Abstract
2-pyridones are among the most widely used nitrogen-containing heterocyclic derivatives in various fields due to their structural properties and biological and therapeutic activities. Therefore, their synthesis has become a major focus of organic chemistry in recent years. In this context, we are interested in the synthesis of N-alkyl-3-cyano-2-pyridones and their derivatives. This work describes a new synthetic method that allows easy and efficient access to these structures under mild conditions through a simple study of their fluorescence applications.
1. Introduction
Heterocyclic compounds represent the largest and most diverse family of organic compounds [1]. Today, there are many heterocyclic compounds; it is known that their number is increasing rapidly day by day due to extensive synthesis research and their synthetic benefits [2]. Heterocycle compounds play a role in most scientific fields such as medicinal chemistry, biochemistry, and other scientific fields [3]. There are many heterocyclic compounds, especially nitrogen heterocyclic compounds; they represent an important and unique class in the applied branch of organic chemistry [4]. A large amount of research has been devoted to the development of new molecular composite materials and has contributed to the development of a lot of organic synthesis protocols finding abundant applications in the chemical sciences [5,6]. Numerous N-heterocyclic compounds that are generally found in nature have both physiological and pharmacological properties and are part of many important biological molecules, particularly 2-pyridone [7,8].
2-pyridone and its derivatives have attracted widespread attention due to their wide application in many fields such as fluorescence [9,10]. Moreover, the 2-pyridone core is common in many natural products as well as synthetically useful compounds that exhibit various biological and therapeutic activities [11,12]. These wide utilities and waves of interest in the 2-pyridone as a key heterocycle, motivated us to look for new developments in the synthesis of 2-pyridone in recent years [13].
In our work, we report the synthesis of a series of 3-cyano-2-pyridone derivatives (Figure 1) via a simple and convenient protocol.
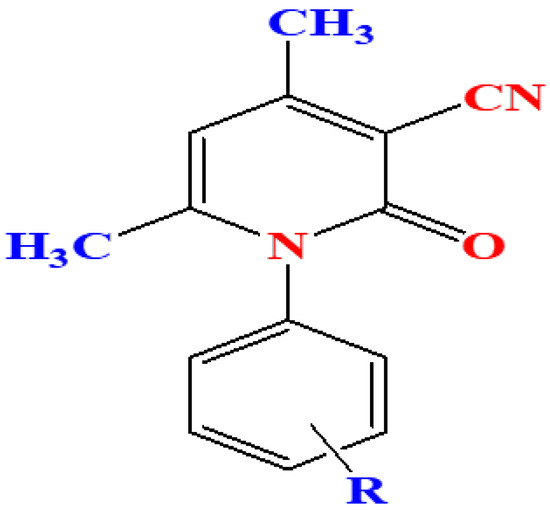
Figure 1.
General structure of 3-cyano-2-pyridone derivatives.
2. Results and Discussion
In this work, we report the synthesis of 3-cyano-2-pyridone derivatives in two steps.
2.1. Synthesis of N-Alkylated-2-cyanoacetamide Derivatives
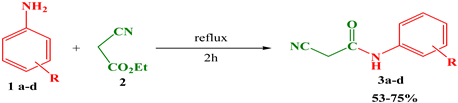
The starting point for our new synthetic approach is to synthesize N-alkylated-2-cyanoacetamide derivatives 3a–d by the reaction of substituted anilines 1a–d and ethyl cyanoacetate 2 refluxed at high temperature for 2h, with the desired products being obtained with good yields (Table 1).

Table 1.
Synthesis of N-alkyled-2-cyanoacetamide derivatives.
The structure of the synthesized compounds 3a–d was confirmed by spectral analysis; the IR spectra (KBr, ν, cm−1) showed the appearance of CN at 2255–2258 cm−1, NH at 3182–3303 cm−1, and CO at 1663–1680 cm−1, the 1H NMR spectra (CDCl3, δ, ppm) showed the appearance of NH stretching at 7.90–7.95 ppm, CH2 stretching at 3.56–3.62 ppm, and HAr at 6.3–7.5 ppm.
2.2. Synthesis of 3-Cyano-2-pyridone Derivatives
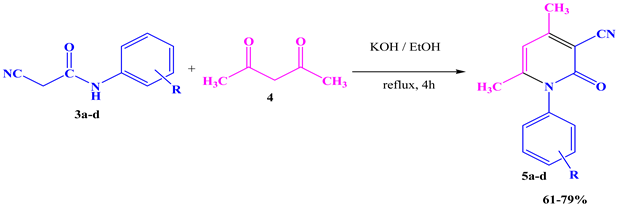
We have synthesized a series of 3-cyano-2-pyridone derivatives 5a–d using the cyanoacetamide 3a–d previously synthesized in the first step with acetylacetone 4 under reflux in the presence of KOH as a base and ethanol as a solvent for 4h. This strategy led to the 3-cyano-2-pyridone derivatives being obtained in excellent yields (Table 2).

Table 2.
Synthesis of 3-cyano-2-pyridone derivatives.
The synthesized compounds 5a–d was confirmed by spectroscopic analysis; the IR spectra (KBr, ν, cm−1) showed the appearance of CN at 2215–2216 cm−1, and CO at 1653–1674 cm−1, the 1H NMR spectra (CDCl3, δ, ppm) showed the appearance of CH stretching at 6.04–6.32 ppm, methyl groups at 2.19–2.20 ppm, and HAr at 7.19–7.82 ppm.
3. Experimental Procedures
3.1. General Synthesis of N-Alkylated-2-cyanoacetamide Derivatives 3a–d
The N-alkylated-2cyanoacetamide derivatives 3a–d were obtained by the reaction between 0.02 mol of anilines derivatives 1a–d and 0.02 mol of ethyl cyanoacetate 2; the reaction mixture was refluxed at high temperature for 2h, and the progress of the reaction was monitored by TLC. The solid obtained was filtered and washed with diethyl ether and ethanol to afford the desired product in good yields (53–75%).
3.2. General Synthesis of 3-Cyano-2-pyridone Derivatives 5a–d
The products 5a–d were prepared using 0.006 mol of cyanoacetamide derivatives 3a–d with 0.006 mol of acetylacetone 4 in the presence of a low quantity of KOH and about 10 mL of ethanol as a solvent; the reaction mixture was stirred and refluxed at 80 °C for 4 h according to TLC, and the precipitate formed after cooling was collected by filtration and washed with ethanol. The required products were obtained with excellent yields (61–79%).
4. Fluorescence Studies of Our 3-Cyano-2-pyridone Derivatives
With the aim of evaluating the interest of our synthesized products 5a–d, we decided to study their molecular fluorescence in three different concentrations, namely 10−4, 10−5 and 10−6, using DCM as a solvent. The fluorescence emission spectra obtained show the influence of the concentration on the intensity (Figure 2).
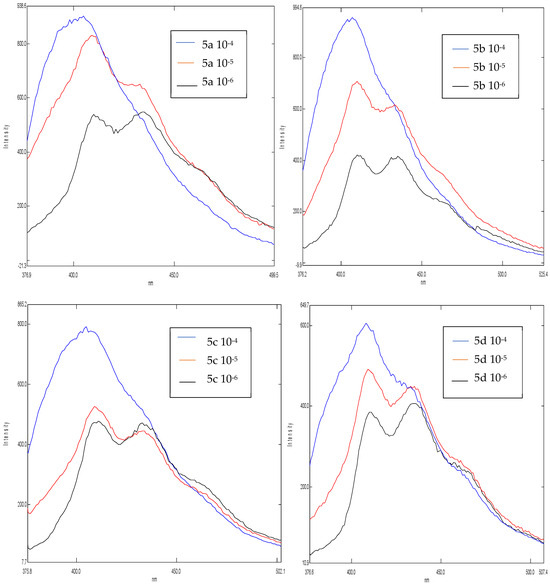
Figure 2.
Emission spectra of the products 5a–d.
We note that the maximum intensity for all products at 10−4 M varies between 610 and 990 u.a and the low intensity at 10−6 M varies between 400 and 530 u.a. Therefore, we conclude that there is a positive correlation between concentration and intensity; when the concentration is decreased, the intensity is decreased with it.
5. Conclusions
We have succeeded in developing a simple, rapid, and efficient synthesis route for 3-cyano-2-pyridone derivatives; this process includes some advantages such as mild operation conditions, simple reactants, and excellent yields. To evaluate the interest of our synthesized products, we studied the influence of the concentration on the intensity of their fluorescence, and we noticed that the best intensity at a concentration of 10−4 corresponds to the product 5b.
Author Contributions
Conceptualization, N.C.-B. and A.K.; validation, F.B., Z.K., J.A.S. and M.P.V.T.; formal analysis, A.K.; investigation, A.K.; writing—original draft preparation, A.K.; writing—review and editing, N.C.-B.; supervision, N.C.-B. All authors have read and agreed to the published version of the manuscript.
Funding
The study is supported by the General Directorate for the Scientific Research and Technological Development (DGRSDT), and the Universities of Tlemcen and Ain Temouchent.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest, financial or otherwise.
References
- Bhattacherjee, D.; Zyryanov, G.V.; Das, P. Recent advances in the synthetic approaches to 2-pyridones (microreview). Chem. Heterocycl. Compd. 2020, 56, 1152–1154. [Google Scholar] [CrossRef]
- Kabir, E.; Uzzaman, M. A review on biological and medicinal impact of heterocyclic compounds. Results Chem. 2022, 4, 100606. [Google Scholar] [CrossRef]
- Amer, M.M.; Aziz, M.A.; Shehab, W.S.; Abdellattif, M.H.; Mouneir, S.M. Recent advances in chemistry and pharmacological aspects of 2-pyridone scaffolds. J. Saudi Chem. Soc. 2021, 25, 101259. [Google Scholar] [CrossRef]
- Bayat, M.; Nasri, S.; Notash, B. Synthesis of new 3-cyanoacetamide pyrrole and 3-acetonitrile pyrrole derivatives. Tetrahedron 2017, 73, 1522–1527. [Google Scholar] [CrossRef]
- Majumdar, P.; Pati, A.; Patra, M.; Behera, R.K.; Behera, A.K. Acid hydrazides, potent reagents for synthesis of oxygen-, nitrogen-, and/or sulfur-containing heterocyclic rings. Chem. Rev. 2014, 114, 2942–2977. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Kishore, D.; Paliwal, S.; Chauhan, R.; Dwivedi, J.; Mishra, A. Transition metal-free one-pot synthesis of nitrogen-containing heterocycles. Mol. Divers. 2016, 20, 185–232. [Google Scholar] [CrossRef] [PubMed]
- Menon, P.K.; Krishnaraj, K.; Anabha, E.; Devaky, K.; Thomas, S.P. Synthesis, crystal structure and electron density analysis of a sulfanyl 2-pyridone analogue: Tautomeric preference and conformation locking by S··· O chalcogen bonding. J. Mol. Struct. 2020, 1222, 128798. [Google Scholar] [CrossRef]
- Bai, H.; Sun, R.; Chen, X.; Yang, L.; Huang, C. Microwave-Assisted, Solvent-Free, Three-Component Domino Protocol: Efficient Synthesis of Polysubstituted-2-Pyridone Derivatives. ChemistrySelect 2018, 3, 4635–4638. [Google Scholar] [CrossRef]
- Kasprzyk, W.; Świergosz, T.; Koper, F. Fluorescence assay for the determination of d-panthenol based on novel ring-fused 2-pyridone derivative. Int. J. Mol. Sci 2020, 21, 8386. [Google Scholar] [CrossRef] [PubMed]
- Ershov, O.V.; Fedoseev, S.V.; Ievlev, M.Y.; Belikov, M.Y. 2-Pyridone-based fluorophores: Synthesis and fluorescent properties of pyrrolo [3, 4-c] pyridine derivatives. Dyes Pigment. 2016, 134, 459–464. [Google Scholar] [CrossRef]
- Hamama, W.S.; Waly, M.; El-Hawary, I.; Zoorob, H.H. Developments in the chemistry of 2-pyridone. Synth. Commun. 2014, 44, 1730–1759. [Google Scholar] [CrossRef]
- Hirano, K.; Miura, M. A lesson for site-selective C–H functionalization on 2-pyridones: Radical, organometallic, directing group and steric controls. Chem. Sci 2018, 9, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Asmaa, K.; Fatima, B.; Zahira, K.; Noureddine, C.-B. Ten Years of Progress in the Synthesis of 2-Pyridone Derivatives via Three/Four Component Reaction. Mini-Rev. Org. Chem 2023, 20, 358–371. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).