Abstract
Fatty esters have wide industrial importance due to their aroma and versatile applications. Enzymatic esterification emerges as a sustainable method, offering high selectivity, fewer byproducts, and mild conditions, which align with the principles of green chemistry. This study focused on synthesizing phenylethyl esters from licuri oil (Syagrus coronata), 2-phenylethanol, and Pseudomonas lipase. The oil composition was characterized by GC-MS, while GC-FID and FTIR confirmed the conversion of the esters formed. The disappearance of the hydroxyl band (3327 cm−1) indicated alcohol consumption, and the carbonyl shift (1743 to 1736 cm−1) revealed new esters. Additional signals at 1240–1150 cm−1 and 1607 cm−1 confirmed the presence of ester groups and the aromatic ring, demonstrating the viability of this enzymatic route.
1. Introduction
Fatty esters represent one of the most important classes of organic compounds due to their industrial applicability, their unique properties, and characteristic aroma. Therefore, the development of methodologies for ester synthesis has gained momentum. In this context, one of the routes for obtaining esters is the reaction between a carboxylic acid and an alcohol, with the elimination of water and in the presence of a catalyst, called esterification [1,2,3,4,5].
In this scenario, biotechnological processes emerge as an excellent alternative for the fatty acid esterification process due to their high catalytic efficiency, mild operating conditions, selectivity, and the synthesis of products classified as natural. Thus, enzyme-catalyzed esterification reactions emerge, which have numerous applications [6,7].
Enzyme-catalyzed esterification offers other advantages, in addition to those already mentioned, such as lower energy consumption, selectivity, specificity, stability under adverse conditions, and reduced waste generation. Among the enzymes capable of performing this reaction, lipases, belonging to the serine hydrolase family, stand out. These enzymes act on the ester bonds of numerous organic compounds. This class of enzymes offers further advantages, such as mild process conditions, short reaction time, catalyst reuse, and the ability to perform the reaction in solvent-free systems [6,7,8,9,10].
In the esterification process, selecting a source rich in fatty acids is essential. Vegetable oils, being natural, renewable, available, and low-cost, represent sustainable alternatives for the synthesis of esters with applications in the chemical, pharmaceutical, food, and biofuel industries [11]. In this context, Syagrus coronata (licuri) stands out, a palm tree of the Arecaceae family, widely distributed in the Brazilian semiarid region, especially in the dry areas of the Caatinga biome. Its fruits have high oil and protein contents, conferring socioeconomic relevance to local communities and potential for applications in various industrial and cultural sectors. Furthermore, because it is rich in fatty acids, this species constitutes a promising source of raw material for the synthesis of esters [12,13,14,15,16].
When choosing a substrate for esterification reactions, it is essential to consider compounds with biological, pharmacological, and industrial potential to generate products with promising applications in different sectors. In this context, 2-phenylethanol stands out because it exhibits antimicrobial and antibacterial activity, as reported in the literature. Furthermore, its characteristic floral rose aroma lends it great interest for use in perfumery, cosmetics, and food, significantly expanding its industrial and biotechnological value [17,18].
Thus, the present work aims at the enzymatic synthesis of phenylethyl esters, through the esterification reaction between licuri oil and 2-phenylethanol, catalyzed by Pseudomonas lipase in a solvent-free system.
2. Materials and Methods
2.1. Chemical Composition of Licuri Fixed Oil
The first step consisted of determining the chemical composition of licuri fixed oil, following the method described by Lima et al. (2013) [19]. This method involves the following steps:
Transesterification: 150 µL of licuri oil was esterified with 2 mL of 0.5 M NaOH in methanol and heated in a water bath at 100 °C for 5 min. Then, 2 mL of 12% BF3 in methanol (1.3 M) was added under an inert atmosphere and refluxed for 30 min. The mixture was cooled, transferred to a separatory funnel with 20 mL of heptane, shaken vigorously for 1 min, and allowed to separate. The organic phase was collected, dried over anhydrous sodium sulfate, and filtered. The resulting fraction was purified by column chromatography using a gradient of eluents (heptane, heptane 1:1 dichloromethane, and dichloromethane; 25/30/15 mL). Finally, the volume of each fraction was reduced under a nitrogen stream at room temperature.
Chromatographic Analysis: Quantitative analysis of fatty acid methyl esters (FAMEs) was performed by gas chromatography–mass spectrometry (GC-MS). Samples (1 µL) were injected directly into the column using hydrogen as the carrier gas at 1 mL/min (splitless). The oven temperature was held at 40 °C for 1 min, then ramped to 150 °C at 55 °C/min and to 220 °C at 1.7 °C/min for 15 min. FAMEs were identified by retention times and coinjection with Mix C4–C24 standards (Sigma-Aldrich).
2.2. Enzymatic Synthesis of Phenethyl Esters
For the enzymatic synthesis, 100 µL of licuri fixed oil, 300 µL of 2-phenylethanol, and 50 mg of Pseudomonas lipase (Sigma-Aldrich, St. Louis, MI, USA) were added to a 5 mL vial and stirred at 50 °C for 24 h. After the reaction, 2 mL of hexane was added, and the enzyme-free supernatant was transferred to a clean vial for purification. Fatty acid esters were purified by column chromatography using an ethyl acetate/hexane gradient (1:4), monitored by thin-layer chromatography (TLC). The isolated esters were analyzed by GC-FID and FTIR to confirm purity. Finally, the aroma of the obtained esters was evaluated organoleptically.
3. Results
3.1. Chemical Composition of the Fixed Oil of Syagrus Coronata
Using the previously described methodology, the chemical composition of the oil was identified. After injecting the sample into the GC-MS, the chromatogram of the methyl esters was obtained, as shown in the Figure 1.
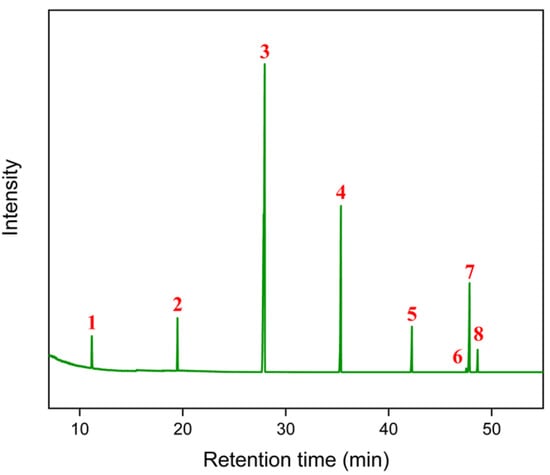
Figure 1.
Chromatogram obtained from methyl esters (FAMEs).
The results obtained allowed, through the comparison of retention times with the Mix C4–C24 standards, the complete identification of the fatty acids present in the fixed licuri oil, as presented in the following table (Table 1).

Table 1.
Fatty acids present in licuri fixed oil.
3.2. Phenylethyl Esters Obtained via Enzymatic Esterification
Before purification, the phenylethyl ester sample was analyzed by GC-FID. The chromatogram (Figure 2) showed peaks corresponding to the esters, along with hexane (solvent) and 2-phenylethanol (substrate). This preliminary analysis is essential for assessing purity and detecting potential residual impurities.
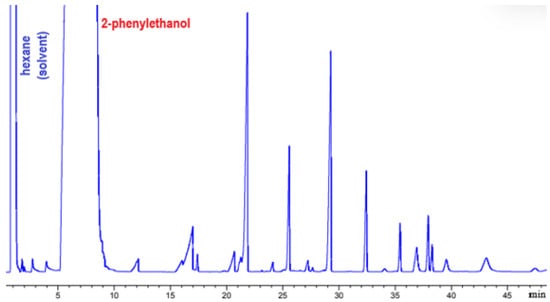
Figure 2.
Sample injected after 24 h in GC-FID.
After purification, GC-FID analysis was repeated, and the chromatogram (Figure 3) confirmed the formation and isolation of the fatty esters from 2-phenylethanol. The peak at the beginning corresponds to hexane, used as a dilution solvent. These results demonstrate the effectiveness of the purification process and ensure accurate subsequent analyses.
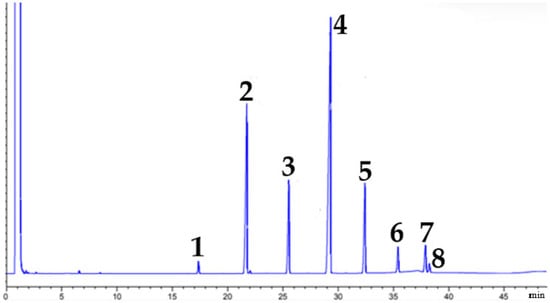
Figure 3.
Separated and purified phenylethyl esters.
Moreover, it was possible to establish a correlation between the peaks and the retention times with the phenylethyl esters formed, as presented in Table 2.

Table 2.
Fatty esters obtained.
The esterification reaction was confirmed by Fourier transform infrared spectroscopy (FTIR), as illustrated in Figure 4. For this analysis, the spectra of 2-phenylethanol, Syagrus coronata oil, and the product (phenylethyl esters) were compared, enabling the identification of the main structural modifications resulting from the reaction.
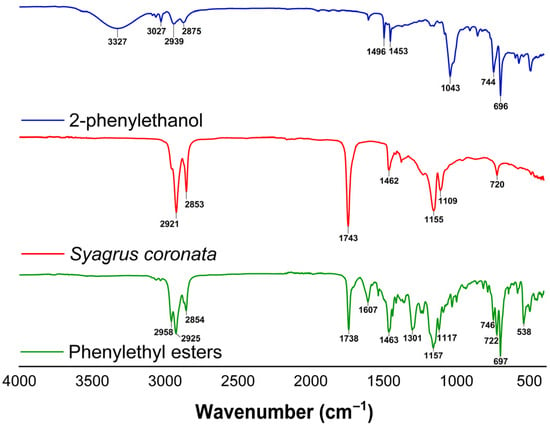
Figure 4.
FTIR spectra of 2-phenylethanol (blue), fixed oil of Syagrus coronata (red), and the obtained phenylethyl esters (green).
4. Discussion
4.1. Chemical Composition Present in Licuri Fixed Oil
As described in the literature, licuri oil is rich in fatty acids, mainly lauric (C12:0) and myristic (C14:0), which correspond to approximately 50% of its composition. While saturated acids caprylic, capric, palmitic, and stearic, and unsaturated acids (linoleic and oleic) make up the remainder of the oil. Saturated fatty acids correspond to approximately 85% of the oil composition, while 15% correspond to unsaturated fatty acids [14,15,19].
Thus, it can be stated that, in addition to the identification of the fatty esters present in the fixed licuri oil based on the standards used, the chemical composition of the oil was consistent with the data previously reported in the literature, demonstrating the conformity of our results with existing studies.
4.2. Chemical Characterization of Phenylethyl Esters
4.2.1. Analysis in GC-FID
GC-FID chromatographic analysis confirmed the effective separation and purification of the ester mixture obtained through enzymatic esterification. Furthermore, as shown in Figure 3 and Table 2, it was possible to identify the different fatty esters formed, with 2-phenylethyl dodecanoate being the most concentrated constituent. This predominance can be attributed to the fact that this ester is derived from the major fatty acid present in licuri oil, lauric acid, thus reflecting the initial composition of the raw material.
Finally, GC-FID analysis confirmed the efficiency of the purification process, since no signals corresponding to residual 2-phenylethanol or remaining fatty acids were detected in the chromatogram, demonstrating that a properly purified material had been obtained.
4.2.2. FTIR Analysis
The spectrum of 2-phenylethanol (blue line) exhibits a broad band at 3327 cm−1, attributed to the axial vibration of the hydroxyl group. The disappearance of this band in the product spectrum (green line) indicates the consumption of alcohol in the esterification reaction. From this perspective, it is noteworthy that no bands corresponding to hydroxyl groups (3200–3600 cm−1) were observed in the FTIR spectrum of Syagrus coronata, indicating the absence of free fatty acids and confirming that they are predominantly present in the form of triacylglycerides.
In the spectrum of Syagrus coronata oil (red line), an intense band is observed at 1743 cm−1, corresponding to the carbonyl of the esters present in the triglycerides. This band is maintained in the spectrum of phenylethyl esters, with a slight shift to 1736 cm−1, suggesting the formation of new esters from the reaction between the oil’s fatty acids and 2-phenylethanol.
Furthermore, bands in the region of 1240–1150 cm−1, associated with the C–O stretching, corroborate the formation of the ester group. The presence of the aromatic ring of 2-phenylethanol in the product is confirmed by the bands at 1607 cm−1 and 746 cm−1, consistent with the characteristic vibrations of the benzene ring (1604 cm−1), indicating its incorporation into the structure of the ester formed.
Furthermore, the fatty esters obtained exhibited a characteristic rose-like aroma, attributed to 2-phenylethanol, which was confirmed by organoleptic analysis. This aspect gives the product broad application potential, considering the importance of aromatized fatty esters in industry.
In summary, the use of the Pseudomonas lipase enzyme as a catalyst proved highly efficient in the synthesis of phenylethyl esters, promoting the esterification of all the fatty acids present in licuri fixed oil. Furthermore, this study represents the first report of the production of phenylethyl esters from the fixed oil of Syagrus coronata, expanding its application potential and reinforcing its socioeconomic and cultural importance for the region of Pernambuco, Brazil.
5. Conclusions
Based on the results obtained, it is concluded that the methodology employed for the enzymatic esterification of phenylethyl fatty acids was effective, allowing not only the characterization of the chemical composition of licuri oil but also the successful production of esters with a floral aroma characteristic of 2-phenylethanol. The process, conducted with Syagrus coronata fixed oil, 2-phenylethanol, and Pseudomonas lipase under the established conditions, proved effective.
Further studies are needed to optimize esterification reaction parameters—temperature, reaction time, and reagent concentrations—to identify the optimal conditions for maximizing yield in Pseudomonas lipase-catalyzed reactions. In parallel, it is recommended to investigate the biological activities of these esters, considering the bioactive potential of licuri oil and 2-phenylethanol, with a view to expanding their industrial and pharmacological applications.
Author Contributions
Conceptualization, D.F.S. and D.d.A.S.; methodology, D.F.S. and D.M.d.A.F.N.; formal analysis, D.F.S.; investigation, D.F.S.; experiment—execution, D.F.S., J.C.R.d.O.F.d.A., D.M.d.A.F.N.; experiment—oil characterization, D.F.S., J.C.R.d.O.F.d.A.; experiment—oil extraction, D.F.S.; data curation, D.F.S.; writing—original draft preparation, D.F.S. and D.d.A.S.; writing—results interpretation and analysis, D.F.S., J.C.R.d.O.F.d.A. and D.M.d.A.F.N.; writing—review and editing, D.F.S. and D.d.A.S.; supervision, D.F.S. and D.d.A.S.; project administration, D.d.A.S.; funding acquisition, D.d.A.S.; final manuscript approval, D.F.S. and D.d.A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financed and supported by PAET-PG (Program of Transversal Strategic Actions of Postgraduate Studies) of the Federal University of Pernambuco (UFPE) With a scholarship provided by CAPES (Coordination for the Improvement of Higher Education Personnel).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request to the corresponding author due to the development of other studies related to this one.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ortega-Requena, S.; Montiel, C.; Máximo, F.; Gómez, M.; Murcia, M.D.; Bastida, J. Esters in the Food and Cosmetic Industries: An Overview of the Reactors Used in Their Biocatalytic Synthesis. Materials 2024, 17, 268. [Google Scholar] [CrossRef] [PubMed]
- Richetti, A. Esterificação Enzimática de Palmitato de 2-Etilexila em Sistema Livre de Solvente. Master’s Thesis, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil, 2009. [Google Scholar]
- Feng, S. Research Progress in the Synthesis of Esters. IOP Conf. Ser. Earth Environ. Sci. 2020, 440, 022019. [Google Scholar] [CrossRef]
- Bassan, I.A.L.; Nascimento, D.R.; Gil, R.A.S.; Silva, M.I.P.; Moreira, C.R.; Gonzalez, W.A.; Faro, A.C.; Onfroy, T.; Lachter, E.R. Esterification of Fatty Acids with Alcohols over Niobium Phosphate. Fuel Process. Technol. 2013, 106, 619–624. [Google Scholar] [CrossRef]
- Voll, F.A.P.; Silva, C.; Rossi, C.C.R.S.; Guirardello, R.; Castilhos, F.; Oliveira, J.V.; Cardozo-Filho, L. Thermodynamic Analysis of Fatty Acid Esterification for Fatty Acid Alkyl Esters Production. Biomass Bioenergy 2011, 35, 781–788. [Google Scholar] [CrossRef]
- Aragão, V.C.; Anschau, A.; Porciuncula, B.D.A.; Thiesen, C.; Kalil, S.J.; Burkert, C.A.V.; Burkert, J.F.M. Síntese Enzimática de Butirato de Isoamila Empregando Lipases Microbianas Comerciais. Quim. Nova 2009, 32, 2268–2272. [Google Scholar] [CrossRef]
- Lortie, R. Enzyme Catalyzed Esterification. Biotechnol. Adv. 1997, 15, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.J. Enzyme Catalysis Advances, Techniques, and Outlooks. Appl. Sci. 2022, 12, 8036. [Google Scholar] [CrossRef]
- Brunschwig, C.; Moussavou, W.; Blin, J. Use of Bioethanol for Biodiesel Production. Prog. Energy Combust. Sci. 2012, 38, 283–301. [Google Scholar] [CrossRef]
- Mukdsi, M.C.A.; Maillard, M.-B.; Medina, R.B.; Thierry, A. Ethyl Butanoate Is Synthesised Both by Alcoholysis and Esterification by Dairy Lactobacilli and Propionibacteria. LWT 2018, 89, 38–43. [Google Scholar] [CrossRef]
- Reda, S.Y.; Carneiro, P.I.B. Oils and Fats: Applications and Implications (Óleos e Gorduras: Aplicações e Implicações). Revista Analytica 2007, 27, 60–67. (In Portuguese) [Google Scholar]
- Guimarães, J.S.; Shiosaki, R.K.; Mendes, M.L.M. Licuri (Syagrus coronata): Características, Importâncias, Potenciais e Perspectivas do Pequeno Coco do Brasil. Desenvolv. Meio Ambiente 2021, 58, 169–192. [Google Scholar] [CrossRef]
- Drumond, M.A. Documentos 199: Licuri Syagrus coronata (Mart.) Becc.; Embrapa Semi-Árido: Petrolina, Brazil, 2007. [Google Scholar]
- Moreira, R.C.; Leonardi, G.R.; Bicas, J.L. Lipase-Mediated Alcoholysis for In Situ Production of Ester Bioaromas in Licuri Oil for Cosmetic Applications. J. Biotechnol. 2024, 392, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Bôas, R.N.V.; Castro, H.F. A Review of Synthesis of Esters with Aromatic, Emulsifying, and Lubricant Properties by Biotransformation Using Lipases. Biotechnol. Bioeng. 2022, 119, 725–742. [Google Scholar] [CrossRef] [PubMed]
- Trevizam, C.J.; Correia, D.; Duarte, V.T.R. Optimization of the Licuri (Syagrus coronata) Oil Extraction Process (Otimização do Processo de Extração do Óleo de Licuri (Syagrus coronata)). Rev. Engenho 2014, 10, 1–19. (In Portuguese) [Google Scholar]
- Rajendran, A.; Palanisamy, A.; Thangavelu, V. Lipase Catalyzed Ester Synthesis for Food Processing Industries. Arch. Biol. Technol. 2009, 52, 207–219. [Google Scholar] [CrossRef]
- Sun, J.; Liu, S.Q. Ester Synthesis in Aqueous Media by Lipase: Alcoholysis, Esterification and Substrate Hydrophobicity. J. Food Biochem. 2015, 39, 12104. [Google Scholar] [CrossRef]
- Lima, L.C.M.; Navarro, D.M.A.F.; Souza-Santos, L.P. Methyl Esters from the Copepod Tisbe biminiensis Assayed by Two Transesterification Methods. Crustaceana 2013, 86, 1343–1353. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).