Abstract
The increase in the investigation of different self-assembled architectures derived from thiosemicarbazone ligands can be attributed to the numerous functional metallosupramolecular architectures with practical applications that can be obtained. The potentially tetradentate organic bisthiosemicarbazone ligand H2L possesses two flexible bidentate [NS] domains separated by a short and rigid spacer, which could stabilize several metal ions and thus different metallosupramolecular architectures. H2L was prepared by the reaction between two equivalents of N-ethylhydrazinecarbothioamide and one equivalent of 1,1′,1″-(benzene-1,3,5-triyl)tris(ethan-1-one) and was fully studied using several techniques, including X-ray diffraction.
1. Introduction
In recent decades, thiosemicarbazone compounds and their derived metal complexes have attracted particular interest due to their diverse biological properties that they exhibit such as antioxidant [1], antimicrobial [2,3] and cytotoxic activities [4,5]. Moreover, bisthiosemicarbazones are versatile organic compounds due to the several donor atoms that are present in their skeleton. For this reason, this type of organic compound is widely used in the field of Coordination Chemistry with the aim of obtaining metal complexes with diverse applications [6,7].
Our research group has broad experience in using bisthiosemicabazone skeletons to obtain different metallosupramolecular architectures [8,9]. To deepen the study of these architectures, herein we report the design, synthesis and structural characterization of a bisthiosemicarbazone ligand named H2L containing a short and rigid spacer that could be a precursor of helicate- and mesocate-type metallosupramolecular architectures.
2. Experimental Section
2.1. Reactants and Solvents
All solvents, 1,3,5-triacetylbenzene and 4-N-ethyl-3-thiosemicarbazide are commercially available and they were used without further purification.
2.2. Synthesis and Characterization of the Bisthiosemicarbazone Ligand H2L
The bisthiosemicarbazone ligand H2L was prepared by reaction between one equivalent of 1,3,5-triacetylbenzene and two equivalents of 4-N-ethyl-3-thiosemicarbazide (Figure 1). First, 0.502 g (2.46 mmol) of 1,3,5-triacetylbenzene and 0.5864 g (4.92 mmol) of 4-N-ethyl-3-thiosemicarbazide was solved in absolute ethanol (ca. 50 mL) using p-toluensulfonic acid as catalyst. The solution was refluxed under magnetic stirring for 4 h and the resulting solution was evaporated (to a volume of ~20 mL) and cooled to 4 °C until the formation of a yellow solid was observed. This solid was filtered off and then washed with diethyl ether. Yield: 0.868 g (87%) elemental analysis, % theoretical (C18H26N6OS2): C, 53.17; H, 6.45; N, 20.67; S, 15.77; % experimental: C, 50.84; H, 6.64; N, 21.34; S, 15.38; IR spectroscopy (KBr, cm−1): ν(N-H) 3238, ν(C=N) 1644, ν(C=O) 1715, ν(C=S) 1108 and 763; mass spectrometry (ESI+, m/z): 407.46 [H2L + H]+; 1H NMR [DMSO]: 10.12 (s, 2H), 8.18 (d, 2H, J = 2.3 Hz), 7.81 (t, 2H, J = 3.0 Hz), 7.68 (t, 2H, J = 8.6 Hz) 3.51 (m, 4H), 2.01 (s, 6H), 1.53 (t, 6H, J = 6.2) ppm.
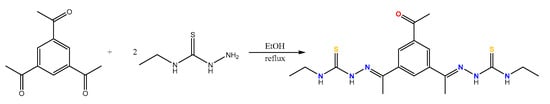
Figure 1.
Synthesis of the bisthiosemicarbazone ligand H2L.
2.3. Crystallographic Data of H2L
H2L: C18H26N6OS2; MW: 406.59 g·mol−1; crystal dimensions 0.24 × 0.16 × 0.14 mm; triclinic; P1; a = 7.4598 (2), b = 11.3924 (3), c = 96.113 (2) Å; α = 96.113 (2); β = 101.988 (10), γ = 98.77 (2) °; V = 989.45 (9) Å3; z = 2; μ = 0.29 mm−1; measured reflections = 14,923; independent reflections [Rint] = 2770 [0.043]; R = 0.055; wR = 0.139.
The main bond distances and angles (Table 1) are within the expected ranges for thiosemicarbazone-derived ligands [10].

Table 1.
Selected bond length and angles for ligand H2L.
3. Results and Discussion
The potentially dianionic and tetradentate bisthiosemicarbazone ligand H2L displays two flexible bidentate [NS] binding domains separated by a short and rigid bencene-derived spacer (Figure 2).
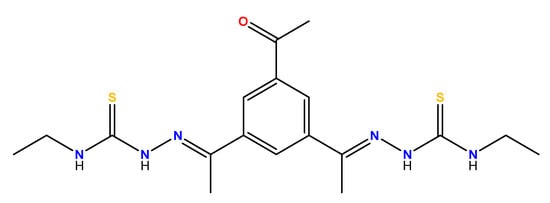
Figure 2.
Bisthiosemicarbazone ligand H2L.
The ligand was obtained by reaction between 1,3,5-triacetylbenzene and 4-N-ethyl-3-thiosemicarbazide in a 1:2 molar ratio, as described in the Experimental Section. The solid product obtained was fully characterized by several techniques. Slow evaporation of the mother liquors from the synthesis of H2L in absolute ethanol allowed us to obtain good-quality crystals for monocrystal X-ray studies. The crystal structure consists of discrete molecules that crystallize in the triclinic P1 system (Figure 3).
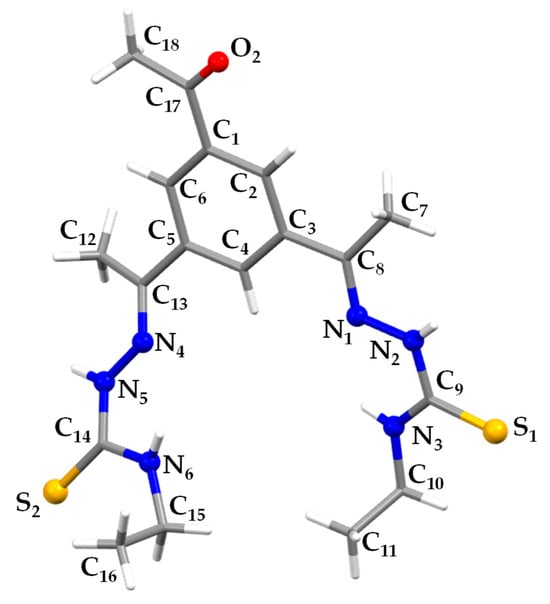
Figure 3.
Crystal structure of H2L.
The thiosemicarbazone arms adopt a syn-close arrangement with both branches directed to the same side and an E conformation in relation to the imine bonds. Thus, the sulfur and nitrogen donor atoms (N1/S1 and N4/S2) are oriented in opposite directions requiring a change in the conformation to form metallosupramolecular helicate- or mesocate-type architectures. The bond distances and angles are in the order of those normally found in thiosemicarbazone ligands and do not merit further discussion.
4. Conclusions
The bisthiosemicarbazone ligand H2L has been successfully synthesized with high purity and yield. Its crystal structure shows that the sulfur and nitrogen donor atoms are oriented in opposite directions in both arms, indicating the need for conformational rotation to coordinate to metal ions and further obtain helicate- and mesocate-type metallosupramolecular architectures.
Author Contributions
Conceptualization, S.F.-F., M.M.-C. and M.M.; methodology, U.B.-S., I.V.-H., M.M.-C., M.M. and S.F.-F.; formal analysis, U.B.-S., I.V.-H. and S.F.-F.; investigation, U.B.-S., I.V.-H., M.M.-C., M.M. and S.F.-F.; resources M.M.; data curation, U.B.-S., I.V.-H., M.M.-C., M.M. and S.F.-F.; writing—original draft preparation, U.B.-S., I.V.-H. and S.F.-F.; writing—review and editing U.B.-S., I.V.-H., M.M.-C., M.M. and S.F.-F.; supervision, M.M.-C, M.M. and S.F.-F.; project administration, M.M.-C and M.M.; funding acquisition, M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the following FEDER co-funded grants. From Consellería de Cultura, Educación e Ordenación Universitaria, Xunta de Galicia, 2018GRCGI-1584 (ED431C2018/13), MetalBIONetwork (ED431D2017/01). From Ministerio de Ciencia e Innovación, Project PID2021-127531NB-I00 (AEI/10.13039/501100011033/FEDER, UE) and METALBIO (RED2022-134091-T).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bingul, M.; Şenkuytu, E.; Saglam, M.F.; Boga, M.; Kandemir, H.; Sengul, I.F. Synthesis, photophysical and antioxidant properties of carbazole-based bis-thiosemicarbazones. Res. Chem. Intermed. 2019, 45, 4487–4499. [Google Scholar] [CrossRef]
- Chikaraishi, N.K.; Onodera, K.; Nakano, S.; Hayashi, K.; Nomiya, K. Syntheses, crystal structures and antimicrobial activities of 6-coordinate antimony(III) complexes with tridentate 2-acetylpyridine thiosemicarbazone, bis(thiosemicarbazone) and semicarbazone ligands. J. Inorg. Chem. 2006, 100, 1176–1186. [Google Scholar]
- Alomar, K.; Landreau, A.; Allain, M.; Bouet, G.; Larcher, G. Synthesis, structure and antifungal activity of thiophene-2,3-dicarboxaldehyde bis(thiosemicarbazone) and nickel(II), copper(II) and cadmium(II) complexes: Unsymmetrical coordination mode of nickel complex. J. Inorg. Biochem. 2013, 126, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Castiñeiras, A.; Fernandez-Hermida, N.; Garcia-Santos, I. Neutral NiII, PdII and PtII ONS-pincer complexes of 5-acetylbarbituric-4Ndimethylthiosemicarbazone: Synthesis, characterization and properties. Dalton Trans. 2012, 41, 13486. [Google Scholar] [CrossRef] [PubMed]
- Anjum, R.; Palanimuthu, D.; Kalinowski, D.S.; Lewis, W.; Park, K.C.; Kovacevic, Z.; Khan, I.U.; Richardson, D.R. Synthesis, Characterization, and In Vitro Anticancer Activity of Copper and Zinc Bis(Thiosemicarbazone) Complexes. Inorg. Chem. 2019, 58, 13709–13723. [Google Scholar] [CrossRef] [PubMed]
- Parrilha, G.L.; dos Santos, R.G.; Beraldo, H. Applications of radiocomplexes with thiosemicarbazones and bis(thiosemicarbazones) in diagnostic and therapeutic nuclear medicine. Coord. Chem. Rev. 2022, 458, 214418. [Google Scholar] [CrossRef]
- Quiroga, A.G.; Ranninger, C.N. Contribution to the SAR field of metallated and coordination complexes: Studies of the palladium and platinum derivatives with selected thiosemicarbazones as antitumoral drugs. Coord. Chem. Rev. 2004, 248, 119–133. [Google Scholar] [CrossRef]
- Martinez-Calvo, M.; Romero, M.J.; Pedrido, R.; Gonzalez-Noya, A.M.; Zaragoza, G.; Bermejo, M.R. Metal self-recognition: A pathway to control the formation of dihelicates and mesocates. Dalton Trans. 2012, 41, 13395. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Fariña, S.; Velo-Heleno, I.; Carballido, R.; Martínez-Calvo, M.; Barcia, R.; Palacios, Ò.; Capdevila, M.; González-Noya, A.M.; Pedrido, R. Exploring the Biological Properties of Zn(II) Bisthiosemicarbazone Helicates. Int. J. Mol. Sci. 2023, 24, 2246. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.J.; Martinez-Calvo, M.; Maneiro, M.; Zaragoza, G.; Pedrido, R.; Gonzalez-Noya, A.M. Selective Metal-Assisted Assembly of Mesocates or Helicates with Tristhiosemicarbazone Ligands. Inorg. Chem. 2019, 58, 881–889. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).