4H-[1,3,5,2]Oxadiazaphospholo[3,4-a][1,5]benzodiazepin-1-amine-1-oxides: Synthesis and Computational Studies †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Instrumentations
2.2. Computational Details
3. Results and Discussion
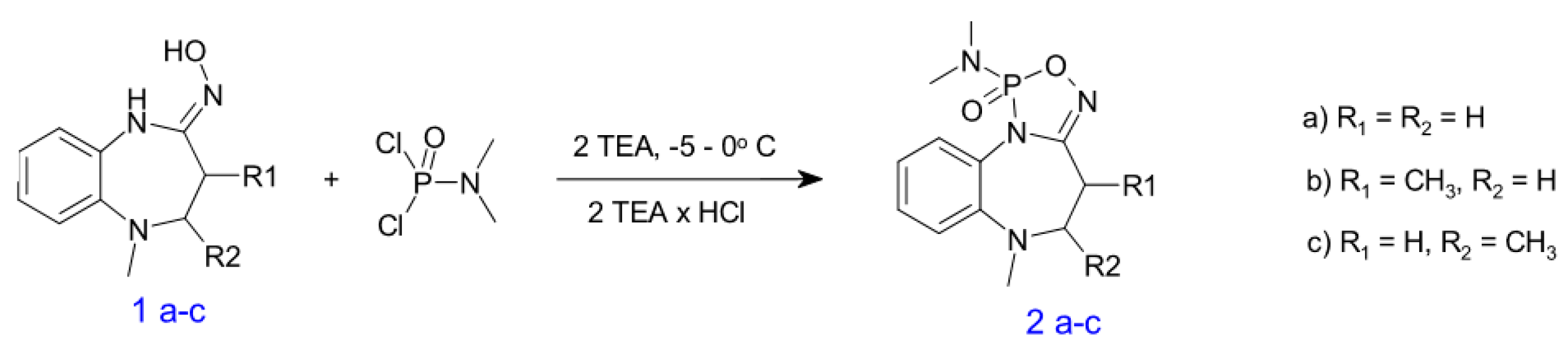
3.1. General Synthetic Procedure
- N,N,6-trimethyl-5,6-dihydro-4H-[1,3,5,2]oxadiazaphospholo[3,4-a][1,5]benzodiazepin-1-amine 1-oxide(2a). White crystals. Yield—17.2%, m.p =163–164 °C (ether); IR, ν: 1609.86 (C=N), 1254.07(P=O), 1004.56 (O-P=O) cm−1; 1H NMR (CDCl3)δ: 2.52 (1H, ddd, J = 7.2 Hz, 11.1 Hz, 14.5 Hz, OCH2); 2.66 (6H, d,JP-H = 10.9 Hz, CH3NCH3); 2.70 (1H, ddd, J = 21 Hz, 6.7 Hz, 14.4 Hz, OCH2); 2.83 (3H, s, CH3N); 3.19 (1H, ddd, J = 2.1 Hz, 7.2 Hz, 10.2 Hz, CH2N); 3.47 (1H, ddd, J = 6.7 Hz, 10.4 Hz, 10.9 Hz CH2N);7.11 (1H, dt, J = 1.4 Hz, 7.6 Hz, H-9); 7.14 (1H, m, H-7); 7.31 (1H, m, H-8); 7.52(1H, m, H-10) ppm; 13C NMR (CDCl3)δ: 24.90 (d, JCP = 3.5 Hz, C-4); 36.25 (d, JCP = 5.5 Hz, C‘-1); 41.61(C-1);56,79 (C-5); 120.75 (C-7); 122.74 (C-8); 123.88 (C-10); 128.00 (C-9); 129.02 (d, JCP = 6.7, C-10a); 142.27 (d, JCP = 4.4 Hz, C-6a);159.96 (d, JCP = 24.9 Hz, C-3a) ppm; 31P NMR (CDCl3) δ = 21.94 ppm.C12H17N4O2P(280,26), Rf = 0.85.Elemental Analysis, %: C, 51.43; H,6.11; N, 19.99; P, 11.05. Found: C, 51.29; H,6.14; N, 20.08; P, 11.09.
- N,N,4,6-tetramethyl-5,6-dihydro-4H-[1,3,5,2]oxadiazaphospholo[3,4-a][1,5]benzodiazepin-1-amine 1-oxide(2b). White crystals. Yield—28.6%, m.p = 158–160 °C (ether); IR, ν:1602.37 (C=N), 1253.89(P=O), 1014.98 (O-P=O) cm−1;1H NMR (CDCl3)δ: 1.22 (3H, d, J = 6.4 Hz, CH3), 2.61 (6H, d, 3JP-H = 10.9 Hz, 2CH3), 2.65–2.74 (1H, m, CH) [centras 2.69], 2.80 (3H, s, CH3), 3.10–3.22 (2H, m, CH2), 7.06–7.58 (4H, m, Ar) ppm;13C NMR (CDCl3)δ: 11.7(4-C); 31.3 (d,3JP-N-C = 3.6 Hz, C-4); 36.3 (2C, 2J = 5.3 Hz, 1-CH3); 41.3 (6-CH3); 65.1 (C-6); 120.2(C-7); 123.0(C-8); 123.8 (C-10); 127.9 (C-9); 128.7 (d, J = 6.5 Hz, C-10a); 143.5 (d, J = 4.9 Hz, C-6a); 162.3 (d, J = 23.6 Hz, C-3a) ppm; 31P NMR (CDCl3) δ = 22.60 ppm.C13H19N4O2P(294,29), Rf = 0.77. Elemental Analysis, %: C, 53.06; H,6.51; N, 19.04; P, 10.52. Found: C, 52.87; H,6.54; N, 19.11; P, 10.58.
- N,N,5,6-tetramethyl-5,6-dihydro-4H-[1,3,5,2]oxadiazaphospholo[3,4-a][1,5]benzodiazepin-1-amine 1-oxide(2c). White crystals. Yield—20.2%, m.p = 172–173 °C (ether); IR, ν: 1604.69 (C=N), 1263.03 (P=O), 1009.25 (O-P=O) cm−1;1H NMR (CDCl3)δ:1.14 (3H, d, J = 7.0 Hz, CH3), 2.78 (6H, d, 3JP-H = 10.8 Hz, 2CH3), 2.77–2.88 (1H, m, CH), 2.79 (3H, s, CH3), 3.10 (1H, dd, 3J = 8.4 Hz, 2J = 10.3 Hz, CH2), 3.30 (1H, dd, 3J = 7.1 Hz, 2J = 10.3 Hz, CH2), 7.03–7.31 (4H, m, Ar) ppm. 13C NMR (CDCl3)δ: 13.4 (5-CH3); 31.0 (d, 3JP-N-C = 3.5 Hz, C-4); 36.1 (2C, d, 2J = 5.3 Hz, 1-CH3); 41.6 (6-CH3); 64.3 (C-5); 120.4 (C-7); 121.6 (C-8); 123.4 (C-10); 127.9(C-9); 128.8 (d, J = 5.7 Hz, C-10a); 144.5 (d, J = 4.0 Hz, C-6a); 162.5 (d, J = 23.4 Hz, C-3a) ppm; 31P NMR (CDCl3) δ = 21.25 ppm. C13H19N4O2P(294.29), Rf = 0.75. Elemental Analysis, %: C, 53.06; H,6.51; N, 19.04; P, 10.52. Found: C, 53.01; H,6.49; N, 19.09; P, 10.46.
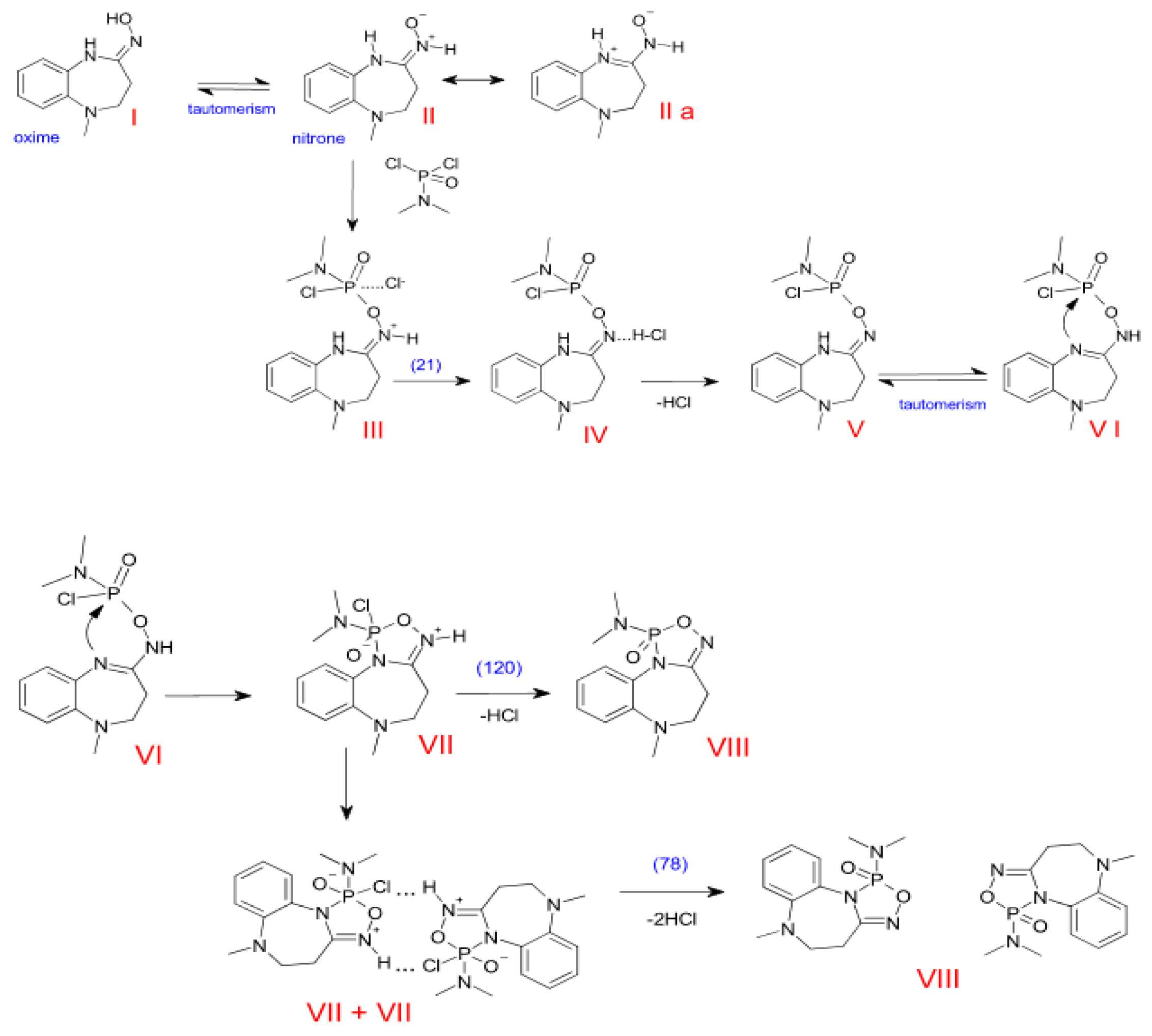
3.2. The Plausible Reaction Pathways
3.3. Prediction of Biological/Drug-like Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arora, N.; Dhiman, P.; Kumar, S.; Singh, G.; Monga, V. Recent advances in synthesis and medicinal chemistry of benzodiazepines. Bioorg. Chem. 2020, 97, 103668. [Google Scholar] [CrossRef] [PubMed]
- Dubovsky, S.L.; Marshall, D. Benzodiazepines remain important therapeutic options in psychiatric practice. Psychother. Psychosom. 2022, 91, 307–334. [Google Scholar] [CrossRef]
- Kosychova, L.; Karalius, A.; Staniulytė, Z.; Sirutkaitis, R.A.; Palaima, A.; Laurynėnas, A.; Anusevičius, Ž. New 1-(3-Nitrophenyl)-5,6-dihydro-4H-[1,2,4]triazolo [4,3-a][1,5]benzodiazepines: Synthesis and Computational Study. Molecules 2015, 20, 5392–5408. [Google Scholar] [CrossRef] [PubMed]
- Zalán, Z.; Martinek, T.; Lazar, L.; Sillanpää, R.; Fulop, F. Synthesis and conformational analysis of tetrahydroisoquinoline- and piperidine-fused 1,3,4,2-oxadiazaphosphinanes, new ring systems. Tetrahedron 2006, 62, 2883–2891. [Google Scholar] [CrossRef]
- Dürüst, Y.; Gözlükaya, Ö.; İvgen, H. Synthesis of some 1,3,5,2-oxadiazaphosphol-2-oxides,3H-1,2,3,5-oxathiadiazol-2-oxides, and 1,3,2-oxazaphospholidn-2-oxides (sulfides). Phosphorus Sulfur Silicon 2014, 189, 215–225. [Google Scholar] [CrossRef]
- Bannwarth, C.; Ehlert, S.; Grimme, S. GFN2-xTB—An Accurate and Broadly Parametrized Self-Consistent Tight-Binding Quantum Chemical Method with Multipole Electrostatics and Density-Dependent Dispersion Contributions. J. Chem. Theory Comput. 2019, 15, 1652–1671. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Wang, H.; Li, C.; Zhang, J.Z.H.; Ji, C. MolGpKa: A web server for small molecule pKa prediction using a graph-convolutional neural network. J. Chem. Inf. Model. 2021, 61, 3159–3165. [Google Scholar] [CrossRef] [PubMed]
- Rudika, A.V.; Dmitrieva, A.V.; Lagunina, A.A.; Filimonova, D.A.; Poroikova, V.V. PASS-based prediction of metabolites detection in biological systems. SAR QSAR Environ. Res. 2019, 30, 751–758. [Google Scholar] [CrossRef]
- Kosychova, L.; Stumbrevičiūtė, Z.; Plečkaitienė, L.; Staniulyte, Z.; Puodžiūnaitė, B.D. Convenient synthesis of novel tetrahydro-1,5-benzodiazepine amide oximes. Chemija 2006, 17, 21–25. [Google Scholar]
- Roca-Lopez, D.; Daru, A.; Tejero, T.; Merino, P. Revisiting oxime–nitrone tautomerism. Evidence of nitrone tautomer participation in oxime nucleophilic addition reactions. RSC Adv. 2016, 6, 22161–22173. [Google Scholar] [CrossRef]
- da Silva, M.M.; Comin, M.; Santos Duarte, T.; Foglio, M.A.; De Carvalho, J.E.; Do Carmo Vieira, M.; Nazari Formagio, A.S. Synthesis, antiproliferative activity and molecular properties predictions of galloyl derivatives. Molecules 2015, 20, 5360–5373. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anusevičius, Ž.; Kosychova, L.; Kairys, V.; Krikštopaitis, K.; Šarlauskas, J. 4H-[1,3,5,2]Oxadiazaphospholo[3,4-a][1,5]benzodiazepin-1-amine-1-oxides: Synthesis and Computational Studies. Chem. Proc. 2023, 14, 22. https://doi.org/10.3390/ecsoc-27-16162
Anusevičius Ž, Kosychova L, Kairys V, Krikštopaitis K, Šarlauskas J. 4H-[1,3,5,2]Oxadiazaphospholo[3,4-a][1,5]benzodiazepin-1-amine-1-oxides: Synthesis and Computational Studies. Chemistry Proceedings. 2023; 14(1):22. https://doi.org/10.3390/ecsoc-27-16162
Chicago/Turabian StyleAnusevičius, Žilvinas, Lidija Kosychova, Visvaldas Kairys, Kastis Krikštopaitis, and Jonas Šarlauskas. 2023. "4H-[1,3,5,2]Oxadiazaphospholo[3,4-a][1,5]benzodiazepin-1-amine-1-oxides: Synthesis and Computational Studies" Chemistry Proceedings 14, no. 1: 22. https://doi.org/10.3390/ecsoc-27-16162
APA StyleAnusevičius, Ž., Kosychova, L., Kairys, V., Krikštopaitis, K., & Šarlauskas, J. (2023). 4H-[1,3,5,2]Oxadiazaphospholo[3,4-a][1,5]benzodiazepin-1-amine-1-oxides: Synthesis and Computational Studies. Chemistry Proceedings, 14(1), 22. https://doi.org/10.3390/ecsoc-27-16162




