Abstract
Sulfonyl derivatives are very important compounds as they can be found in sulfones and sulfonamides, two classes of compounds with prominent biological and pharmacological activities. This study explores a copper-catalyzed cascade heterocyclization/sulfonylation reaction for the controlled preparation of sulfonyl oxazinones. Surprisingly, in this work we have isolated a great variety of vinyl sulfones with high selectivity instead of the expected cyclization. These sulfones are obtained by the reaction between N-Boc-allenes and aromatic sodium sulfinates. These results emphasize the reactivity of allenes toward the formation of bis(γ-amino-functionalized vinyl sulfones) in the presence of copper salts under radical conditions.
1. Introduction
Allenamides are a subgroup of allenes that show interesting reactivity because the free electron pair of nitrogen can be delocalized with the amide group, reducing its donating ability towards the allenic center and giving it greater stability compared to other subgroups such as allenamines. Allenyl carbamates is one type of allenamides where the Boc group is used as a protecting group and can be removed under mild conditions. These allenamides are interesting in organic synthesis since they can be used as starting materials in cyclization reactions catalyzed by transition metals and cycloaddition reactions, among others [1].
Sulfonated compounds are of great importance in Organic Chemistry since they exhibit a wide range of biological activities, including antimicrobial, antiviral, antidiabetic, and anticancer properties [2,3].
Vinylsulfones and gamma-amino vinylsulfones (Figure 1) are compounds known for their varied pharmacological activity, such as being reversible inhibitors of many types of cysteine proteases, inhibiting other types of enzymes such as HIV-1 integrase and antibacterial agents, among others [4,5].
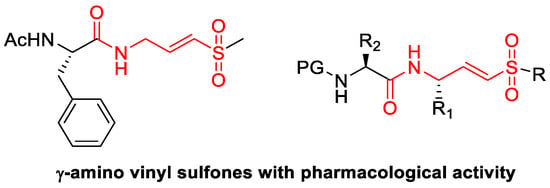
Figure 1.
Importance of sulfonated compounds.
Despite their high biological importance, they also play an important role in Organic Synthesis, acting as efficient Michael acceptors and 2π donors in cycloaddition reactions. Our research group has reported a gold(I)-catalyzed cyclization of allenic carbamates to obtain 1,3-oxazinan-2-ones and the synthesis of sulfonated heterocycles from α-allenols by an oxycyclization/sulfonylation reaction [6]. The use of copper salts in organic synthesis is desirable because of low toxicity and low cost. In this context, we decided to study a copper-catalyzed cascade heterocyclization/sulfonylation for the controlled preparation of sulfonyl compounds [7].
2. Results and Discussion
According to our working plan, the preparation of allenyl carbamates 5 was carried out following a procedure described in the literature (Scheme 1) [8]. Thus, alkynyl carbamates 1a–b were prepared through reductive amination of the appropriate aromatic aldehyde and propargylamine in the presence of anhydrous magnesium sulfate, which acts as a dehydrating agent. The treatment of the corresponding N-substituted prop-2-yn-1-amines with Boc2O afforded the desired compounds with good yields. Finally, the terminal alkynes 4 were conveniently converted into allenic carbamates 5 by treatment with paraformaldehyde in the presence of diisopropylamine and copper(I) bromide (Crabbé homologation reaction).
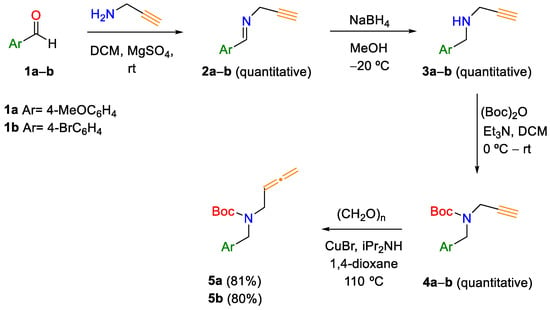
Scheme 1.
Synthesis of allenic carbamates 5.
Next, when starting materials were synthesized, the study of the cyclization/sulfonylation cascade reaction for obtaining sulfonated oxazinones was developed. The allenyl carbamate 5a was used as model substrate. The reaction conditions, previously optimized by the research group for cyclization reactions with incorporation of the arylsulfonyl group, were tested (2 equivalents of sodium sulfinate 6a, 2 equivalents of silver nitrate as the oxidizing agent, and copper(II) acetate as the catalyst) [9]. The solvent used was acetonitrile, and the reaction was achieved in a sealed tube at 100 °C (Scheme 2). However, after purifying the product and obtaining its 1H-NMR spectrum, it did not correspond to the sulfonated oxazinone 7.
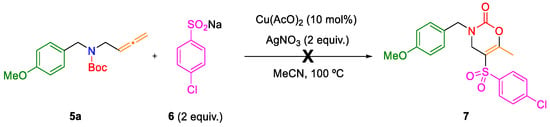
Scheme 2.
Oxycyclization/sulfonylation sequence of allene 5a with benzenesulfinate 6.
It is important to note that the 1H-NMR spectrum at 25 °C showed more aromatic signals. However, due to overlapping resonance signals, the integration values did not correspond to the heterocyclization/sulfonylation reaction. For this reason, NMR experiments were conducted at different temperatures with the aim to have a good resolution of peaks that were indistinguishable at room temperature (Figure 2). This structure was studied through variable-temperature 1H NMR (300 MHz), including 1D and 2D experiments, in C2D2Cl4. The higher resolution of the 1H NMR spectrum at 65 °C compared to 25 °C is clearly visible. The multiplet (6.61 ppm, signal orange, A) well-resolved signal at 65 °C (t, J = 6.3 Hz) indicates the presence of a double bond. This triplet is expected because the proton couples with two protons, N-CH2-CH (3.83 ppm, signal green, C) (d, J = 6.3 Hz). Finally, the singlet resonance (4.32 ppm, signal blue, B) can be assigned to benzylic protons.

Figure 2.
1H NMR (300 MHz) spectrum of 8a in C2D2Cl4. at 25 °C and 65 °C.
This experiment revealed a sulfonylation/homodimerization sequence of the allenyl carbamate 5a and the exact mass spectrum confirmed this dimeric compound.
Although the desired compound 7 was not generated, the product obtained has an attractive pharmacological and biological potential as γ-amino vinylsulfone.
The scope of the copper-catalyzed sulfonylation/homodimerization of allenyl carbamates was explored. Allenyl carbamates 5a–c with electron-donating (MeO), moderately electron-withdrawing (Br) or electron-withdrawing (CO2Me) substituents at para position of the phenyl ring and sulfinate 6 provided the required homodimeric γ-amino vinyl sulfones 8 in fair yields (Scheme 3).
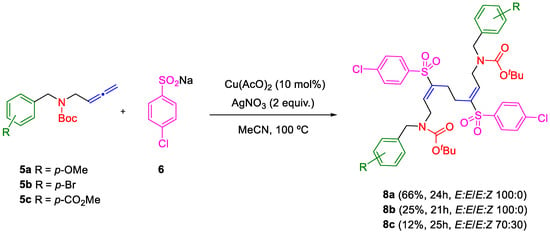
Scheme 3.
Sulfonylation/homodimerization of allenyl carbamates 5a–c.
The variation in yields was notable for the different products. The highest yield was obtained for product 8a as single (E,E)-isomer (66% yield). Nevertheless, when the ring of the allenyl carbamate 5c is substituted with an electron-withdrawing group, the dimer 8c was obtained as a mixture of (E,E) and (E,Z) isomers. These results show a regioselective synthesis (sulfonylation in the central carbon atom of the allene) and stereoselective synthesis (exclusive or majority formation of the E:E isomer) of a variety of bis(γ-amino vinylsulfones) that incorporate different substituents in the aromatic ring with different stereoelectronic effects.
3. Experimental Section
3.1. General Information, Intrumentation and Chemicals
General methods: 1H NMR and 13C NMR spectra were recorded on a Bruker Avance-300 spectrometer. NMR spectra were recorded in (CCl2D)2 solutions, (CCl2D)2 (1H, 5.91 ppm; 13C, 74.2 ppm). Low- and high-resolution mass spectra were taken on an AGILENT 6520 Accurate-Mass QTOF LC/MS spectrometer using the electrospray mode (ES) unless otherwise stated. IR spectra were recorded on a Bruker Tensor 27 spectrometer. All commercially available compounds were used without further purification.
3.2. General Procedure
To a solution of the appropriate allenyl carbamates 5a–c (1.0 mmol) in acetonitrile (10 mL) was added Cu(OAc)2 (10 mol %), the corresponding sodium sulfinate 6 (2.0 mmol) and AgNO3 (2.0 mmol). The reaction was stirred at 100 °C in a sealed tube until completion, which was monitored by TLC. The mixture was cooled to room temperature and was diluted with ethyl acetate (3 × 5 mL). The organic extract was washed with brine (3 × 3 mL), dried over anhydrous MgSO4 and concentrated under reduced pressure. Chromatography of the residue using hexanes/ethyl acetate mixtures gave analytically pure compounds. Spectroscopic and analytical data for pure forms of compounds 8 follow.
3.3. Spectral Data
Bis(γ-amino vinyl sulfone) 8a. From 25 mg (0.10 mmol) of allene 5a, and after chromatography of the residue using hexanes/ethyl acetate (4:1) as eluent gave compound 6ba (31 mg, 66%) as a colorless solid; mp 147.1–148.6 °C; 1H NMR (300 MHz, (CCl2D)2, 65 °C): δ = 7.56 (d, 4H, J = 8.7 Hz, ArH), 7.39 (d, 4H, J = 8.7 Hz, ArH), 7.06 (d, 4H, J = 8.7 Hz, ArH), 6.80 (d, 4H, J = 8.7 Hz, ArH), 6.61 (t, 2H, J = 6.3 Hz, CH=), 4.32 (s, 4H, N-CH2), 3.83 (d, 4H, J = 6.3 Hz, N-CH2-CH), 3.74 (s, 6H, O-CH3), 2.21 (s, 4H, CH2-CH2), 1.39 (s, 18H, t-Bu); 13C NMR (75 MHz, (CCl2D)2, 55 °C): δ = 159.4 (2C), 155.4 (2C), 141.0 (2C), 140.5 (2C), 140.2 (2C), 137.6 (2C), 129.9 (Ar, 4CH), 129.8 (Ar, 4CH), 129.7 (2C), 129.2 (Ar, 4CH), 114.6 (Ar, 4CH), 81.0 (2C), 55.7 (2CH3-O), 51.2 (2CH2), 44.8 (2CH2), 28.7 (6CH3), 26.2 (2CH2); IR (AcOEt): ν = 1690 (C=O), 1611, 1247 cm−1; HRMS (ES): calcd for C46H54Cl2N2NaO10S2 [M + Na]+: 951.2489; found: 951.2517.
Bis(γ-amino vinyl sulfone) 8b. From 45 mg (0.13 mmol) of allene 5b, and after chromatography of the residue using hexanes/ethyl acetate [6:1]⟶[2:1] as eluent gave compound 6ca (17 mg, 25%) as a white solid; mp 181.1–182.5 °C; 1H NMR (300 MHz, (CCl2D)2, 65 °C): δ = 7.58 (d, 4H, J = 8.3 Hz, ArH), 7.41 (m, 8H, ArH), 7.02 (d, 4H, J = 8.1 Hz, ArH), 6.63 (t, 2H, J = 6.3 Hz, CH=), 4.33 (s, 4H, N-CH2), 3.87 (d, 4H, J = 6.4 Hz, N-CH2-CH), 2.26 (s, 4H, CH2-CH2), 1.38 (s, 18H, t-Bu); 13C NMR (75 MHz, (CCl2D)2, 65 °C): δ = 155.3 (2C), 140.9 (2C), 140.6 (2C), 140.4 (2C), 137.7 (2C), 137.0 (2C), 132.2 (Ar, 4CH), 129.9 (Ar, 4CH), 129.7 (Ar, 4CH), 129.5 (Ar, 4CH), 121.7 (2C), 81.3 (2C), 51.3 (2CH2), 45.3 (2CH2), 28.6 (6CH3), 26.3 (2CH2); IR (AcOEt): ν = 1690 (C=O), 1475, 1146 cm−1; HRMS (ES): calcd C44H48Br2Cl2N2NaO8S2 [M + Na]+: 1047.0488; found: 1047.0498.
Bis(γ-amino vinyl sulfone) 8c. From 49.6 mg (0.16 mmol) of allene 5c, and after chromatography of the residue using hexanes/ethyl acetate [2:1] as eluent gave compound 8c (13.2 mg, 12%) as a yellow oil; 1H NMR (300 MHz, (CCl2D)2, 65 °C): δ = 7.92 (d, 8H, J = 8.3 Hz, ArH, M+m), 7.70–7.44 (m, 8H, ArH, M+m), 7.34–7.09 (m, 16H, ArH, M+m), 6.93 (t, 2H, J = 6.8 Hz, CH=, m), 6.61 (t, 2H, J = 6.3 Hz, CH=, M), 4.47 (m, 8H, N-CH2, M+m), 3.84 (m, 8H, N-CH2-CH, M+m y 12H, O-CH3, M+m), 2.15 (s, 8H, CH2-CH2, M+m), 1.35 (m, 36H, t-Bu, M+m); IR (AcOEt): ν = 1696 (C=O), 1595, 1155 cm−1; HRMS (ES): calcd C48H54Cl2N2O12S2 [M]+: 985.9820. This compounds did not ionize well.
4. Conclusions
In this work the copper-catalyzed cascade heterocyclization/sulfonylation for the controlled preparation of sulfonyl oxazinones from allenyl-carbamates and sodium sulfinates did not take place. Instead, a selective copper-catalyzed synthesis of dimeric vinylsulfones was developed, where the products could present pharmacology activities due to the nature of these compounds.
On the other hand, the electronic nature and the steric hindrance of the substituents of the aromatic ring of the allenyl-carbamates influence the performance and selectivity of the process.
Author Contributions
Conceptualization, P.A. and A.L.; methodology, P.A. and A.L.; validation, P.A. and A.L.; formal analysis, D.H. and I.P.; investigation, D.H. and I.P.; resources, P.A.; data curation, D.H., I.P. and A.L.; writing—original draft preparation, A.L.; writing—review and editing, P.A.; visualization, A.L.; supervision, P.A.; project administration, P.A.; funding acquisition, P.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by MCIN/AEI/10.13039/501100011033/FEDER (Project PID2021-122183NB-C21) and UCM (PR3/23-30806).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Li, X.; Liu, Y.; Ding, N.; Tan, X.; Zhao, Z. Recent progress in transition-metal-free functionalization of allenamides. RSC Adv. 2020, 10, 36818–36827. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Malakar, S. Synthesis of sulfonamide and their synthetic and therapeutic applications: Recent advances. Tetrahedron 2020, 76, 131662. [Google Scholar] [CrossRef]
- Trost, B.M.; Kalnmals, C.A. Sulfones as Chemical Chameleons: Versatile Synthetic Equivalents of Small-Molecule Synthons. Chem. Eur. J. 2019, 25, 11193–11213. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Cunningham, L.; Evans, P. Asymmetric Synthesis of γ-Amino-Functionalised Vinyl Sulfones: De Novo Preparation of Cysteine Protease Inhibitors. Synthesis 2022, 54, 1753–1764. [Google Scholar]
- Ahmadi, R.; Emami, S. Recent applications of vinyl sulfone motif in drug design and discovery. Eur. J. Med. Chem. 2022, 234, 114255. [Google Scholar] [CrossRef] [PubMed]
- Meadows, D.C.; Gervay-Hague, J. Vinyl sulfones: Synthetic preparations and medicinal chemistry applications. Med. Res. Rev. 2006, 26, 793–814. [Google Scholar] [CrossRef] [PubMed]
- Esteban, P.; Herrera, F.; San Martín, D.; Luna, A.; Almendros, P. Regioselectivity Switch Based on the Stoichiometry: Stereoselective Synthesis of Trisubstituted Vinyl Epoxides by Cu-Catalyzed 3-exo-trig Cyclization of α-Allenols. Adv. Synth. Catal. 2022, 364, 3289–3294. [Google Scholar] [CrossRef]
- Alcaide, B.; Almendros, P.; Quirós, M.T.; Fernández, I. Gold-catalyzed oxycyclization of allenic carbamates: Expeditious synthesis of 1,3-oxazin-2-ones. Beilstein J. Org. Chem. 2013, 9, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Herrera, F.; Esteban, P.; Luna, A.; Almendros, P. Metal-Catalyzed Reactivity Reversal in the Sulfonylation Reaction of α-Allenols: Controlled Synthesis of 4-(Arylsulfonyl)-2,5-Dihydrofurans. Adv. Synth. Catal. 2021, 363, 3952–3956. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).