Abstract
The extraction and complexing properties of a series of p-tert-butylcalix[4]arene derivatives containing N-methoxycarbonylmonoaza-12-crown-4 as the substituents were evaluated using liquid–liquid extraction and spectrophotometric titration methods. It has been demonstrated that monosubstituted calixarene is an effective and highly selective extractant toward sodium cations. Both tri- and tetrasubstituted calixarenes form mononuclear complexes with sodium cations and complexes of 1:1 and 1:2 compositions (L–M) with barium and strontium cations. The ability of calix[4]arene with four crown ether substituents to form binuclear complexes with cations of transition metals—copper, nickel, cobalt and cadmium—has also been demonstrated.
1. Introduction
Calixarene derivatives are a new generation of highly selective molecular receptors capable of recognizing and binding ions and organic molecules of various natures. The introduction of a second complexing center, such as a crown ether, into the calixarene molecule can make it possible to obtain compounds that demonstrate interesting steric and cooperative effects, which, in turn, can lead to the increased complexing abilities and higher ion selectivity of the resulting supramolecular ensembles [1,2]. It should be noted that in the literature devoted to the properties of calixarenes, there is still a limited number of publications with systematic data on the dependence of the complexing properties of calixarenes on the nature and size of the intramolecular cavity formed by a different number of identical substituents or substituents representing fragments of molecules that make up homologous series.
Previously, we presented a comparison of the extraction properties of a series of p-tert-butylcalix[4]arene derivatives with crown-5 ether substituents: aza-15, benzoaza-15 and amidobenzo-15-crown-5 [3]. This work presents a study of the extraction and complexing properties of the p-tert-butylcalix[4]arene series with N-carbonylmonoaza-12-crown-4 substituents at the lower rim. This choice is based on results demonstrating the ability of small crown ethers like 12-crown-4 to participate in the binding of not only s-elements cations but transition metals [4], and the well-known contribution of the amide group, which is part of the molecules of calixarene derivatives, to increased-stability complexes with metal salts [5,6].
2. Materials and Methods
2.1. Chemistry
Calixarenes 5, 6 and 8 were synthesized according to the procedures described in [7,8].
The 1H and 13C NMR spectra were recorded from 10% solutions in chloroform-d using a Bruker Avance DRX 500 spectrometer and using tetramethylsilane as an internal reference. The mass spectra were recorded using an Agilent 6530 Accurate Mass Q-TOF spectrometer with an LC/MS system. Absorption spectra in the UV region were recorded using a spectrophotometer SPECORD 250 Plus. All of metal chlorides were of analytical grade.
5,11,17,23-tetra-tert-butyl-25,26,27-tris[(1,4,7-trioxa-10-aza-cyclododec-10-yl)carbonylmethoxy]-28-hydroxycalix[4]arene (7). White solid; yield 85%. 1H NMR: δ 7.7 (s, 1H, OH), 7.2 (d, J = 2.56 Hz, 2H, arom.), 7.12 (s, 2H, arom.), 7.08 (d, 2H, arom.), 6.79 (s, 2H, arom.), 4.89 (s, 6H, CH2CO), 4.52 (d, J = 13.45 Hz, 2H, ArCH2Ar), 4.39 (d, J = 12.76 Hz, 2H, ArCH2Ar), 4.0 (t, 6H, -CH2CH2-N), 3.8 (t, 6H, -CH2CH2-N), 3.65 (t, 6H, -CH2CH2-N), 3.52 (m, 18H, O-CH2CH2-O, -CH2CH2-N), 3.23 (m, 4H, ArCH2Ar), 1.18 (s, 9H, (CH3)3C), 1.14 (s, 18H, (CH3)3C), 0.97 (s, 9H, (CH3)3C). 13C NMR: δ 171.23 (C=O), 168.54 (C=O), 153.81, 153.11, 152.53, 146.82, 146.75, 142.48, 134.18, 134.10, 131.87, 126.89, 126.16, 72.24, 70.65, 70.43, 70.32, 64.78, 49.69, 44.62, 34.45, 34.79, 33.28, 31.86, 31.82, 31.76, 31.56, 29.87. MS ESI(+): m/z 1293.7615 [M+1]+, 1316.7487 [M+Na]+.
2.2. Metal Picrate Extraction
After mutual saturation of the solvents, equal volumes (5 mL) of aqueous solutions of the metal picrates (2.5 × 10−4 M) and solutions of the calixarenes (2.5 × 10−4 M) in CHCl3 were mechanically stirred for 1 h at 20 °C. After complete phase separation, the absorbance A of picrate ions remaining in the aqueous phase after extraction was determined spectrophotometrically at 350–380 nm. For each cation–calixarene system, the absorbance measurements were repeated at least four times. Blank experiments without calixarene were run under the same conditions, yielding an absorbance A0. The percentages of extraction of the metal picrates (%E) were calculated as the ratio 100 × (A0 − A)/A0 as already described in detail [9,10]. The results of preliminary measurements of the distribution coefficient D for calixarenes 5-8 in the concentration range 4 × 10−5–4 × 10−4 M indicate the absence of “significant” solubility of calixarenes in water.
2.3. Stability Constant Determination
UV–vis titration experiments. A solution of the calixarenes 5–8 (concentration about 2 × 10−5–3.5 × 10−5 M) in methanol was treated with increasing amounts of metal chloride solution (concentration about 1–4 × 10−4 M) containing a proper ligand of the same concentration at 20 °C. The host concentration was maintained constant and the molar ratio of the guest increased with respect to the host over the range 0.1:1 to 10(20): 1 during the titration. The absorbance measurements were carried out at 6-10 wavelengths, at which spectral changes were the most notable (220–320 nm) simultaneously, and sets of the obtained experimental values (4 × 21 points) were used for joint computer processing [11,12]. The data were processed using the nonlinear least squares fitting SIRKO software [13].
In the case of studies involving transition metal salts, a solution of a ligand of a certain concentration was prepared with the addition of 0.01 M tris(hydroxymethyl)aminomethane (TRIS) in methanol.
3. Results and Discussion
To introduce substituents containing an amide fragment into the calixarene molecule, a previously developed approach based on the acylation of amino components using carboxy-substituted calixarenes (1–4) under the conditions of the activated ester method in the presence of DCC and hydroxybenzotriazole (HOBt) was implemented [3,7]. Thus, the series was synthesized: mono- (5), di- (6), tri- (7) and tetra- (8) [(1,4,7-trioxa-10-aza-cyclododec-10-yl)carbonylmethoxy]-p-tert-butylcalix[4]arenes (Scheme 1). The 1H NMR spectra indicate that the molecules of all calixarenes adopt a cone conformation.
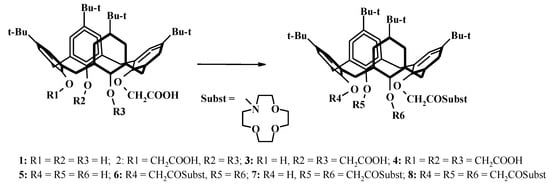
Scheme 1.
Synthesis of p-tert-butylcalix[4]arene derivatives 5–8 with N-carbonyl-monoaza-12-crown: DCC, HOBt, monoaza-12-crown-4, CHCl3, r.t., 12–25 h.
In order to evaluate the ability of calix[4]arenes with crown ether substituents to recognize metal ions, a liquid–liquid extraction experiment of some alkali, alkaline earth and transition metal picrates was carried out. The obtained results are given in Table 1.

Table 1.
Extraction (%) of picrates using calixarene derivatives 5–8 at ligand–metal ratio 1:1.
The results of studying the extraction properties of calixarenes 5–8 indicate that monosubstituted calixarene 5 demonstrates high selectivity and efficiency toward sodium cations, extracting sodium picrate at 77% (SNa/K 4.88) from aqueous solutions. And only with sodium cations is the process of complex formation with the participation of a monosubstituted macrocycle observed under spectrophotometric titration conditions. For the di- 6 and tri- 7 substituted calixarenes, comparable and relatively low percent extraction values for most of the metals studied are observed. The exception is the results of the extraction of sodium and iron cations with trisubstituted calixarene 7, which are quite difficult to explain. Tetrasubstituted calixarene 8 is highly effective as an extractant of alkali and alkaline earth metals, as well as cadmium and iron (III) cations in the series of transition metals; however, this compound does not exhibit the properties of a selective extractant. This behavior can be explained by the large size of the intramolecular cavity formed by the substituents, containing donor centers of different natures, which can take part in the coordination of both “hard” and “soft” cations, and by the absence of serious steric hindrances in the interactions under consideration.
Note that in the course of studying the extraction properties toward magnesium and barium cations for all four calixarenes, a re-extraction process is observed, with a partial transition of the resulting complexes into the aqueous phase.
The results of studying the complexing properties of calixarenes toward s-element cations indicate the ability of disubstituted calixarene 6 to form 1:1 complexes with most of the metals studied. On the contrary, the cesium cation forms with the disubstituted calixarene a biligand mononuclear complex with a high lgK21 > 6 value (Table 2).

Table 2.
Stability constants (lgKn) of the complexes of calixarenes 5–8 with alkali and alkaline earth metal cations in MeOH.
For trisubstituted calixarene 7, the highest values of the stability constants of complexes in the series of alkali metals are observed for the sodium cation, with very high sodium selectivity: 3.5–5 orders of logarithmic units. Complexes of calixarene 7 with alkaline earth metal cations are characterized by higher stability constants compared to the data obtained for the series of alkali metals (Table 2). At the same time, calcium selectivity is observed in the series of alkaline earth metals, which are about two logarithmic units. With the sodium, cesium and calcium cations, calixarene 7 forms complexes of only 1:1 composition; in other cases, along with 1:1 complexes, there are complexes of 1:2 composition (L:M). Tetrasubstituted analog 8 exhibits the same behavior as trisubstituted calixarene toward the cations of alkaline earth metals, but with lower stability constants of the complexes. It should be noted that only tetrasubstituted calixarene 8 acts as an effective extractant for lithium, forming 1:2 (L:M) complexes with lithium cations, while not demonstrating affinity to cations with a larger ionic radius: rubidium and cesium.
As is known, the optimal ratio of the effective diameter of the macrocycle to the ionic radius of the metal cation, which is 0.7–0.9:1, plays an important role in the process of the complexation of crown ethers with cations of s-elements [14,15]. In the case of the interaction of crown ethers with transition metal salts, this model is not always realized, due to the possible participation of water molecules from the outer sphere in coordination with macrocycle heteroatoms [15]. Therefore, crown ethers exhibit low affinity to transition metal cations. The introduction of several aza-12-crown-4 ether residues into the preorganized structure of calix[4]arene in the cone conformation will, in our opinion, make it possible to create a polytopic receptor for transition metal ions. Since the calix[4]arene molecule is a relatively rigid framework, and the crown ethers are connected to it by a rather short linker, the formation of sandwich- or pseudosandwich-type complexes with metal cations of a large ionic radius can be expected. To test this assumption, we studied the interaction of tetrasubstituted calixarene 8 with transition metal cations using spectrophotometric titration. As follows from Table 3, tetrasubstituted calixarene molecules are capable of forming binuclear complexes with cations of copper, nickel, cobalt and cadmium, and the greatest stability of the complexes in this series is observed in the case of the interaction with cadmium ions (Table 3).

Table 3.
Stability constants (lgKn) of the complexes of calixarene 8 with cations of some transition metals (ionic rad.) in MeOH.
4. Conclusions
Thus, a change in the extraction and complexing properties of calix[4]arenes with azacrown ether substituents—potentially complexones—has been demonstrated, depending on the number of crown ether substituents included in the calixarene molecule.
Author Contributions
Conceptualization, E.A.; methodology, E.A. and E.K.; investigation, E.A. and E.K.; writing—original draft, E.A.; writing—review and editing, T.K.; project administration, T.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Iglesias-Sanchez, J.C.; Wang, W.; Ferdani, R.; Prados, P.; de Mendoza, J.; Gokel, G.W. Synthetic cation transporters incorporating crown ethers and calixarenes as headgroups and central relays: A comparison of sodium and chloride selectivity. New. J. Chem. 2008, 32, 878–890. [Google Scholar] [CrossRef] [PubMed]
- Baldini, L.; Bracchini, C.; Cacciapaglia, R.; Casnati, A.; Mandoloni, L.; Ungaro, R. Catalysis of Acyl Group Transfer by a Double Displacement Mechanism: The Cleavage of Aryl Esters Catalyzed by Calixcrown-Ba2+ Complexes. Chem. Eur. J. 2000, 6, 1322–1330. [Google Scholar] [CrossRef]
- Alekseeva, E.A.; Basok, S.S. Synthesis and extraction properties of p-tert-butylcalix[4]arenes with crown-5 ether substituents. Macroheterocycles 2017, 10, 221–225. [Google Scholar] [CrossRef]
- Junk, P.C.; Smith, M.K.; Steed, J.W. Anion-induced structural diversity in 12-crown-4 complexes of transition metal salts. Polyhedron 2001, 20, 2979–2988. [Google Scholar] [CrossRef]
- Beer, P.D.; Drew, G.B.; Knubley, R.J.; Ogden, M.I. Synthesis and Coordination Chemistry of a Novel Bis(Benzo Crown Ether) Substituted Calix[4]arene that can Simultaneously Complex Cations and Anions. J. Chem. Soc., Dalton Trans. 1995, 19, 3117–3124. [Google Scholar] [CrossRef]
- Roymon, J.; Chebrolu, P.R. Ion and molecular recognition by lower rim 1,3-di-conjugates of calix[4]arene as receptors. Chem. Rev. 2011, 111, 4658–4702. [Google Scholar] [CrossRef]
- Alekseeva, E.A.; Basok, S.S.; Mazepa, A.V.; Luk’yanenko, A.P.; Snurnikova, O.V.; Gren, A.I. p-tert-Butylcalix[4]arenes containing azacrown ether substituents at the lower rim as potential polytopic receptors. Rus. J. Gen. Chem. (Engl. Transl.) 2013, 83, 1738–1743. [Google Scholar] [CrossRef]
- Ivanova, E.A.; Prokhorova, P.E.; Morzherin, Y.Y.; Lukyanenko, A.P.; Alekseeva, E.A.; Basok, S.S. 4-tert-Butylcalix[4]arenes containing azacrown ether substituents at the narrow rim as membrane carriers. Bull. Acad. Sci. USSR, Div. Chem. 2015, 64, 905–908. [Google Scholar] [CrossRef]
- Aggarwal, R.C.; Singh, N.K. Synthesis and characterization of some first row transition metal picrates. def. Sci. J. 1975, 25, 153–158. [Google Scholar]
- Bosque-Sendra, J.M.; Almansa-López, E.; García-Campaña, A.M.; Cuadros-Rodríguez, L. Data analysis in the determination of stoichiometries and stability constants of complexes. Anal. Sci. 2003, 19, 1431–1440. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Beck, M.; Nagypal, I. Chemistry of Complex Equilibria; Academiai Kiado: Budapest, Romania, 1989; p. 402. [Google Scholar]
- Connors, K.A. Binding Constants: The Measurement of Molecular Complex Stability; Wiley-Interscience: New York, NY, USA, 1987; p. 432. [Google Scholar]
- Vetrogon, V.I.; Lukyanenko, N.G.; Schwing-Weill, M.-J.; Arnaud-Neu, F. A PC compatible computer program for the calculation of equilibrium constants by the simultaneous processing of different sets of experimental results. Talanta 1994, 41, 2105–2112. [Google Scholar] [CrossRef] [PubMed]
- Steed, J.W. First- and second-sphere coordination chemistry of alkali metal crown ether complexes. Coord. Chem. Rev. 2001, 215, 171–221. [Google Scholar] [CrossRef]
- Izatt, R.M.; Pawlak, K.; Bradshaw, J.S.; Bruening, R.L. Thermodinamic and kinetic data for macrocycle interaction with cations, anions, and neutral molecules. Chem. Rev. 1995, 95, 2529–2586. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).