Abstract
Using the Bingel–Hirsch reaction, new hexakis methanofullerenes containing strained polycyclic hydrocarbons were synthesized. Organic field-effect transistors were manufactured based on the obtained compounds.
1. Introduction
One of the most popular and promising areas for the use of fullerene derivatives is the creation of organic field-effect transistors due to the covalent binding of fullerenes with electron-donating or photoactive macromolecules, which forms a molecular heterojunction [1].
The most selective method for the synthesis of hexakis methanofullerenes is the Bingel–Hirsch reaction [2,3], which makes it possible to obtain fullerene C60 [4,5,6] hexaadducts with Th-symmetry [4] that can be used as n-type acceptor materials with a high charge carrier mobility and electrical stability [7,8,9,10,11]. The search for organic semiconductors for creating field-effect thin-film transistors is an urgent task. It is known from the literature [12,13] that manufactured organic field-effect transistors based on styrylfullerenes are significantly superior in efficiency to devices created on the basis of PCBM. Based on the above, it follows that C60 fullerene derivatives are promising objects for creating organic systems that can act as n-type semiconductors.
2. Results and Discussion
Previously, we [14] were the first to synthesize hybrid molecules from C60 fullerene and strained polycyclic hydrocarbons, on the basis of which organic field-effect transistors (OFETs) with high-quality films were created. In development of these studies, according to the well-known method [4], we carried out the selective synthesis of C60 hexaadducts containing six SPH (strained polycyclic hydrocarbon) addends in order to increase the solubility of new hybrid molecules, as well as their promising direction associated with the creation of methanofullerenes, which are an effective n-type organic semiconductor, due to a larger number of covalently attached SPH groups to the C60 carbon cage. Thus, using the reaction of nucleophilic addition to fullerene C60 of α-halocarbanions generated in situ by the interaction of esters 1–3 with CBr4 in the presence of the base 1,8-diazabicyclo [5.4.0]undec-7-ene (DBU) in the ratio 1:10:100:10, respectively, under the developed conditions (orthodichlorobenzene, 20 °C, 24 h) (Scheme 1), the target fullerene hexaadducts 4–6 were synthesized with a yield of ~96%.
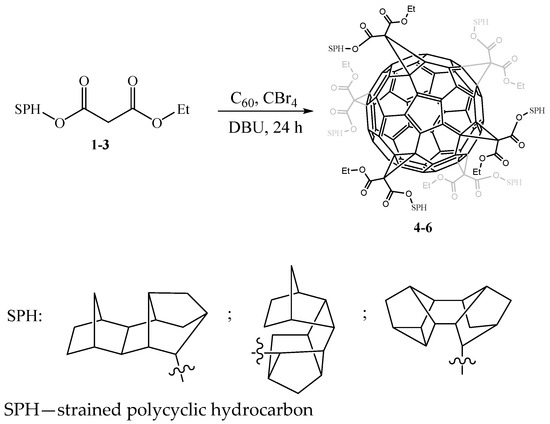
Scheme 1.
Synthesis of hexakisadduct methanofullerenes.
To manufacture transistors, we used a method described earlier [14]; the structure of a field-effect transistor is shown in Figure 1. In a similar way, the current–voltage characteristics were measured and the mobility of charge carriers in the active layer of μ OFETs was calculated (Table 1).
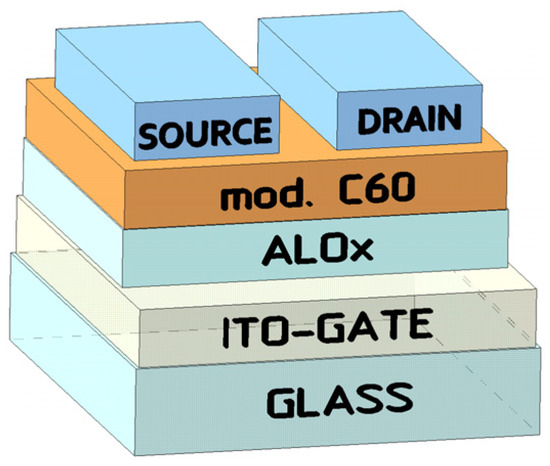
Figure 1.
Structure of a field-effect transistor with films of fullerene derivatives.

Table 1.
Charge carrier mobility values.
Current–voltage characteristics (Figure 2, Figure 3 and Figure 4) were measured at room temperature without an inert atmosphere. Under the application of a positive gate voltage, the current exhibits an increase, indicating the prevalence of electronic conductivity in the transport channel of the OFETs. The dependences are non-linear, lacking saturation regions in the output characteristics of the devices. The absence of saturation sections in the output characteristics may be attributed to the existence of leakage currents.
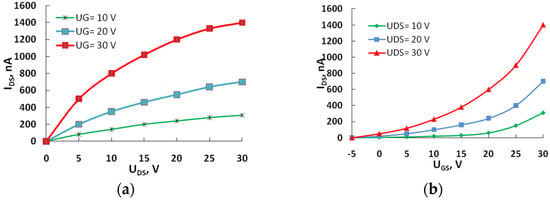
Figure 2.
The output (a) and transfer characteristics (b) of the field-effect transistor with active layer 4.
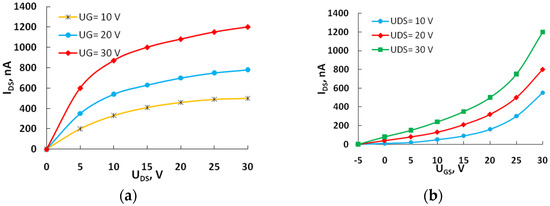
Figure 3.
The output (a) and transfer characteristics (b) of the field-effect transistor with active layer 5.
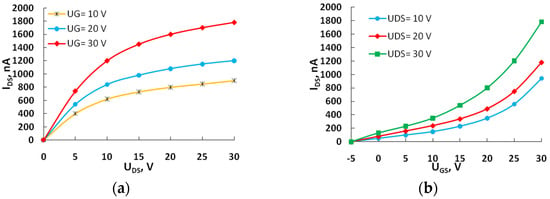
Figure 4.
The output (a) and transfer characteristics (b) of the field-effect transistor with active layer 6.
Current–voltage characteristics were measured at room temperature without an inert atmosphere.
3. Materials and Methods
All reactions were performed under an argon atmosphere and in anhydrous solvent. The solvents and reagents were dried or refined according to the literature procedures. Commercially available [60]fullerene (99.5% pure, Sigma-Aldrich, 9402 Alberene Drive, Houston, TX, USA) was used. The reaction products were analyzed on an HPLC chromatograph Shimadzu SPD-20A (1900 SE 4th Avenue, Canby, OR, USA) equipped with a UV detector at 313 or 340 nm. The mixtures were separated on a preparative column Cosmosil Buckyprep Waters (250 × 10 mm) at ~20 °C. Toluene was used as an eluent; the flow rate was 3.0 mL·min–1. The 1H and 13C NMR spectra were procured on a Bruker Avance-500 spectrometer at 500.17 and 125.78 MHz, respectively. A mixture of CDCl3 and CS2 (1:5) was used as a solvent. The chemical shifts are reported as δ values in parts per million relative to the internal standard Me4Si. The coupling constants (J) are reported in Hertz. The mass spectra were obtained on an UltraFlex III TOF/TOF (Bruker Daltonik GmbH, Karlsruhe, Germany) operating in linear (TOF) and reflection (TOF/TOF) positive and negative ion modes. S8 and DCTB (trans-2-[3-(4-tert-butylphenyl)-2-methyl-2-propenyliden]malononitrile) were used as the matrix. For the application on a metal target, toluene solutions of the samples were used.
For transistor creation, ITO-coated glass substrates were used. A 400 nm-thick AlOx film was spin-coated onto the ITO layer. Then, using the vacuum thermal evaporation method, aluminum electrodes with a thickness of 500 nm were deposited onto the AlOx dielectric layer. After this, fullerene derivatives with a thickness of 150 nm were deposited using the centrifugation method. To prepare a solution for centrifugation, 5 mg of the fullerene derivative and 200 μl of toluene were used. The size of the source and drain contacts with a gap of 2 mm between them was 4 mm. To carry out the process, an SM-6M centrifuge, a vacuum unit (VUP5), an HY3005D-3 direct current source, a GDM 8245 multimeter (microammeter), an AFM Nanoeducator II and Gwyddion software (http://gwyddion.net/ accessed on 3 December 2023) were used. Fullerene derivatives 4–6 were synthesized using a known procedure [6].
3.1. Hexamethanofullerene 4
Brown powder. 1H NMR (500 MHz, CDCl3): δ (ppm) = 4.43–4.34 (m, 12H, CH2), 2.55 (b, m, 12H, CH), 2.17–2.02 (m, 12H, CH), 1.97–1.93 (m, 12H, CH), 1.83–1.74 (m, 12H, CH), 1.61–1.55 (m, 12H, CH), 1.38–1.34 (m, 18H, 6CH3), 1.13–1.07 (m, 12H, CH), 0.93–0.77 (m, 12H, CH). 13C NMR (125 MHz, CDCl3): δ (ppm) = 163.90, 163.35, 145.99, 145.54, 145.41, 141.29, 141.03, 140.99, 89.56, 69.11, 62.75, 57.63, 54.43, 53.89, 46.88, 43.57, 41.78, 39.45, 36.89, 36.00, 34.86, 29.36, 29.07, 14.14. MALDI TOF [M]− calcd. for C174H144O24 2616.9981; Found 2616.9978. Yield 48 mg, 96%.
3.2. Hexamethanofullerene 5
Brown powder. 1H NMR (500 MHz, CDCl3): δ (ppm) = 4.41–4.35 (m, 12H, CH2), 2.54 (b, m, 12H, CH), 2.28–2.24 (m, 12H, CH), 2.17–2.11 (m, 12H, CH), 2.05–1.99 (m, 12H, CH), 1.89–1.82 (m, 12H, CH), 1.46–1.43 (m, 12H, CH), 1.37–1.31 (m, 18H, 6CH3), 0.92–0.84 (m, 12H, CH), 0.79–0.77 (m, 12H, CH). 13C NMR (125 MHz, CDCl3): δ (ppm) = 163.87, 163.40, 147.15, 145.57, 141.30, 140.92, 89.85, 69.11, 62.74, 54.59, 53.43, 50.64, 50.08, 46.87, 42.30, 41.87, 40.45, 38.98, 36.93, 36.41, 24.04, 14.12. MALDI TOF [M]− calcd. for C174H144O24 2616.9981; Found 2616.9985. Yield 44 mg, 95%.
3.3. Hexamethanofullerene 6
Brown powder. 1H NMR (500 MHz, CDCl3): δ (ppm) =4.99–4.90 (m, 12H, CH2), 2.33–2.32 (m, 12H, CH), 2.31–2.30 (m, 12H, CH), 2.13–2.11 (m, 12H, CH), 2.05–1.95 (m, 12H, CH), 1.93–1.77 (m, 12H, CH), 1.66–1.63 (m, 12H, CH), 1.55–1.47(m, 12H, CH), 1.36–1.32 (m, 18H, 6CH3), 1.23–1.20 (m, 12H, CH), 1.02–0.98 (m, 12H, CH). 13C NMR (125 MHz, CDCl3): δ (ppm) = 163.67, 162.81, 147.19, 145.49, 141.48, 86.07, 84.25, 69.22, 52.42, 44.65, 42.94, 41.40, 40.06, 36.63, 35.66, 34.61, 33.64, 32.32, 30.12, 15.83, 14.95, 14.82. MALDI TOF [M]− calcd. for C174H132O24 2604.9042; Found 2604.9039. Yield 46 mg, 95%.
4. Conclusions
The calculated charge carrier mobilities reflect the corresponding changes for pairs (a and b). Hybrid molecules of hexakis adducts of C60 fullerene 4 and 6 contain six fragments of a strained polycyclic hydrocarbon; the best charge carrier mobility μ result for the device shown in Figure 1 was shown by methanofullerene 6, where μ = 0.012 cm2/V·s, and methanofullerenes 4 and 5, with μ = 0.005 cm2/V·s and μ = 0.007 cm2/V·s, respectively. The manufactured organic field-effect transistors showed a high electrical stability; the current value remained the same during repeated measurements.
Author Contributions
Conceptualization, data curation, synthetic investigation, writing—original draft, review and editing, A.R.A., R.I.A., Z.R.S., R.B.S., I.N.M. and T.R.S.; supervision, R.B.S. All authors have read and agreed to the published version of the manuscript.
Funding
Fullerene derivatives were obtained in the framework of the Ministry of Science and Higher Education within the State assignment Institute of Petrochemistry and Catalysis of RAS State Registration FMRS-2022-0075, FMRS-2022-0076 and the research was funded with the support of a state assignment (scientific code FZWU-2023-0002).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Structural studies were performed using the equipment of the Collective Usage Centre ‘Agidel’ at the Institute of Petrochemistry and Catalysis of the Russian Academy of Sciences.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cravino, A.; Sariciftci, N.S. Double-cable polymers for fullerene based organic optoelectronic applications. J. Mater. Chem. 2002, 7, 1931–1943. [Google Scholar] [CrossRef]
- Bingel, C. Cyclopropanierung von Fullerenen. Chem. Berichte 1993, 126, 1957–1959. [Google Scholar] [CrossRef]
- Camps, X.; Hirsch, A. Efficient cyclopropanation of C60 starting from malonates. J. Chem. Soc. Perkin Trans. 1 1997, 11, 1595–1596. [Google Scholar] [CrossRef]
- Hirsch, A.; Vostrowsky, O. C60 Hexakisadducts with an Octahedral Addition Pattern—A New Structure Motif in Organic Chemistry. Eur. J. Org. Chem. 2001, 5, 829–848. [Google Scholar] [CrossRef]
- Yan, W.; Seifermann, S.M.; Pierrat, P.; Bräse, S. Synthesis of highly functionalized C60 fullerene derivatives and their applications in material and life sciences. Org. Biomol. Chem. 2015, 13, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, P.; Hirsch, A. Photoswitchable Norbornadiene–Quadricyclane Interconversion Mediated by Covalently Linked C60. Chem. Eur. J. 2020, 26, 5220–5230. [Google Scholar] [CrossRef] [PubMed]
- Tuktarov, A.R.; Salikhov, R.B.; Khuzin, A.A.; Popod’ko, N.R.; Safargalin, I.N.; Mullagaliev, I.N.; Dzhemilev, U.M. Photocontrolled organic field effect transistors based on the fullerene C60 and spiropyran hybrid molecule. RSC Adv. 2019, 9, 7505–7508. [Google Scholar] [CrossRef] [PubMed]
- Robin, M.; Harnois, M.; Molard, Y.; Jacques, E. Improvement of n-type OTFT electrical stability by gold electrode modification. Org. Electr. 2016, 39, 214–221. [Google Scholar] [CrossRef]
- Zhou, K.; Dong, H.; Zhang, H.L.; Hu, W. High performance n-type and ambipolar small organic semiconductors for organic thin film transistors. Phys. Chem. Chem. Phys. 2014, 16, 22448–22457. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Dong, H.; Hu, W.; Liu, Y.; Zhu, D. Semiconducting π-Conjugated Systems in Field-Effect Transistors: A Material Odyssey of Organic Electronics. Chem. Rev. 2012, 112, 2208–2267. [Google Scholar] [CrossRef] [PubMed]
- Sadretdinova, Z.R.; Akhmetov, A.R.; Salikhov, R.B.; Mullagaliev, I.N.; Salikhov, T.R. 1,2,3-Triazolylfullerene-based n-type semiconductor materials for organic field-effect transistors. Mend. Comm. 2023, 33, 320–322. [Google Scholar] [CrossRef]
- Tuktarov, A.R.; Chobanov, N.M.; Budnikova, Y.H.; Dudkina, Y.B.; Dzhemilev, U.M. Synthesis and electrochemical properties of fullerenylstyrenes. J. Org. Chem. 2019, 84, 16333–16337. [Google Scholar] [CrossRef] [PubMed]
- Tuktarov, A.R.; Chobanov, N.M.; Sadretdinova, Z.R.; Salikhov, R.B.; Mullagaliev, I.N.; Salikhov, T.R.; Dzhemilev, U.M. New n-type semiconductor material based on styryl fullerene for organic field—Effect transistors. Mendeleev Commun. 2021, 31, 641–643. [Google Scholar] [CrossRef]
- Akhmetov, A.R.; Aminov, R.I.; Mullagaliev, I.N.; Salikhov, R.B. Synthesis of hybrid molecules based on strained polycyclic hydrocarbons and fullerene C60: Application of thin films based on them in organic electronics. Russ. J. Gen. Chem. 2023, 9, 1315–1325. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).