Thiohydrazides in the Synthesis of Functionalized Extranuclear Heterosteroids †
Abstract
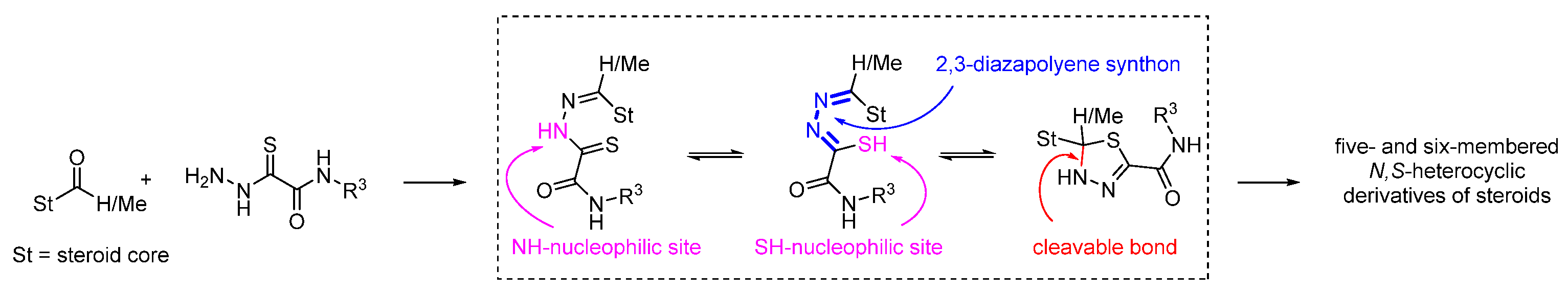
1. Introduction
2. Results and Discussion
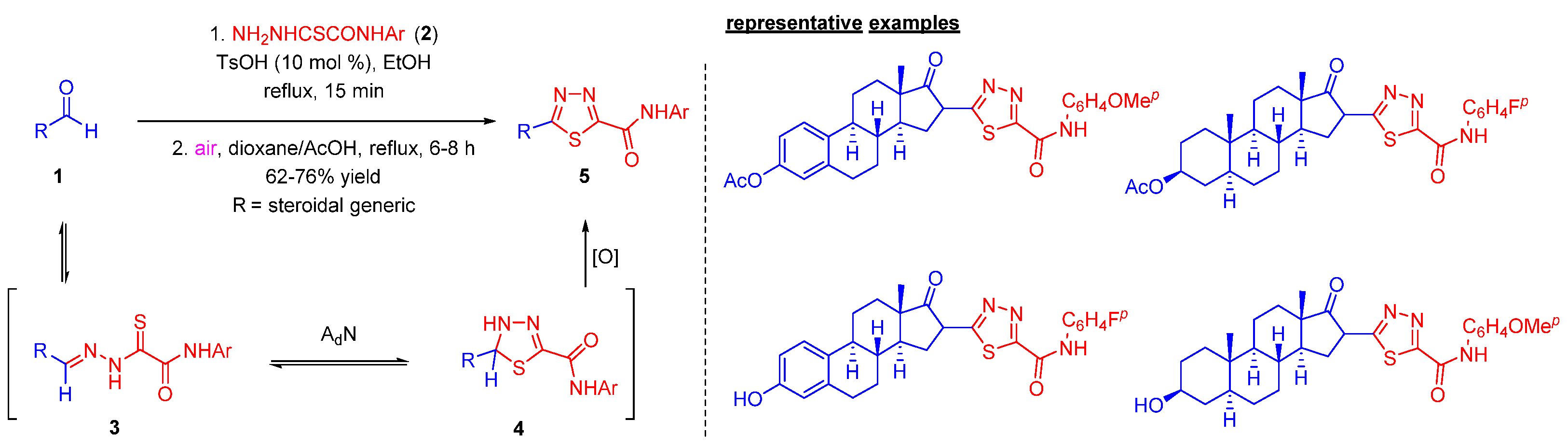
2.1. Steroidal 1,3,4-thiadiazoles
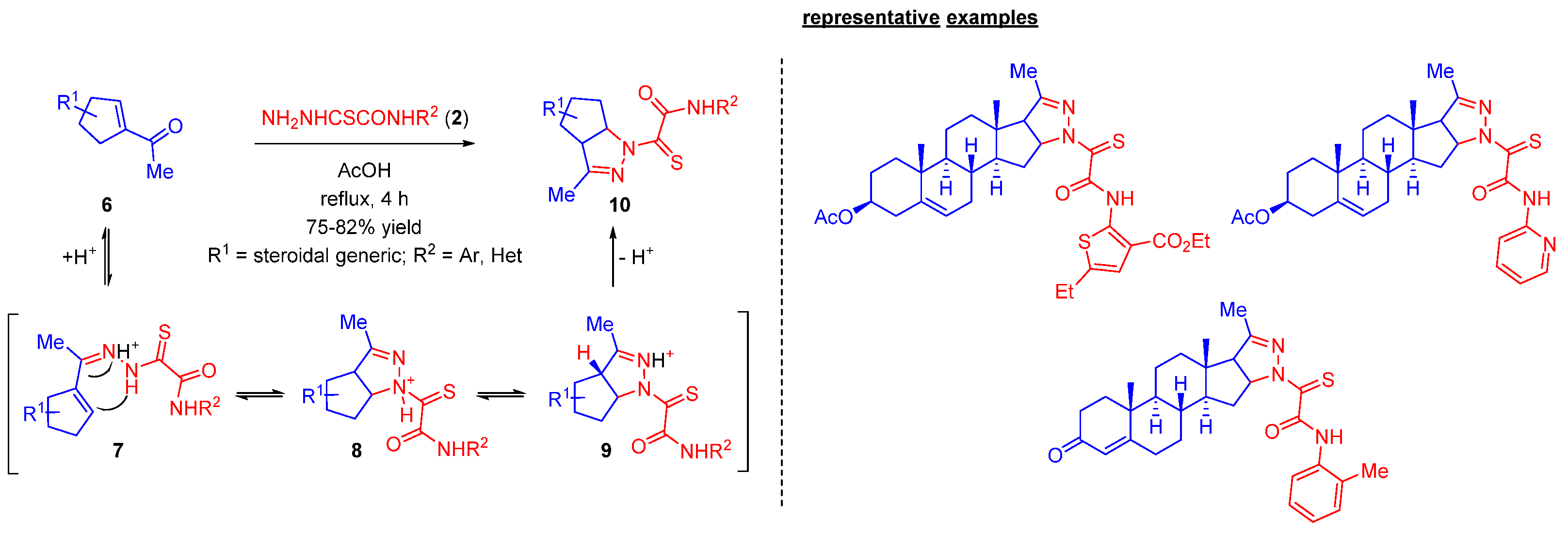
2.2. Steroidal Pyrazolines
2.3. Steroidal Pyridazines
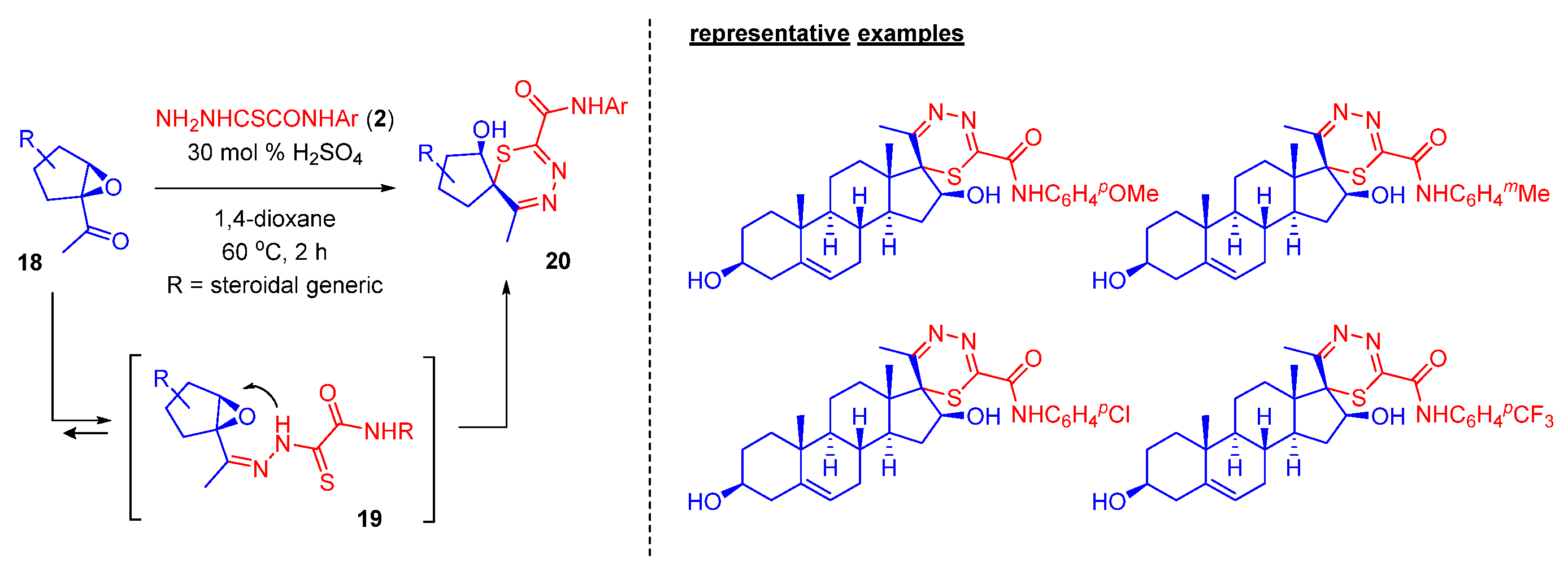
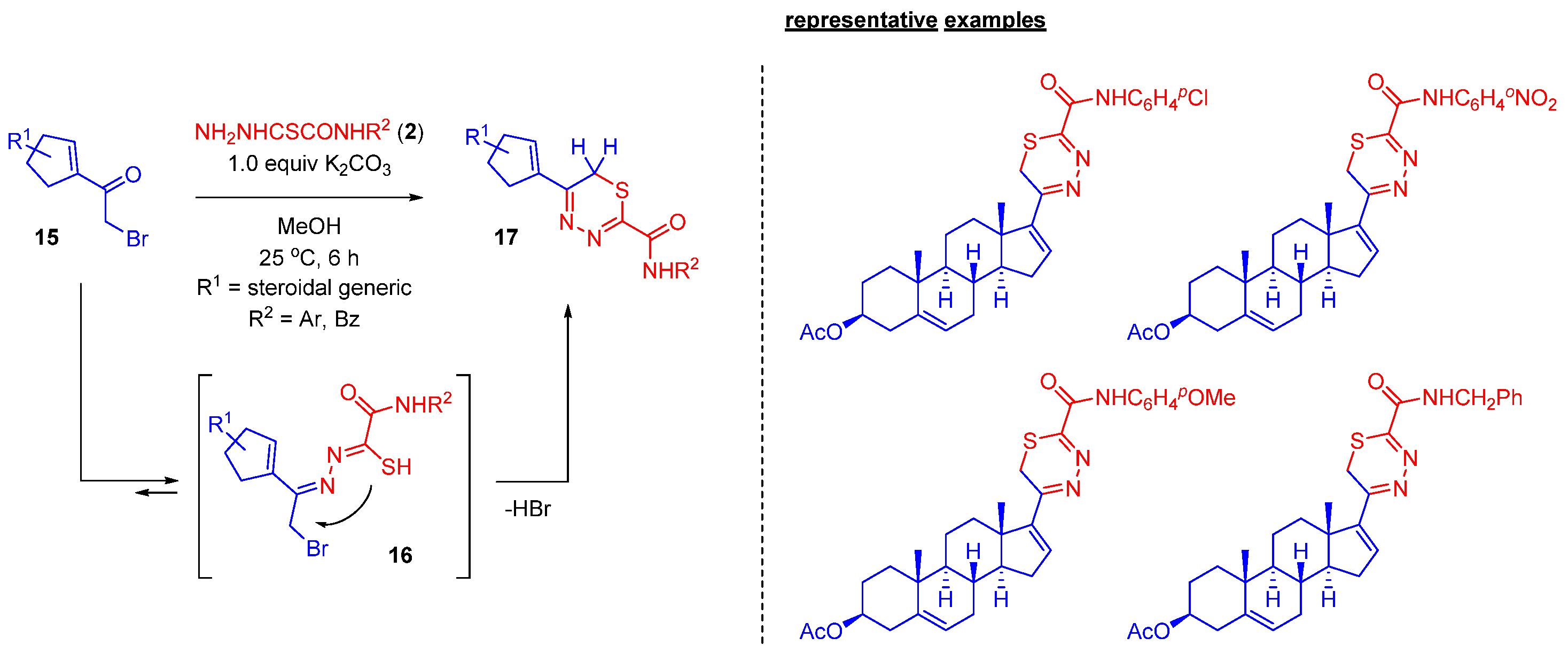
2.4. Steroidal 1,3,4-thiadiazines
2.5. Antiproliferative Activity
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, B.; Cai, Z.; Wang, S.; Liu, H. Recent advances on the synthesis and antitumor evaluation of exonuclear heterosteroids. Chin. J. Org. Chem. 2017, 37, 1952. [Google Scholar] [CrossRef]
- Singh, H.; Jindal, D.P.; Yadav, M.R.; Kumar, M. 5 Heterosteroids and drug research. In Progress in Medicinal Chemistry; Ellis, G.P., West, G.B., Eds.; Elsevier: Amsterdam, The Netherlands, 1991; Volume 28, pp. 233–300. [Google Scholar]
- Abiraterone acetate. Drugs RD 2010, 10, 261–269. [CrossRef] [PubMed][Green Version]
- Hughes, E.; Brown, J.; Tiffin, G.; Vandekerckhove, P. Danazol for unexplained subfertility. Cochrane Database Syst. Rev. 2007, Cd000069. [Google Scholar] [CrossRef] [PubMed]
- Arif, T.; Dorjay, K.; Adil, M.; Sami, M. Dutasteride in androgenetic alopecia: An update. Curr. Clin. Pharmacol. 2017, 12, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Farhan, A.; Alshamusi, Q.; Jebur, M. Synthesis of steroid bearing heterocyclic derivatives and biological activity. Review 2014–2020. J. Phys. Conf. Ser. 2021, 1853, 012057. [Google Scholar] [CrossRef]
- Ibrahim-Ouali, M.; Dumur, F. Recent syntheses of steroidal derivatives containing heterocycles. ARKIVOC-Online J. Org. Chem. 2020, 2019, 304–339. [Google Scholar] [CrossRef]
- Sharma, K.; Kumar, H. Formation of nitrogen-containing six-membered heterocycles on steroidal ring system: A review. Steroids 2022, 191, 109171. [Google Scholar] [CrossRef]
- Pawar, S.; Kumar, K.; Gupta, M.K.; Rawal, R.K. Synthetic and medicinal perspective of fused-thiazoles as anticancer agents. Anti-Cancer Agents Med. Chem. 2021, 21, 1379–1402. [Google Scholar] [CrossRef]
- Matiadis, D.; Sagnou, M. Pyrazoline hybrids as promising anticancer agents: An up-to-date overview. Int. J. Mol. Sci. 2020, 21, 5507. [Google Scholar] [CrossRef]
- Hammouda, M.M.; Elattar, K.M.; Rashed, M.M.; Osman, A.M.A. Synthesis and biological activities of bicyclic pyridines integrated steroid hybrid. Steroids 2023, 199, 109287. [Google Scholar] [CrossRef]
- Elattar, K.M.; El-Mekabaty, A. Heterocyclic steroids: Synthetic routes and biological characteristics of steroidal fused bicyclic pyrimidines. J. Heterocycl. Chem. 2021, 58, 389–414. [Google Scholar] [CrossRef]
- Iqbal, A.; Siddiqui, T. A review on synthesis and biological activities of D-ring modified pregnenolone. Steroids 2021, 170, 108827. [Google Scholar] [CrossRef] [PubMed]
- Huo, H.; Li, G.; Shi, B.; Li, J. Recent advances on synthesis and biological activities of C-17 aza-heterocycle derived steroids. Bioorganic Med. Chem. 2022, 69, 116882. [Google Scholar] [CrossRef] [PubMed]
- Raičević, V.; Radulović, N.; Sakač, M. Toward selective anticancer agents: Ferrocene-steroid conjugates. Eur. J. Inorg. Chem. 2022, 2022, e202100951. [Google Scholar] [CrossRef]
- Nahar, L.; Sarker, S.D. A review on steroid dimers: 2011–2019. Steroids 2020, 164, 108736. [Google Scholar] [CrossRef] [PubMed]
- Paquin, A.; Reyes-Moreno, C.; Bérubé, G. Recent advances in the use of the dimerization strategy as a means to increase the biological potential of natural or synthetic molecules. Molecules 2021, 26, 2340. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim-Ouali, M.; Hamze, K. A click chemistry approach to secosteroidal macrocycles. Steroids 2014, 80, 102–110. [Google Scholar] [CrossRef]
- Le Bideau, F.; Dagorne, S. Synthesis of transition-metal steroid derivatives. Chem. Rev. 2013, 113, 7793–7850. [Google Scholar] [CrossRef]
- Yarovenko, V.; Shirokov, A.; Krupinova, O.; Zavarzin, I.; Krayushkin, M. Synthesis of oxamic acids thiohydrazides and carbamoyl-1, 3, 4-thiadiazoles. Russ. J. Org. Chem. 2003, 39, 1133–1139. [Google Scholar] [CrossRef]
- Komendantova, A.S.; Lyssenko, K.A.; Zavarzin, I.V.; Volkova, Y.A. Iodine-promoted synthesis of pyrazoles from 1, 3-dicarbonyl compounds and oxamic acid thiohydrazides. Org. Chem. Front. 2020, 7, 1640–1646. [Google Scholar] [CrossRef]
- Komkov, A.V.; Komendantova, A.S.; Menchikov, L.G.; Chernoburova, E.I.; Volkova, Y.A.; Zavarzin, I.V. A straightforward approach toward multifunctionalized pyridazines via imination/electrocyclization. Org. Lett. 2015, 17, 3734–3737. [Google Scholar] [CrossRef] [PubMed]
- Komendantova, A.S.; Ivanova, K.A.; Lyssenko, K.V.; Volkova, Y.A.; Zavarzin, I.V. Facile Synthesis of Carboxamide-Substituted 1, 3, 4-Thiadiazines and 5, 6-Dihydro-4 H-1, 3, 4-Thiadiazin-5-Ols. Chem. Heterocycl. Compd. 2019, 55, 665–671. [Google Scholar] [CrossRef]
- Krayushkin, M.; Yarovenko, V.; Zavarzin, I. Synthesis and reactivity of monothiooxamides and thiohydrazides of oxamic acids. Russ. Chem. Bull. 2004, 53, 517–527. [Google Scholar] [CrossRef]
- Volkova, Y.A.; Chernoburova, E.I.; Petrova, A.S.; Shtil, A.A.; Zavarzin, I.V. Reactions of hydrazones derived from oxamic acid thiohydrazides. Phosphorus Sulfur Silicon Relat. Elem. 2017, 192, 237–240. [Google Scholar] [CrossRef]
- Krayushkin, M.; Yarovenko, V.; Zavarzin, I. Synthesis of heterocyclic compounds based on oxamic acid monothiooxamides and thiohydrazides. Russ. Chem. Bull. 2019, 68, 1143–1163. [Google Scholar] [CrossRef]
- Vorontsova, S.K.; Yadykov, A.V.; Scherbakov, A.M.; Minyaev, M.E.; Zavarzin, I.V.; Mikhaevich, E.I.; Volkova, Y.A.; Shirinian, V.Z. Novel D-annulated pentacyclic steroids: Regioselective synthesis and biological evaluation in breast cancer cells. Molecules 2020, 25, 3499. [Google Scholar] [CrossRef]
- Samanta, S.; Ghosh, A.K.; Ghosh, S.; Ilina, A.A.; Volkova, Y.A.; Zavarzin, I.V.; Scherbakov, A.M.; Salnikova, D.I.; Dzichenka, Y.U.; Sachenko, A.B. Fe (III)-Catalyzed synthesis of steroidal imidazoheterocycles as potent antiproliferative agents. Org. Biomol. Chem. 2020, 18, 5571–5576. [Google Scholar] [CrossRef]
- Volkova, Y.A.; Kozlov, A.S.; Kolokolova, M.K.; Uvarov, D.Y.; Gorbatov, S.A.; Andreeva, O.E.; Scherbakov, A.M.; Zavarzin, I.V. Steroidal N-sulfonylimidates: Synthesis and biological evaluation in breast cancer cells. Eur. J. Med. Chem. 2019, 179, 694–706. [Google Scholar] [CrossRef]
- Scherbakov, A.M.; Zavarzin, I.V.; Vorontsova, S.K.; Hajra, A.; Andreeva, O.E.; Yadykov, A.V.; Levina, I.S.; Volkova, Y.A.; Shirinian, V.Z. Synthesis and evaluation of the antiproliferative activity of benzylidenes of 16-dehydroprogesterone series. Steroids 2018, 138, 91–101. [Google Scholar] [CrossRef]
- Scherbakov, A.M.; Komkov, A.V.; Komendantova, A.S.; Yastrebova, M.A.; Andreeva, O.E.; Shirinian, V.Z.; Hajra, A.; Zavarzin, I.V.; Volkova, Y.A. Steroidal pyrimidines and dihydrotriazines as novel classes of anticancer agents against hormone-dependent breast cancer cells. Front. Pharmacol. 2018, 8, 979. [Google Scholar] [CrossRef]
- Rassokhina, I.V.; Volkova, Y.A.; Kozlov, A.S.; Scherbakov, A.M.; Andreeva, O.E.; Shirinian, V.Z.; Zavarzin, I.V. Synthesis and antiproliferative activity evaluation of steroidal imidazo[1,2-a]pyridines. Steroids 2016, 113, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Birukova, V.; Scherbakov, A.; Ilina, A.; Salnikova, D.; Andreeva, O.; Dzichenka, Y.; Zavarzin, I.; Volkova, Y. Discovery of highly potent proapoptotic antiestrogens in a series of androst-5,16-dienes D-modified with imidazole-annulated pendants. J. Steroid Biochem. Mol. Biol. 2023, 231, 106309. [Google Scholar] [CrossRef] [PubMed]
- Zavarzin, I.; Antonov, Y.S.; Chernoburova, E.; Shchetinina, M.; Kolotyrkina, N.; Shashkov, A. Interaction of 16-hydroxymethylidene derivatives of androstane and estrone with thiohydrazides of oxamic acids. Russ. Chem. Bull. 2013, 62, 2603–2608. [Google Scholar] [CrossRef]
- Zavarzin, I.V.; Kamernitsky, A.; Chertkova, V.V.; Chernoburova, E.I.; Yarovenko, V.N.; Kraushkin, M.M.; Kachala, V.V. Synthesis of 1’-arylcarbamoylthiocarbonyl-3’-methyl-3-oxoandrost-4-eno[16α,17α-d]pyrazolines. Arkivoc 2008, 4, 62–70. [Google Scholar] [CrossRef]
- Kamernitsky, A.; Chernoburova, E.; Chertkova, V.; Zavarzin, I.; Yarovenko, V.; Krayushkin, M. Acylhydrazones of 20-keto steroids and their transformations: I. Synthesis and properties of 1′-acyl-substituted 3′-methylandrosteno [16,17-d] pyrazolines. Russ. J. Bioorganic Chem. 2007, 33, 315–319. [Google Scholar] [CrossRef]
- Kamernitskii, A.; Chernoburova, E.; Chertkova, V.; Yarovenko, V.; Zavarzin, I.; Krayushkin, M. Effect of ω-substituents in the hydrazones of conjugated pregnane 20-ketosteroids on their ability to cyclize to pyrazolines. Russ. Chem. Bull. 2006, 55, 2117–2118. [Google Scholar] [CrossRef]
- Volkova, Y.A.; Antonov, Y.S.; Komkov, A.V.; Scherbakov, A.M.; Shashkov, A.S.; Menchikov, L.G.; Chernoburova, E.I.; Zavarzin, I.V. Access to steroidal pyridazines via modified thiohydrazides. RSC Adv. 2016, 6, 42863–42868. [Google Scholar] [CrossRef]
- Komendantova, A.S.; Fakhrutdinov, A.N.; Menchikov, L.G.; Sukhorukov, A.Y.; Zavarzin, I.V.; Volkova, Y.A. Cyclization of β-chlorovinyl thiohydrazones into pyridazines: A mechanistic study. Eur. J. Org. Chem. 2019, 2019, 527–536. [Google Scholar] [CrossRef]
- Komendantova, A.S.; Scherbakov, A.M.; Komkov, A.V.; Chertkova, V.V.; Gudovanniy, A.O.; Chernoburova, E.I.; Sorokin, D.V.; Dzichenka, Y.U.; Shirinian, V.Z.; Volkova, Y.A. Novel steroidal 1, 3, 4-thiadiazines: Synthesis and biological evaluation in androgen receptor-positive prostate cancer 22Rv1 cells. Bioorganic Chem. 2019, 91, 103142. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volkova, Y.; Scherbakov, A.; Zavarzin, I. Thiohydrazides in the Synthesis of Functionalized Extranuclear Heterosteroids. Chem. Proc. 2023, 14, 74. https://doi.org/10.3390/ecsoc-27-16179
Volkova Y, Scherbakov A, Zavarzin I. Thiohydrazides in the Synthesis of Functionalized Extranuclear Heterosteroids. Chemistry Proceedings. 2023; 14(1):74. https://doi.org/10.3390/ecsoc-27-16179
Chicago/Turabian StyleVolkova, Yulia, Alexander Scherbakov, and Igor Zavarzin. 2023. "Thiohydrazides in the Synthesis of Functionalized Extranuclear Heterosteroids" Chemistry Proceedings 14, no. 1: 74. https://doi.org/10.3390/ecsoc-27-16179
APA StyleVolkova, Y., Scherbakov, A., & Zavarzin, I. (2023). Thiohydrazides in the Synthesis of Functionalized Extranuclear Heterosteroids. Chemistry Proceedings, 14(1), 74. https://doi.org/10.3390/ecsoc-27-16179