Abstract
A hybrid compound based on (5Z,9Z)-eicosa-5,9-dienoic acid and vanillin was synthesized in high yield (94%) using a new intermolecular cross-cyclomagnesiation reaction of aliphatic and O-containing 1,2-dienes catalyzed by Cp2TiCl2.
1. Introduction
Vanillin (4-hydroxy-3-methoxybenzaldehyde) isolated from orchids (Vanilla planifolia, V. pompona or V. tahitiensis) is attracting attention for several reasons. First, vanillin as a flavoring agent is used in the food, nutraceutical, and pharmaceutical industries. Secondly, vanillin has a simple chemical structure, which can simplify its synthesis to some extent. Third, vanillin has been shown to have various biological activities, such as antitumor, antioxidant, and antimicrobial properties [1,2]. In addition, vanillin exhibited a neuroprotective effect in an experimental model of Huntington’s disease and ischemia [3].
An analysis of the literature showed that vanillin derivatives also exhibit versatile biological activity. In the studies of Boiko Y. A., the analgesic and anti-inflammatory activity of vanillin and its derivatives was established, which is associated with the effect of these substances on the TRPA-1 and TRPV-1 ion channels [4]. Scipioni M. and colleagues synthesized a number of vanillin derivatives and simultaneously demonstrated that their antioxidant activity is similar to the reference antioxidant Trolox [5]. Vanillin derivatives have a high antioxidant potential and a protective effect against oxidative stress in neuroblastoma cells [6]. Li’s research group reported the synthesis of a number of dendrimers from vanillin that have antioxidant properties and protective effects on fatty acids, DNA, and lipoproteins [7]. Vanillin derivatives are also used as multipurpose drugs for the treatment of atopic dermatitis with positive pharmacokinetic and pharmacodynamic results. Mourtzinos and colleagues have shown that carboxylic acid, obtained by oxidation of vanillin, increases its antibacterial effect [8].
Given the high biomedical potential of vanillin derivatives, we put forward the idea of synthesizing a hybrid compound based on 5Z,9Z-eicosa-5,9-dienoic acid and vanillin. We have previously shown that (5Z,9Z)-eicosa-5,9-dienoic acid has a high inhibitory activity of topoisomerases I (hTop1) and II (hTop2α) in vitro [9,10].
2. Results and Discussion
Using the cross-molecular cyclomagnesiation reaction of trideca-1,2-diene 1 with 2-(hepta-5,6-dien-1-yloxy)tetrahydro-2H-pyran 2 with EtMgBr in the presence of a Cp2TiCl2 catalyst gave 2,5-dialkylidenemagnezacyclopentane 3. Acid hydrolysis of cyclomagnesiation product 3 and oxidation of the formed tetrahydropyranyl ether 5Z with the Jones reagent, 9Z-diene 4, leads to 5Z,9Z-eicosadienoic acid 5. The esterification reaction of (5Z,9Z)-eicosa-5,9-dienoic acid 5 with vanillin 6 was carried out in CH2Cl2 at 0 °C in the presence of 4-dimethylaminopyridine and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride; as a result, the target product was obtained with a yield of 94% (Scheme 1).
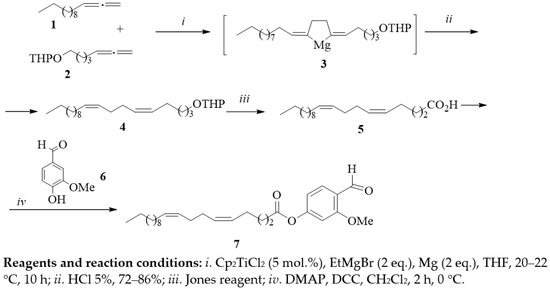
Scheme 1.
Synthesis of a hybrid compound based on (5Z,9Z)-eicosa-5,9-dienoic acid and vanillin.
3. Conclusions
Thus, we have synthesized a hybrid compound based on natural biologically active (5Z,9Z)-eicosa-5,9-dienoic acid and vanillin with high yield (94%), using a new reaction of intermolecular cross-cyclomagnesiation of aliphatic and O-containing 1,2-dienes catalyzed by Cp2TiCl2 at the key stage.
4. Experimental Procedure
4-formyl-3-methoxyphenyl (5Z,9Z)-icosa-5,9-dienoate (7). 1H NMR (400 MHz, CDCl3) δ: 0.90 (t, J = 6.6 Hz, 3H), 1.45–1.28 (m, 16H), 2.03–1.77 (m, 2H), 2.22–2.12 (m, 8H), 265–2.60 (m, 2H), 3.91 (s, 3H), 5.51–5.34 (m, 4H), 7.51–7.20 (m, 3H), 9.96 (s, 1H). 13C NMR (100.62 MHz, CDCl3) δ: 191.03, 171.23, 152.01, 145.08, 135.16, 130.72, 130.60, 128.90, 128.62, 124.75, 123.42, 110.79, 56.04, 33.35, 31.92, 29.74, 29.65, 29.57, 29.50, 29.35, 29.27, 29.03, 27.42, 27.30, 24.85, 22.69, 14.12. MS (MALDI-TOF), m/z: 442 [M]+. C28H42O4. Found (%): C 75.79; H 9.41.Calcd for C28H42O4 (%): C 75.98; H 9.56.
Author Contributions
Conceptualization, U.M.D. and L.U.D.; methodology, A.A.M.; validation, E.K.M., resources, E.K.M.; data curation, U.M.D.; writing—original draft preparation, E.K.M., A.A.M.; writing—review and editing, U.M.D. and L.U.D.; visualization, E.K.M.; supervision, U.M.D.; project administration, A.A.M.; funding acquisition, A.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was done within approved plans for research projects at the IPC RAS State Registration No. FMRS-2022-0075.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sinha, A.K.; Sharma, U.K.; Sharma, N. A comprehensive review on vanilla flavor: Extraction, isolation and quantification of vanillin and others constituents. Int. J. Food Sci. Nutr. 2008, 59, 299–326. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, D.J.; Stratford, M.; Gasson, M.J.; Ueckert, J.; Bos, A.; Narbad, A. Mode of antimicrobial action of vanillin against Escherichia coli, Lactobacillus plant and Listeria innocua. J. Appl. Microbiol. 2004, 97, 104–133. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, D.J.; Stratford, M.; Gasson, M.J.; Narbad, A. Structure-function analysis of the vanillin molecule and its antifungal properties. J. Agric. Food Chem. 2005, 53, 1769–1775S. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Sharma, B. Pharmacological benefits of agomelatine and vanillin in experimental model of Huntington’s disease. Pharmacol. Biochem. Behav. 2014, 122, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Scipioni, M.; Kay, G.; Megson, I.; Kong Thoo Lin, P. Synthesis of novel vanillin derivatives: Novel multi-targeted scaffold ligands against Alzheimer’s disease. MedChemComm 2019, 10, 764–777. [Google Scholar] [CrossRef] [PubMed]
- Blaikie, L.; Kay, G.; Kong Thoo Lin, P. Synthesis and in vitro evaluation of vanillin derivatives as multi-target therapeutics for the treatment of Alzheimer’s disease. Bioorganic Med. Chem. Lett. 2020, 30, 127505. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Sharma, A.; Uzarski, R.L.; Cheong, J.E.; Xu, H.; Held, R.A.; Upadhaya, S.K.; Nelson, J.L. Potent antioxidant dendrimers lacking pro-oxidant activity. Free Radical Biol. Med. 2011, 50, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Mourtzinos, I.; Konteles, S.; Kalogeropoulos, N.; Karathanos, V.T. Thermal oxidation of vanillin affects its antioxidant and antimicrobial properties. Food Chem. 2009, 114, 791–797. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Makarov, A.A.; Dzhemileva, L.U.; Makarova, E.K.; Khusnutdinova, E.K.; Dzhemilev, U.M. The facile synthesis of the 5Z,9Z-dienoic acids and their topoisomerase I inhibitory activity. Chem. Commun. 2013, 49, 8401–8403. [Google Scholar] [CrossRef] [PubMed]
- D’yakonov, V.A.; Dzhemileva, L.U.; Makarov, A.A.; Mulyukova, A.R.; Baev, D.S.; Khusnutdinova, E.K.; Tolstikova, T.G.; Dzhemilev, U.M. nZ,(n+4)Z-Dienoic Fatty Acids: A New Method for the Synthesis and Inhibitory Action on Topoisomerase I and IIα. Med. Chem. Res. 2016, 25, 30–39. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).