Halogenated Cinnamanilides and Their Activity Against Selected Gram-Negative Bacteria †
Abstract
1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. Chemistry
3.2. In Vitro Antibacterial Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO Antimicrobial Resistance. Available online: https://www.who.int/health-topics/antimicrobial-resistance (accessed on 3 September 2025).
- Bertagnolio, S.; Dobreva, Z.; Centner, C.M.; Olaru, I.D.; Dona, D.; Burzo, S.; Huttner, B.D.; Chaillon, A.; Gebreselassie, N.; Wi, T.; et al. WHO global research priorities for antimicrobial resistance in human health. Lancet Microbe. 2024, 5, 100902. [Google Scholar] [CrossRef]
- Sati, H.; Carrara, E.; Savoldi, A.; Hansen, P.; Garlasco, J.; Campagnaro, E.; Boccia, S.; Castillo-Polo, J.A.; Magrini, E.; Garcia-Vello, P.; et al. The WHO Bacterial Priority Pathogens List 2024: A prioritisation study to guide research, development, and public health strategies against antimicrobial resistance. Lancet Infect. Dis. 2025, 25, 1033–1043. [Google Scholar] [CrossRef]
- GBD 2021 Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef]
- Gigante, V.; Alm, R.A.; Melchiorri, D.; Rocke, T.; Arias, C.A.; Czaplewski, L.; Fernandes, P.; Franceschi, F.; Harbarth, S.; Kozlov, R.; et al. Multi-year analysis of the global preclinical antibacterial pipeline: Trends and gaps. Antimicrob. Agents Chemother. 2024, 68, e0053524. [Google Scholar] [CrossRef]
- WHO Global Action Plan on Antimicrobial Resistence. Available online: https://iris.who.int/bitstream/handle/10665/193736/9789241509763_eng.pdf?sequence=1 (accessed on 3 September 2025).
- WHO Prioritization of Pathogens to Guide Discovery, Research and Development of New Antibiotics for Drug-Resistant Bacterial Infections, Including Tuberculosis. Available online: https://www.who.int/publications/i/item/WHO-EMP-IAU-2017.12 (accessed on 3 September 2025).
- 2023 Antibacterial Agents in Clinical and Preclinical Development: An Overview and Analysis. Available online: https://www.who.int/publications/i/item/9789240094000 (accessed on 3 September 2025).
- Gajic, I.; Tomic, N.; Lukovic, B.; Jovicevic, M.; Kekic, D.; Petrovic, M.; Jankovic, M.; Trudic, A.; Mitic Culafic, D.; Milenkovic, M.; et al. A comprehensive overview of antibacterial agents for combating multidrug-resistant bacteria: The current landscape, development, future opportunities, and challenges. Antibiotics 2025, 14, 221. [Google Scholar] [CrossRef]
- WHO Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance. Available online: https://www.who.int/publications/i/item/9789240093461 (accessed on 3 September 2025).
- Widmer, A.F. Emerging antibiotic resistance: Why we need new antibiotics! Swiss. Med. Wkly. 2022, 152, 40032. [Google Scholar] [CrossRef]
- Muteeb, G.; Rehman, M.T.; Shahwan, M.; Aatif, M. Origin of antibiotics and antibiotic resistance, and their impacts on drug development: A narrative review. Pharmaceuticals 2023, 16, 1615. [Google Scholar] [CrossRef]
- Ho, C.S.; Wong, C.T.H.; Aung, T.T.; Lakshminarayanan, R.; Mehta, J.S.; Rauz, S.; McNally, A.; Kintses, B.; Peacock, S.J.; de la Fuente-Nunez, C.; et al. Antimicrobial resistance: A concise update. Lancet Microbe. 2025, 6, 100947. [Google Scholar] [CrossRef]
- Blaskovich, M.A.T.; Cooper, M.A. Antibiotics re-booted-time to kick back against drug resistance. NPJ Antimicrob. Resist. 2025, 3, 47. [Google Scholar] [CrossRef]
- Gargate, N.; Laws, M.; Rahman, K.M. Current economic and regulatory challenges in developing antibiotics for Gram-negative bacteria. NPJ Antimicrob. Resist. 2025, 3, 50. [Google Scholar] [CrossRef]
- Jampilek, J. Drug repurposing to overcome microbial resistance. Drug Discov. Today 2022, 27, 2028–2041. [Google Scholar] [CrossRef]
- Choudhary, M.I.; Romling, U.; Nadeem, F.; Bilal, H.M.; Zafar, M.; Jahan, H.; Ur-Rahman, A. Innovative strategies to overcome antimicrobial resistance and tolerance. Microorganisms 2023, 11, 16. [Google Scholar] [CrossRef]
- Jacobowski, A.C.; Boleti, A.P.A.; Cruz, M.V.; Santos, K.F.D.P.; de Andrade, L.R.M.; Frihling, B.E.F.; Migliolo, L.; Paiva, P.M.G.; Teodoro, P.E.; Teodoro, L.P.R.; et al. Combating antimicrobial resistance: Innovative strategies using peptides, nanotechnology, phages, quorum sensing interference, and CRISPR-Cas systems. Pharmaceuticals 2025, 18, 1119. [Google Scholar] [CrossRef]
- Gagare, S.; Patil, P.; Jain, A. Natural product-inspired strategies towards the discovery of novel bioactive molecules. Futur. J. Pharm. Sci. 2024, 10, 55. [Google Scholar] [CrossRef]
- Bharate, S.B.; Lindsley, C.W. Natural products driven medicinal chemistry. J. Med. Chem. 2024, 67, 20723–20730. [Google Scholar] [CrossRef]
- Woo, S.; Marquez, L.; Crandall, W.J.; Risener, C.J.; Quave, C.L. Recent advances in the discovery of plant-derived antimicrobial natural products to combat antimicrobial resistant pathogens: Insights from 2018–2022. Nat. Prod. Rep. 2023, 40, 1271–1290. [Google Scholar] [CrossRef]
- Feng, J.; Zheng, Y.; Ma, W.; Ihsan, A.; Hao, H.; Cheng, G.; Wang, X. Multitarget antibacterial drugs: An effective strategy to combat bacterial resistance. Pharmacol. Ther. 2023, 252, 108550. [Google Scholar] [CrossRef]
- Yan, H.; Xin, Z.; Sang, Z.; Li, X.; Xie, J.; Wu, J.; Pang, S.; Wen, Y.; Wang, W. A rational multi-target combination strategy for synergistic improvement of non-ribosomal peptide production. Nat. Commun. 2025, 16, 1883. [Google Scholar] [CrossRef]
- Maharramov, E.; Czikkely, M.S.; Szili, P.; Farkas, Z.; Grezal, G.; Daruka, L.; Kurko, E.; Meszaros, L.; Daraba, A.; Kovacs, T.; et al. Exploring the principles behind antibiotics with limited resistance. Nat. Commun. 2025, 16, 1842. [Google Scholar] [CrossRef]
- Gaikwad, N.; Nanduri, S.; Madhavi, Y.V. Cinnamamide: An insight into the pharmacological advances and structure-activity relationships. Eur. J. Med. Chem. 2019, 181, 111561. [Google Scholar] [CrossRef]
- Ruwizhi, N.; Aderibigbe, B.A. Cinnamic acid derivatives and their biological efficacy. Int. J. Mol. Sci. 2020, 21, 5712. [Google Scholar] [CrossRef]
- Tian, Y.; Jiang, X.; Guo, J.; Lu, H.; Xie, J.; Zhang, F.; Yao, C.; Hao, E. Pharmacological potential of cinnamic acid and derivatives: A comprehensive review. Pharmaceuticals 2025, 18, 1141. [Google Scholar] [CrossRef]
- Pospisilova, S.; Kos, J.; Michnova, H.; Kapustikova, I.; Strharsky, T.; Oravec, M.; Moricz, A.M.; Bakonyi, J.; Kauerova, T.; Kollar, P.; et al. Synthesis and spectrum of biological activities of novel N-arylcinnamamides. Int. J. Mol. Sci. 2018, 19, 2318. [Google Scholar] [CrossRef] [PubMed]
- Kos, J.; Bak, A.; Kozik, V.; Jankech, T.; Strharsky, T.; Swietlicka, A.; Michnova, H.; Hosek, J.; Smolinski, A.; Oravec, M.; et al. Biological activities and ADMET-related properties of novel set of cinnamanilides. Molecules 2020, 25, 4121. [Google Scholar] [CrossRef] [PubMed]
- Strharsky, T.; Pindjakova, D.; Kos, J.; Vrablova, L.; Michnova, H.; Hosek, J.; Strakova, N.; Lelakova, V.; Leva, L.; Kavanova, L.; et al. Study of biological activities and ADMET-related properties of novel chlorinated N-arylcinnamamides. Int. J. Mol. Sci. 2022, 23, 3159. [Google Scholar] [CrossRef] [PubMed]
- Strharsky, T.; Pindjakova, D.; Kos, J.; Vrablova, L.; Smak, P.; Michnova, H.; Gonec, T.; Hosek, J.; Oravec, M.; Jendrzejewska, I.; et al. Trifluoromethylcinnamanilide Michael acceptors for treatment of resistant bacterial infections. Int. J. Mol. Sci. 2022, 23, 15090. [Google Scholar] [CrossRef]
- Kos, J.; Strharsky, T.; Tosso, R.; Gutierrez, L.; Kos, D.; Jurica, J.; Zendulka, O.; Pes, O.; Gregorova, J.; Degotte, G.; et al. Trifluoromethylcinnamanilides—Effective dual inhibitors of Mycobacterium smegmatis and Plasmodium falciparum. Bioorg. Chem. 2025, 154, 107957. [Google Scholar] [CrossRef]
- Kos, J.; Degotte, G.; Pindjakova, D.; Strharsky, T.; Jankech, T.; Gonec, T.; Francotte, P.; Frederich, M.; Jampilek, J. Insights into antimalarial activity of N-phenyl-substituted cinnamanilides. Molecules 2022, 27, 7799. [Google Scholar] [CrossRef]
- Bak, A.; Kos, J.; Degotte, G.; Swietlicka, A.; Strharsky, T.; Pindjakova, D.; Gonec, T.; Smolinski, A.; Francotte, P.; Frederich, M.; et al. Towards arginase inhibition: Hybrid SAR protocol for property mapping of chlorinated N-arylcinnamamides. Int. J. Mol. Sci. 2023, 24, 3611. [Google Scholar] [CrossRef]
- American Type Culture Collection (ATCC). Available online: https://www.atcc.org/ (accessed on 5 September 2025).
- Dolejska, M.; Villa, L.; Dobiasova, H.; Fortini, D.; Feudi, C.; Carattoli, A. Plasmid content of a clinically relevant Klebsiella pneumoniae clone from the Czech Republic producing CTX-M-15 and QnrB1. Antimicrob. Agents Chemother. 2013, 57, 1073–1076. [Google Scholar] [CrossRef]
- Annuur, R.M.; Triana, D.; Ernawati, T.; Murai, Y.; Aswad, M.; Hashimoto, M.; Tachrim, Z.P. A Review of cinnamic acid’s skeleton modification: Features for antibacterial-agent-guided derivatives. Molecules 2024, 29, 3929. [Google Scholar] [CrossRef]
- Guzman, J.D. Natural cinnamic acids, synthetic derivatives and hybrids with antimicrobial activity. Molecules 2014, 19, 19292–19349. [Google Scholar] [CrossRef]
- Mingoia, M.; Conte, C.; Di Rienzo, A.; Dimmito, M.P.; Marinucci, L.; Magi, G.; Turkez, H.; Cufaro, M.C.; Del Boccio, P.; Di Stefano, A.; et al. Synthesis and biological evaluation of novel cinnamic acid-based antimicrobials. Pharmaceuticals 2022, 15, 228. [Google Scholar] [CrossRef]
- Leitao, M.M.; Goncalves, A.S.C.; Sousa, S.F.; Borges, F.; Simoes, M.; Borges, A. Two cinnamic acid derivatives as inhibitors of Pseudomonas aeruginosa las and pqs quorum-sensing systems: Impact on biofilm formation and virulence factors. Biomed. Pharmacother. 2025, 187, 118090. [Google Scholar] [CrossRef]
- Rajkumari, J.; Borkotoky, S.; Murali, A.; Suchiang, K.; Mohanty, S.K.; Busi, S. Cinnamic acid attenuates quorum sensing associated virulence factors and biofilm formation in Pseudomonas aeruginosa PAO1. Biotechnol. Lett. 2018, 40, 1087–1100. [Google Scholar] [CrossRef]
- Pan, D.; Wu, H.; Li, J.J.; Wang, B.; Jia, A.Q. Two cinnamoyl hydroxamates as potential quorum sensing inhibitors against Pseudomonas aeruginosa. Front. Cell. Infect. Microbiol. 2024, 14, 1424038. [Google Scholar] [CrossRef] [PubMed]
- Maher, C.; Hassan, K.A. The Gram-negative permeability barrier: Tipping the balance of the in and the out. mBio 2023, 14, e0120523. [Google Scholar] [CrossRef] [PubMed]
- Gaurav, A.; Bakht, P.; Saini, M.; Pandey, S.; Pathania, R. Role of bacterial efflux pumps in antibiotic resistance, virulence, and strategies to discover novel efflux pump inhibitors. Microbiology 2023, 169, 001333. [Google Scholar] [CrossRef] [PubMed]
- Macedo, N.S.; de Sousa Silveira, Z.; Dantas, D.M.; dos Santos Barbosa, C.R.; de Morais Oliveira-Tintino, C.D.; Tintino, S.R.; Goncalves Alencar, G.; Siqueira, G.M.; da Silva, T.F.; Machado Marinho, M.; et al. Evaluation of the antibacterial and efflux inhibitory activity of trans-cinnamic acid in Staphylococcus aureus: Experimental assays and in silico modeling. Phytomedicine Plus 2025, 5, 100816. [Google Scholar] [CrossRef]
- Tetard, A.; Zedet, A.; Girard, C.; Plesiat, P.; Llanes, C. Cinnamaldehyde induces expression of efflux pumps and multidrug resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2019, 63, e01081-19. [Google Scholar] [CrossRef]
- National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 11th ed.; M07; NCCLS: Wayne, PA, USA, 2018. [Google Scholar]
- Schwalbe, R.; Steele-Moore, L.; Goodwin, A.C. Antimicrobial Susceptibility Testing Protocols; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Markuliak, M.; Kos, D.; Vojackova, V.; Horvath, B.; Svajdlenka, E.; Leskovska, J.; Pisarcik, M.; Krystof, V.; Cizek, A.; Jampilek, J.; et al. Preparation and biological activity of isoperrottetin A and its phosphonium salts derivatives. Eur. J. Pharm. Sci. 2025, 212, 107167. [Google Scholar] [CrossRef]
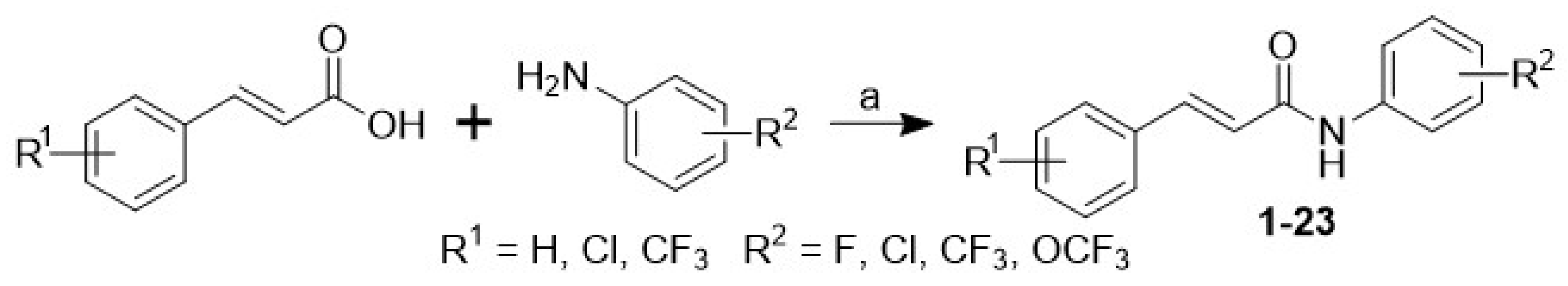
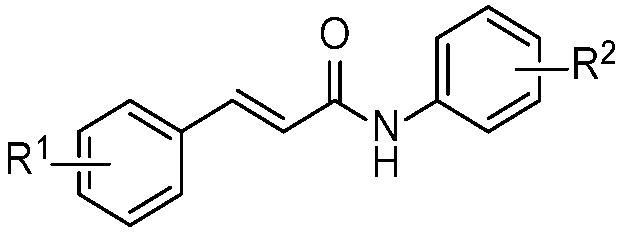 | |||||
|---|---|---|---|---|---|
| No. | R1 | R2 | MIC [µg/mL] | ||
| E. coli ATCC 25922 | P. aeruginosa ATCC 27859 | K. pneumoniae 797 | |||
| 1 | H | 3-CF3 | >256 | >256 | >256 |
| 2 | H | 3,4-Cl | >256 | >256 | >256 |
| 3 | H | 3,5-Cl | >256 | >256 | >256 |
| 4 | H | 3,5-CF3 | >256 | >256 | >256 |
| 5 | H | 3-F-4-CF3 | >256 | >256 | >256 |
| 6 | 4-Cl | 3-CF3 | >256 | >256 | >256 |
| 7 | 4-Cl | 4-CF3 | >256 | >256 | >256 |
| 8 | 4-Cl | 3,5-Cl | >256 | >256 | >256 |
| 9 | 4-Cl | 3,5-CF3 | >256 | >256 | >256 |
| 10 | 3,4-Cl | 3,5-CF3 | >256 | >256 | >256 |
| 11 | 2-CF3 | 3-CF3 | >256 | >256 | >256 |
| 12 | 2-CF3 | 4-CF3 | >256 | >256 | >256 |
| 13 | 2-CF3 | 3,5-Cl | >256 | >256 | >256 |
| 14 | 2-CF3 | 3,5-CF3 | >256 | >256 | >256 |
| 15 | 2-CF3 | 4-OCF3 | >256 | >256 | >256 |
| 16 | 3-CF3 | 3-CF3 | >256 | >256 | >256 |
| 17 | 3-CF3 | 4-CF3 | >256 | >256 | >256 |
| 18 | 3-CF3 | 3,5-Cl | >256 | >256 | >256 |
| 19 | 3-CF3 | 3,5-CF3 | >256 | >256 | >256 |
| 20 | 4-CF3 | 3-CF3 | >256 | >256 | >256 |
| 21 | 4-CF3 | 4-CF3 | >256 | >256 | >256 |
| 22 | 4-CF3 | 3,5-Cl | >256 | >256 | >256 |
| 23 | 4-CF3 | 3,5-CF3 | >256 | >256 | >256 |
| ciprofloxacin | 0.125 | 0.125 | 1.00 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simurdova, M.; Strharsky, T.; Kos, J.; Gonec, T.; Cizek, A.; Jampilek, J. Halogenated Cinnamanilides and Their Activity Against Selected Gram-Negative Bacteria. Chem. Proc. 2025, 18, 22. https://doi.org/10.3390/ecsoc-29-26718
Simurdova M, Strharsky T, Kos J, Gonec T, Cizek A, Jampilek J. Halogenated Cinnamanilides and Their Activity Against Selected Gram-Negative Bacteria. Chemistry Proceedings. 2025; 18(1):22. https://doi.org/10.3390/ecsoc-29-26718
Chicago/Turabian StyleSimurdova, Michaela, Tomas Strharsky, Jiri Kos, Tomas Gonec, Alois Cizek, and Josef Jampilek. 2025. "Halogenated Cinnamanilides and Their Activity Against Selected Gram-Negative Bacteria" Chemistry Proceedings 18, no. 1: 22. https://doi.org/10.3390/ecsoc-29-26718
APA StyleSimurdova, M., Strharsky, T., Kos, J., Gonec, T., Cizek, A., & Jampilek, J. (2025). Halogenated Cinnamanilides and Their Activity Against Selected Gram-Negative Bacteria. Chemistry Proceedings, 18(1), 22. https://doi.org/10.3390/ecsoc-29-26718







