | 11,119 | 1.97 |
| SZ169 | Flow b | Toluene | 120 | 4 | 32 | 3156 | 6040 | 1.91 |
| SZ170 | Flow b | Toluene | 120 | 6 | 37 | 3253 | 6485 | 1.99 |
| SZ171 | flow b | Toluene | 140 | 4 | 27 | 6153 | 13,415 | 2.18 |
| SZ172 | flow b | Toluene | 140 | 6 | 42 | 5544 | 10,849 | 1.96 |
| FT110 | MWA b | Toluene | 140 | 1.7 | 32 | 4952 | 15,668 | 3.16 |
| FT111 | MWA b | DMF | 160 | 1.2 | 54 | 5894 | 26,743 | 4.54 |
| FT112 | MWA b | DMF/toluene = 1:2 | 160 | 1.7 | 49 | 5367 | 11,072 | 2.06 |
| FT113 | MWA b | DMF/toluene = 1:10 | 160 | 2.3 | 55 | 6241 | 16,044 | 2.57 |
| Polymer | λabsmax | λemmax * |
|---|---|---|
| SZ169 | 625 | 760 |
| SZ170 | 643 | 760 |
| SZ171 | 658 | 775 |
| SZ172 | 653 | 760 |
| SZ173 | 660 | 755 |
| FT110 | 634 | 775 |
| FT111 | 660 | 800 |
| FT112 | 652 | 795 |
| FT113 | 652 | 795 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villafiorita-Monteleone, F.; Squeo, B.M.; Turco, F.; Boccia, A.C.; Giacometti Schieroni, A.; Piovani, D.; Pasini, M.; Zappia, S. Sustainable Conjugated Polymer Synthesis in OPV: A Case Study from Conventional to Flow and Microwave-Assisted Synthesis. Chem. Proc. 2025, 18, 24. https://doi.org/10.3390/ecsoc-29-26719
Villafiorita-Monteleone F, Squeo BM, Turco F, Boccia AC, Giacometti Schieroni A, Piovani D, Pasini M, Zappia S. Sustainable Conjugated Polymer Synthesis in OPV: A Case Study from Conventional to Flow and Microwave-Assisted Synthesis. Chemistry Proceedings. 2025; 18(1):24. https://doi.org/10.3390/ecsoc-29-26719
Chicago/Turabian StyleVillafiorita-Monteleone, Francesca, Benedetta Maria Squeo, Federico Turco, Antonella Caterina Boccia, Alberto Giacometti Schieroni, Daniele Piovani, Mariacecilia Pasini, and Stefania Zappia. 2025. "Sustainable Conjugated Polymer Synthesis in OPV: A Case Study from Conventional to Flow and Microwave-Assisted Synthesis" Chemistry Proceedings 18, no. 1: 24. https://doi.org/10.3390/ecsoc-29-26719
APA StyleVillafiorita-Monteleone, F., Squeo, B. M., Turco, F., Boccia, A. C., Giacometti Schieroni, A., Piovani, D., Pasini, M., & Zappia, S. (2025). Sustainable Conjugated Polymer Synthesis in OPV: A Case Study from Conventional to Flow and Microwave-Assisted Synthesis. Chemistry Proceedings, 18(1), 24. https://doi.org/10.3390/ecsoc-29-26719










