Efficient Solvent Extraction of Phenol Using Imidazolium-Based Ionic Liquids †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instruments and Reagents
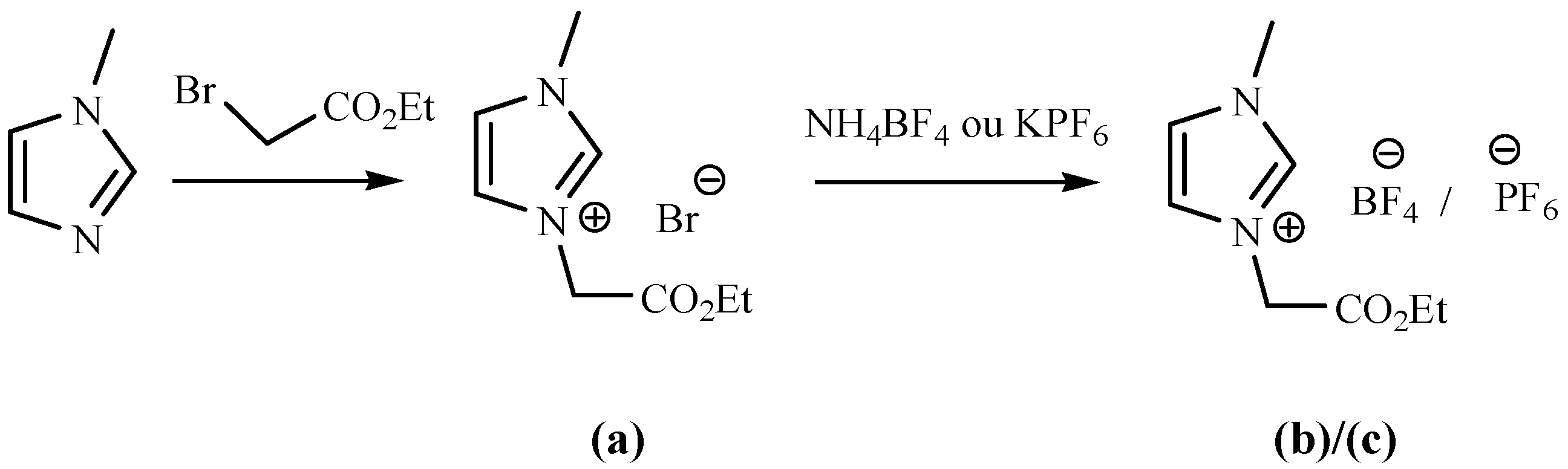
2.2. Synthesis of ILs (a, b and c)
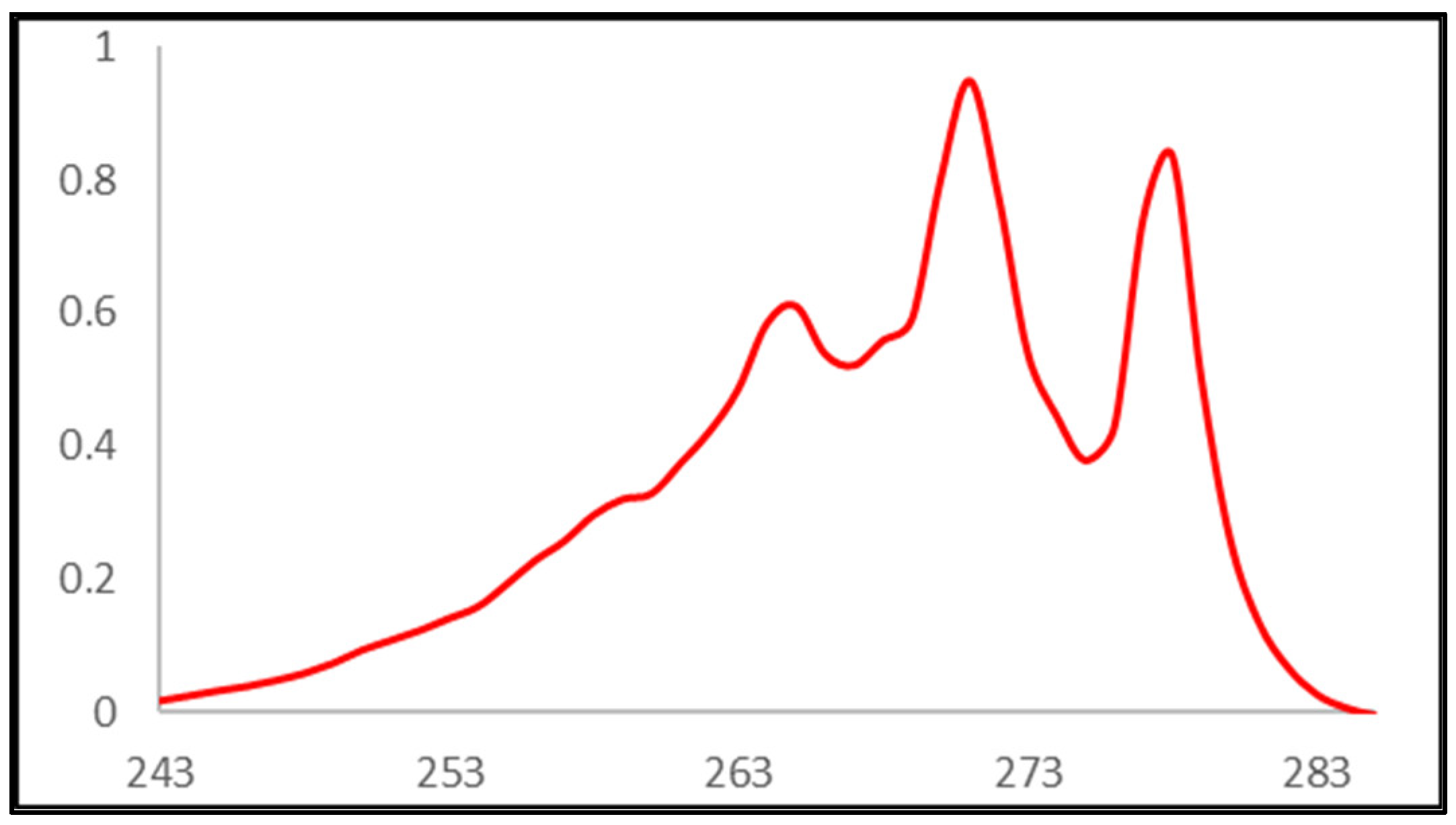
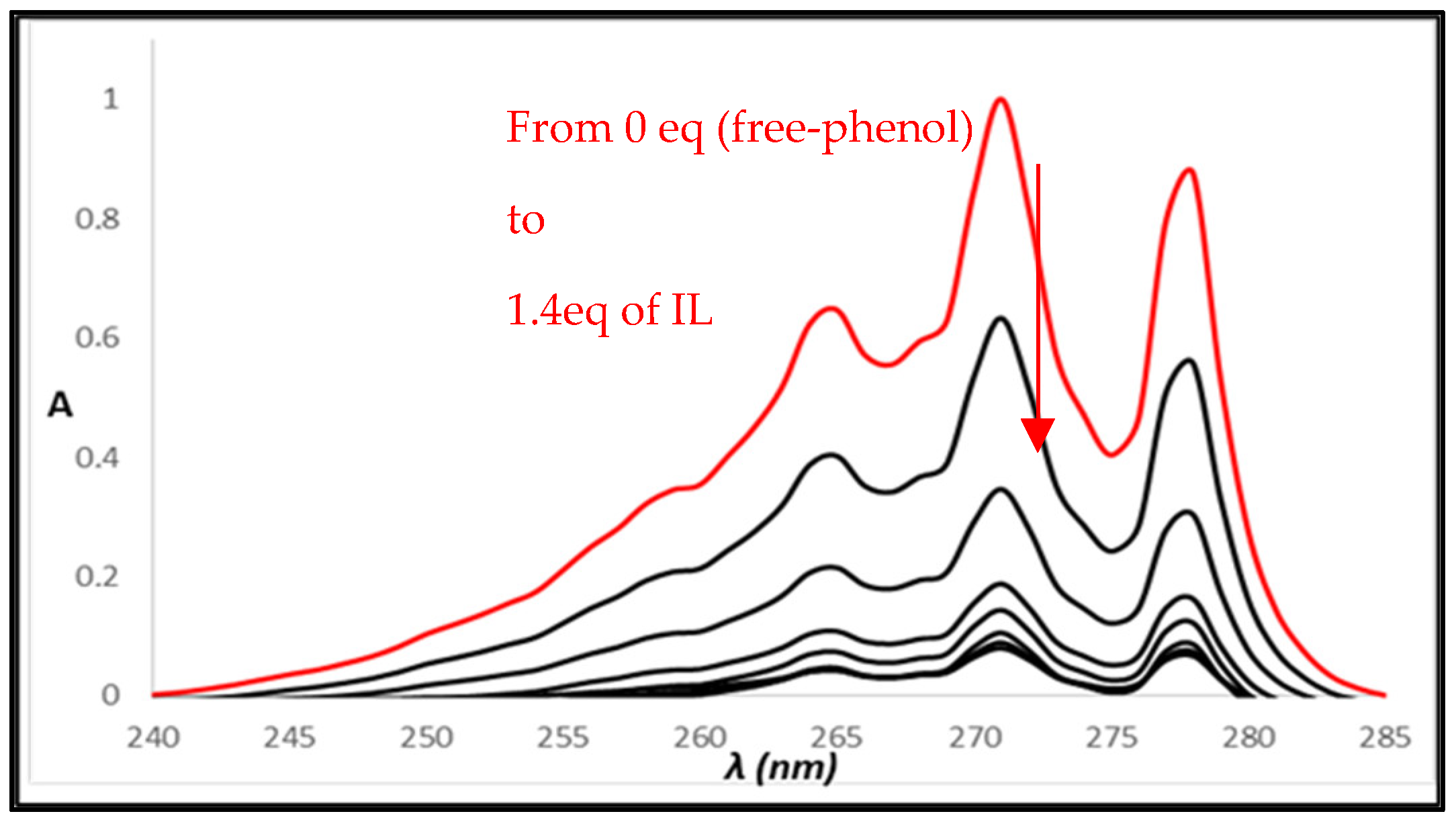
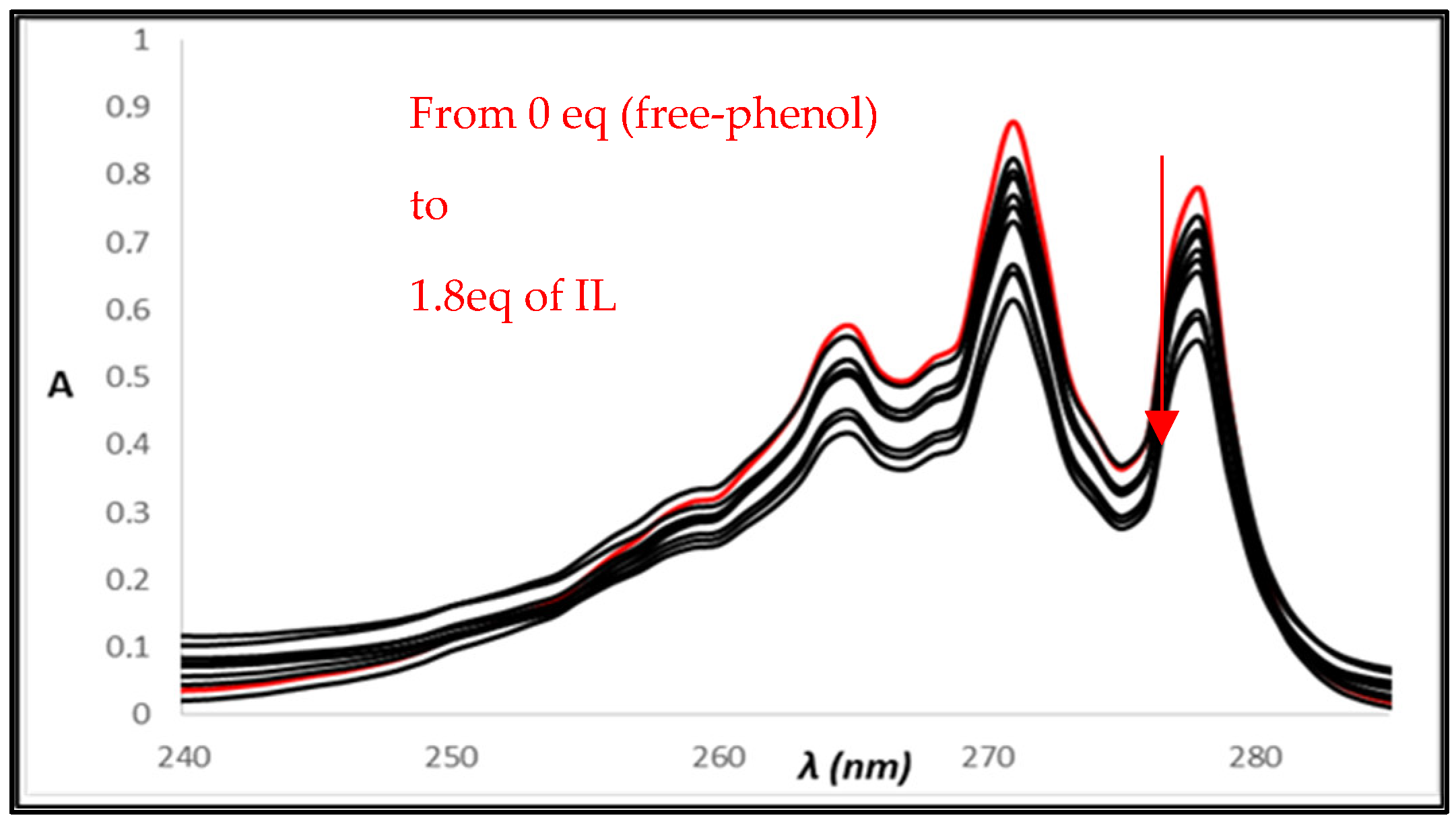
2.3. UV-Vis Titrations
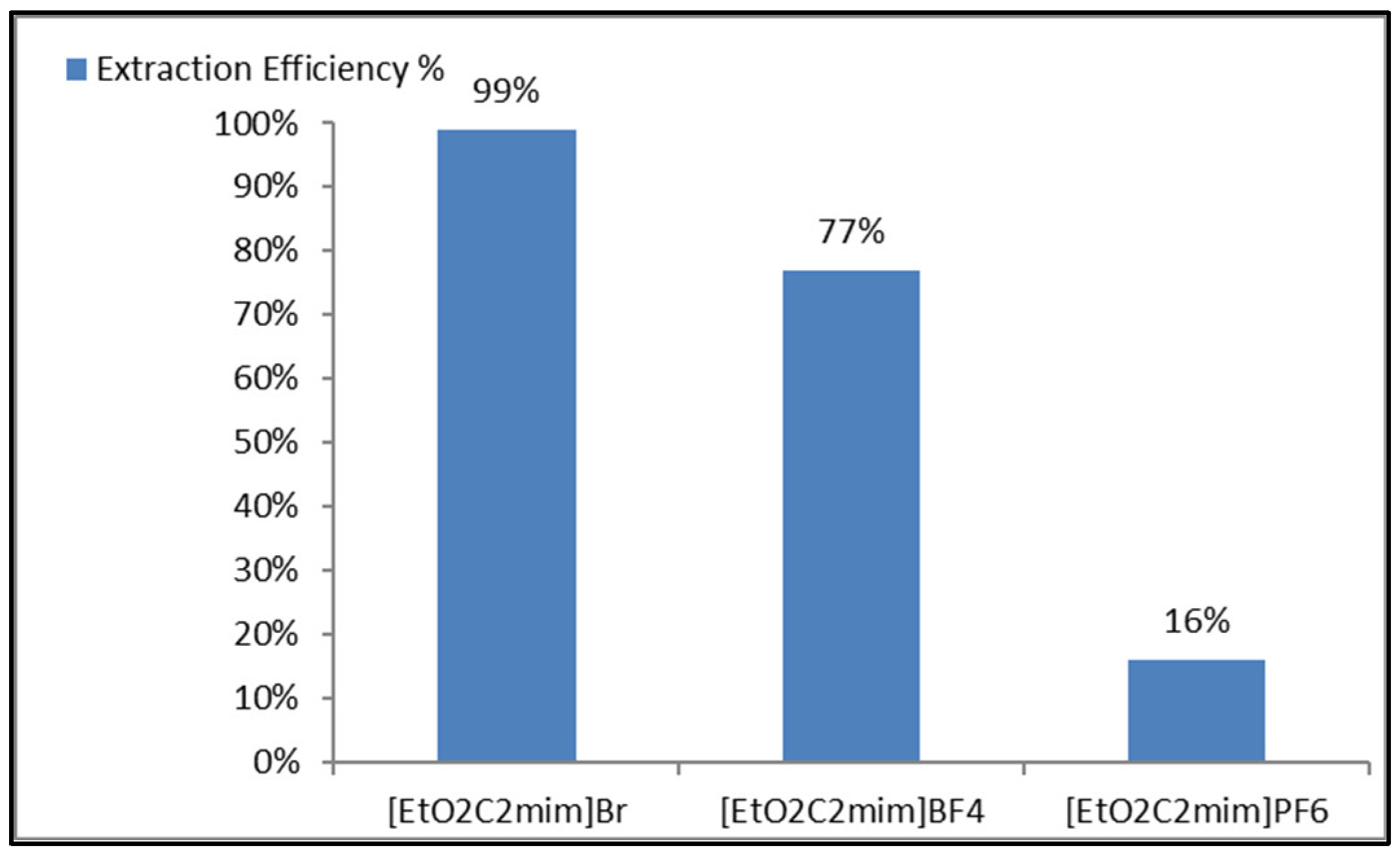
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, A.; Mishra, A.K. Biological importance of phenol derivatives as potent bioactive compound: A review. Lett. Org. Chem. 2018, 15, 251–264. [Google Scholar] [CrossRef]
- Davidson, P.M.; Branden, A.L. Antimicrobial activity of non-halogenated phenolic compounds. J. Food Prot. 1981, 44, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Bruce, R.M.; Santodonato, J.; Neal, M.W. Summary review of the health effects associated with phenol. Toxicol. Ind. Health 1987, 3, 535–568. [Google Scholar] [CrossRef] [PubMed]
- Bukowska, B.; Kowalska, S. The presence and toxicity of phenol derivatives-their effect on human erythrocytes. Curr. Top. Biophys. 2003, 27, 43–51. [Google Scholar]
- Busca, G.; Berardinelli, S.; Resini, C.; Arrighi, L. Technologies for the removal of phenol from fluid streams: A short review of recent developments. J. Hazard. Mater. 2008, 160, 265–288. [Google Scholar] [CrossRef] [PubMed]
- Pang, K.; Hou, Y.; Wu, W.; Guo, W.; Peng, W.; Marsh, K.N. Efficient separation of phenols from oils via forming deep eutectic solvents. Green Chem. 2012, 14, 2398–2401. [Google Scholar] [CrossRef]
- Hou, Y.; Ren, Y.; Peng, W.; Ren, S.; Wu, W. Separation of phenols from oil using imidazolium-based ionic liquids. Ind. Eng. Chem. Res. 2013, 52, 18071–18075. [Google Scholar] [CrossRef]
- Xu, X.; Li, A.; Zhang, T.; Zhang, L.; Xu, D.; Gao, J.; Wang, Y. Efficient extraction of phenol from low-temperature coal tar model oil via imidazolium-based ionic liquid and mechanism analysis. J. Mol. Liq. 2020, 306, 112911. [Google Scholar] [CrossRef]
- Xu, D.; Wang, S.; Zhang, T.; Peng, L.; Bing, X.; Zhang, L.; Wang, Y. Extraction and interaction insights for enhanced separation of phenolic compounds from model coal tar using a hydroxyl-functionalized ionic liquid. Chem. Eng. Res. Des. 2022, 178, 567–574. [Google Scholar] [CrossRef]
- Dupont, J.; Consorti, C.S.; Suarez, P.A.Z.; de Souza, R.F. Preparation of 1-Butyl-3-Methyl Imidazolium-Based Room Temperature Ionic Liquids. Org. Synth. 2002, 79, 236, Erratum in Org. Synth. 2004, 10, 184. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamzi, I.; Bouchakour, S.; Mostefa-Kara, B.; Villemin, D. Efficient Solvent Extraction of Phenol Using Imidazolium-Based Ionic Liquids. Chem. Proc. 2023, 14, 25. https://doi.org/10.3390/ecsoc-27-16151
Hamzi I, Bouchakour S, Mostefa-Kara B, Villemin D. Efficient Solvent Extraction of Phenol Using Imidazolium-Based Ionic Liquids. Chemistry Proceedings. 2023; 14(1):25. https://doi.org/10.3390/ecsoc-27-16151
Chicago/Turabian StyleHamzi, Imane, Souad Bouchakour, Bachir Mostefa-Kara, and Didier Villemin. 2023. "Efficient Solvent Extraction of Phenol Using Imidazolium-Based Ionic Liquids" Chemistry Proceedings 14, no. 1: 25. https://doi.org/10.3390/ecsoc-27-16151
APA StyleHamzi, I., Bouchakour, S., Mostefa-Kara, B., & Villemin, D. (2023). Efficient Solvent Extraction of Phenol Using Imidazolium-Based Ionic Liquids. Chemistry Proceedings, 14(1), 25. https://doi.org/10.3390/ecsoc-27-16151




