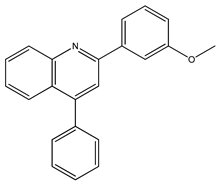
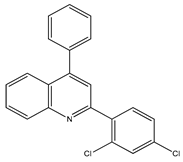
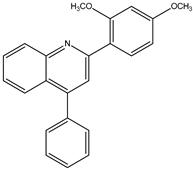
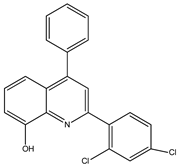
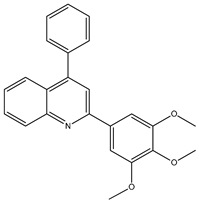
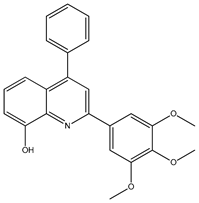
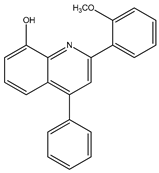
Abstract
Depression is a debilitating neuropsychiatric disorder and a leading cause of disability worldwide, with current therapeutic options often limited by delayed onset of action, inadequate efficacy, and undesirable side effects. The quinoline scaffold, a privileged structure in medicinal chemistry, has been reported to possess a wide spectrum of pharmacological properties, including central nervous system (CNS) modulation. In this study, two novel 2,4-diphenylquinoline derivatives—CMPD1 [2-(4-methoxyphenyl)-4-phenylquinoline] and CMPD2 [2-(2,4-dichlorophenyl)-4-phenylquinoline]—were rationally designed based on structure–activity relationship (SAR) insights and synthesized via the Friedländer condensation of appropriately substituted anilines with carbonyl precursors. Purification was achieved through recrystallization, and structural confirmation was performed using Fourier-transform infrared (FT-IR) spectroscopy, proton nuclear magnetic resonance (NMR), and carbon-13 NMR spectroscopy, confirming the expected chemical shifts and diagnostic signals for quinoline derivatives. The pharmacological activity was evaluated using murine models for antidepressant screening: the Forced Swim Test (FST) and Tail Suspension Test (TST). Both compounds produced statistically significant reductions in immobility time compared to the control group (p < 0.05), with CMPD2 showing slightly enhanced activity. The results suggest that electron-donating and electron-withdrawing substituents influence antidepressant potency, potentially through modulation of CNS receptor binding. These findings validate 2,4-diphenylquinoline derivatives as promising antidepressant leads, meriting further optimization, in vivo pharmacokinetic studies, and mechanistic investigations to establish their clinical translation potential.
1. Introduction
Depression is a severe mental health disorder characterized by poor treatment outcomes and notable adverse effects linked to traditional antidepressant medication. The demand for novel antidepressant agents with improved safety and efficacy profiles has driven attention toward heterocyclic scaffolds, particularly quinoline derivatives. Quinolines exhibit diverse pharmacological properties, including antimicrobial, anticancer, and neuroactive activities, making them attractive frameworks for CNS drug discovery. Previous computational and experimental studies suggest that functionalized quinolines can modulate neurotransmitter pathways such as monoamine oxidase inhibition and serotonin reuptake, thereby contributing to antidepressant effects. This work aimed to synthesize, characterize, and evaluate 2,4-diphenylquinoline derivatives as potential antidepressant agents.
2. Materials and Methods
2.1. Design and Synthesis of 2,4-Diphenylquinoline Derivatives
The compound library was designed to explore the antidepressant potential of diphenyl quinoline derivatives through systematic structural modifications. The primary objective was to investigate how variations in functional groups and substitution patterns influence binding affinities to two critical targets: the serotonin transporter (SERT) and monoamine oxidase A (MAO-A). The aim was to identify compounds with improved binding characteristics, enhanced pharmacokinetic profiles, and reduced toxicity, making them viable candidates for antidepressant drug development.
The library consists of 22 compounds, all featuring a diphenyl quinoline scaffold with variations in substituents on the phenyl rings. Substituents included electron-donating groups (e.g., methoxy) and electron-withdrawing groups (e.g., chlorine) to evaluate their effects on activity. The quinoline core was retained for its established bioactivity in modulating central nervous system receptors, while phenyl ring substitutions were guided by structure-activity relationship (SAR) insights from the prior literature. These compounds are listed in the Table 1.

Table 1.
List of compounds from the previous work.
CMPD1 and CMPD2 were synthesized via a three-component reaction involving nitrobenzene, aromatic aldehydes, and phenylacetylene in the presence of indium catalyst under dilute HCl. The crude products were purified by column chromatography (n-hexane/ethyl acetate, 8:2) and then recrystallized to yield pure compounds. The synthesized compounds were characterized through FT-IR, 1H NMR, and 13C NMR spectroscopy, alongside determination of melting points, Rf values, and percentage yields.
Figure 1 and Figure 2 below show the elaborate schemes for the synthesis of Target Molecules, respectively.
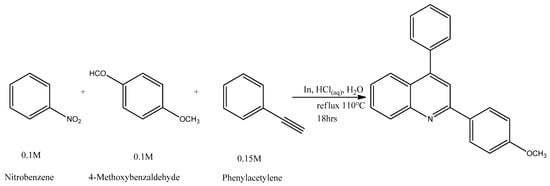
Figure 1.
Scheme for the synthesis of 2-(4-methoxyphenyl)-4-phenylquinoline (CMPD 1).
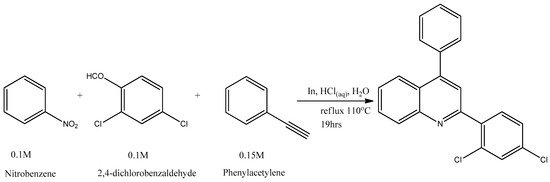
Figure 2.
Scheme for the synthesis of 2-(2, 4-dichlorophenyl)-4-phenylquinoline (CMPD 2).
Spectroscopic Analysis
Detailed structural analysis of the synthesized compounds was performed using Fourier transform infrared spectroscopy (FT-IR), and, nuclear magnetic resonance
FT-IR data were reported as frequency of absorption cm−1. Data for 1H NMR and 13C NMR were reported as chemical shift (ppm).
2.2. Pharmacological Evaluation
Antidepressant activity was evaluated using the Forced Swim Test (FST) and the Tail Suspension Test (TST) in animal models. CMPD1 and CMPD2 were administered at test doses, and immobility times were compared with a standard antidepressant control.
3. Results and Discussion
3.1. Synthesis and Characterization
The synthesis yielded CMPD1 (2-(4-methoxyphenyl)-4-phenylquinoline) and CMPD2 (2-(2,4-dichlorophenyl)-4-phenylquinoline) in satisfactory yields. FT-IR spectra confirmed characteristic quinoline bands, while 1H NMR and 13C NMR spectra validated the expected proton and carbon environments. The physical data (melting point, Rf values) were consistent with the literature, confirming compound purity.
The Table 2 below shows the yield and some physical properties of the synthesized compounds.

Table 2.
Yield and some physical properties of the synthesized compounds.
The FTIR spectrum of CMPD1 showed an aromatic C-H stretching signal at 3063.9 cm−1, consistent with typical literature values ranging from 3000 to 3100 cm−1 for aromatic compounds [1]. The C=N stretching band, observed around 1500 cm−1, aligns with the documented range of 1450–1600 cm−1 for quinoline derivatives [2]. Additionally, the methoxy group’s O-C-O stretching vibration appeared at 1200 cm−1, matching reported values of 1150–1250 cm−1 for methoxy-substituted aromatics [3]. For CMPD2, the distinct C-Cl stretching band between 600 and 800 cm−1 corroborates studies on halogen-substituted aromatic compounds [4].
In the 1H NMR spectrum, the aromatic proton multiplets for CMPD1 in the range of 7.0 and 8.0 ppm are consistent with quinoline derivatives, which typically resonate around 7.0 and 8.5 ppm [5]. The methoxy group in CMPD1 showed a singlet at 3.8 ppm, which matches expected chemical shifts for -OCH3 groups on aromatic rings [6]. For CMPD2, the protons near the C-Cl group resonated between 7.307.70 ppm, reflecting the electron-withdrawing effect of chlorine, as noted in halogen-substituted quinoline studies [7]. The 13C NMR spectrum provided additional confirmation, with aromatic carbons in both compounds appearing between 128 and 162 ppm, consistent with standard values for aromatic phenyl and quinoline rings [8]. The methoxy carbon in CMPD1 resonated at 56 ppm, aligning with literature reports for methoxy-substituted aromatics [9], while the chlorinated carbon in CMPD2 appeared at 140 ppm, typical of chlorinated aromatic systems [10]. Lastly, carbons near the nitrogen atom in the quinoline ring resonated between 153.6 and 162.5 ppm, showing deshielding effects characteristic of nitrogen-adjacent carbons in quinoline structures [11].
CMPD1
1H NMR (600 MHz): δ 3.89 (3H, s), 7.05 (2H, ddd, J = 8.9, 1.6, 0.4 Hz), 7.41–7.63 (4H, 7.48 (dddd, J = 7.9, 7.5, 1.5, 0.5 Hz), 7.51 (tdd, J = 7.5, 1.6, 1.5 Hz), 7.56 (ddd, J = 8.3, 7.3, 1.8 Hz)), 7.66–7.89 (5H, 7.73 (ddd, J = 8.9, 1.6, 0.4 Hz), 7.76 (ddd, J = 8.0, 7.3, 1.8 Hz), 7.82 (dddd, J = 7.9, 1.5, 1.5, 0.5 Hz)), 8.02–8.15 (2H, 8.09 (dddd, J = 8.3, 1.8, 0.4, 0.4 Hz), 8.09 (ddd, J = 8.0, 1.8, 0.4 Hz)), 8.56 (1H, d, J = 0.4 Hz).
13C NMR: δ 55.3 (1C, s), 114.3 (2C, s), 118.2 (1C, s), 124.1 (1C, s), 126.7 (1C, s), 127.5 (1C, s), 128.2 (1C, s), 128.5 (2C, s), 128.9 (2C, s), 129.3 (2C, s), 129.5 (1C, s), 130.5 (1C, s), 131.7 (1C, s), 138.4 (1C, s), 147.7 (1C, s), 148.6 (1C, s), 158.1 (1C, s), 159.9 (1C, s).
CMPD2
1H NMR (600 MHz): δ 7.35 (1H, dd, J = 1.8, 0.4 Hz), 7.41–7.91 (9H, 7.49 (dddd, J = 7.8, 7.5, 1.5, 0.5 Hz), 7.52 (tdd, J = 7.5, 1.6, 1.4 Hz), 7.62 (ddd, J = 8.4, 7.4, 1.9 Hz), 7.71 (dd, J = 8.5, 0.4 Hz), 7.77 (ddd, J = 7.7, 7.4, 1.8 Hz), 7.82 (dd, J = 8.5, 1.8 Hz), 7.85 (dddd, J = 7.8, 1.5, 1.5, 0.5 Hz)), 8.04–8.22 (2H, 8.11 (dddd, J = 8.4, 1.8, 0.4, 0.4 Hz), 8.16 (ddd, J = 7.7, 1.9, 0.4 Hz)), 8.60 (1H, d, J = 0.4 Hz).
13C NMR: δ 118.2 (1C, s), 124.1 (1C, s), 126.7–126.9 (2C, 126.7 (s), 126.8 (s)), 127.5 (1C, s), 128.2 (1C, s), 128.5 (2C, s), 128.9 (2C, s), 129.4–129.6 (2C, 129.5 (s), 129.6 (s)), 130.5 (1C, s), 131.5 (1C, s), 132.1 (1C, s), 132.7 (1C, s), 134.3 (1C, s), 138.4 (1C, s), 147.7 (1C, s), 148.6 (1C, s), 155.3 (1C, s).
3.2. Pharmacological Studies
In vivo behavioral studies demonstrated that both CMPD1 and CMPD2 significantly reduced immobility time in FST and TST, indicative of antidepressant-like activity. There was a significant (p < 0.05) reduction in duration of immobility at all the tested doses (20, 40, and 80 mg/Kg) as compared to the distilled water group in the forced swim test.
Interestingly, both compounds demonstrated significant antidepressant activity without the pronounced motor stimulation observed with psychostimulants, as evidenced by controlled behavioral patterns during the tests [12].
4. Conclusions
This study successfully designed, synthesized, and characterized two novel quinoline derivatives, CMPD1 and CMPD2. Spectral data confirmed their structures, and pharmacological evaluation revealed significant antidepressant activity, especially for CMPD2. In silico results corroborated their drug-like properties and target interactions. These findings position 2,4-diphenylquinoline derivatives as promising candidates for further antidepressant development.
Author Contributions
Conceptualization, A.S.Y.; methodology, A.N.H. and R.B.; software, A.N.H.; validation, A.S.Y., I.A. and I.Y.A.; formal analysis, A.S.Y.; investigation, M.A.; data curation, I.U.; writing—original draft preparation, A.S.Y.; writing—review and editing, I.U. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Öztürk, N.; Özdemir, T.; Alpaslan, Y.B.; Gökce, H.; Alpaslan, G. Experimental (FT-IR, Raman and NMR) and Theoretical (B3LYP, B3PW91, M06-2X and CAM-B3LYP) Analyses of P-Tert-Butylphenyl Salicylate. Bilge Int. J. Sci. Technol. Res. 2018, 2, 56–73. [Google Scholar] [CrossRef]
- Kumru, M.; Küçük, V.; Kocademir, M.; Alfanda, H.M.; Altun, A.; Sari, L. Experimental and theoretical studies on IR, Raman, and UV-Vis spectra of quinoline-7-carboxaldehyde. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2015, 134, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Collier, W.E.; Schultz, T.P.; Kalasinsky, V.F. Infrared Study of Lignin: Reexamination of Aryl-Alkyl Ether C-O Stretching Peak Assignments. Holzforschung 1992, 46, 523–528. [Google Scholar] [CrossRef]
- Sevvanthi, S.; Muthu, S.; Raja, M.; Aayisha, S.; Janani, S. PES, molecular structure, spectroscopic (FT-IR, FT-Raman), electronic (UV-Vis, HOMO-LUMO), quantum chemical and biological (docking) studies on a potent membrane permeable inhibitor: Dibenzoxepine derivative. Heliyon 2020, 6, e04724. [Google Scholar] [CrossRef] [PubMed]
- Katariya, K.D. Synthesis and Characterization of Some New Oxazole Containing Heterocyclic Compounds and Study of Their Biological Activities. Doctoral Dissertation, Maharaja Sayajirao University of Baroda, Vadodara, India, 2018. [Google Scholar]
- Hasan, A.H.; Abdulrahman, F.A.; Obaidullah, A.J.; Alotaibi, H.F.; Alanazi, M.M.; Noamaan, M.A.; Murugesan, S.; Amran, S.I.; Bhat, A.R.; Jamalis, J. Discovery of Novel Coumarin-Schiff Base Hybrids as Potential Acetylcholinesterase Inhibitors: Design, Synthesis, Enzyme Inhibition, and Computational Studies. Pharmaceuticals 2023, 16, 971. [Google Scholar] [CrossRef] [PubMed]
- Ökten, S. Synthesis of aryl-substituted quinolines and tetrahydroquinolines through Suzuki–Miyaura coupling reactions. J. Chem. Res. 2019, 48, 274–280. [Google Scholar] [CrossRef]
- Fatma, S.; Bishnoi, A.; Verma, A.K. Synthesis, spectral analysis (FT-IR, 1H NMR, 13C NMR and UV-visible) and quantum chemical studies on molecular geometry, NBO, NLO, chemical reactivity and thermodynamic properties of novel 2-amino-4-(4-(dimethylamino)phenyl)-5-oxo-6-phenyl-5,6-dihydro-4H-pyrano[3,2-c]quinoline-3-carbonitrile. J. Mol. Struct. 2015, 1095, 112–124. [Google Scholar]
- Boček, I.; Starčević, K.; Novak Jovanović, I.; Vianello, R.; Hranjec, M. Novel imidazo[4,5-b]pyridine derived acrylonitriles: A combined experimental and computational study of their antioxidative potential. J. Mol. Liq. 2021, 342, 117527. [Google Scholar] [CrossRef]
- von der Heiden, D.; Vanderkooy, A.; Erdélyi, M. Halogen bonding in solution: NMR spectroscopic approaches. Coord. Chem. Rev. 2020, 407, 213147. [Google Scholar] [CrossRef]
- Khalid, M.; Ullah, M.A.; Adeel, M.; Usman Khan, M.; Tahir, M.N.; Braga, A.A.C. Synthesis, crystal structure analysis, spectral IR, UV–Vis, NMR assessments, electronic and nonlinear optical properties of potent quinoline based derivatives: Interplay of experimental and DFT study. J. Saudi Chem. Soc. 2019, 23, 546–560. [Google Scholar] [CrossRef]
- Hassan, Z.; Bosch, O.G.; Singh, D.; Narayanan, S.; Kasinather, B.V.; Seifritz, E.; Kornhuber, J.; Quednow, B.B.; Müller, C.P. Novel psychoactive substances-recent progress on neuropharmacological mechanisms of action for selected drugs. Front. Psychiatry 2017, 8, 152. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).