1. Introduction
Environmental pollution is one of the main concerns of contemporary research.
The content of all metal ions becomes toxic when it exceeds the acceptable threshold.
The recognition of toxic metal ions is receiving particular attention, as they cause serious damage to human health and the environment [
1,
2].
Over the last few decades, several small-molecule-based fluorescence chemosensors have been developed [
3].
There is a wide range of highly electronegative fluorescent heteroaromatics (nitrogen, oxygen, etc.) [
4]. In addition, pyridine ring molecules are used as nonlinear optical materials, electrical materials and chelating agents in metal ligand chemistry [
5].
From the literature, it is clear that among the fluorescent sensors developed for cation detection, those sensitive to mercury, lead, chromium, nickel, cobalt and thallium are of crucial interest due to their high toxicity [
1,
3,
6,
7,
8].
In the present work, we report on the synthesis of chemosensors containing the pyrinidic moiety as the fluorophore unit, so we have studied their influence on the detection of toxic cations, including chromium, iron, cobalt, nickel and copper, in acetonitrile/water (1:1) mixtures given the importance of developing water-soluble probes for metal detection in biological and environmental systems.
2. Materials and Methods
These fluorometric chemosensors were analyzed at room temperature. The fluorescence excitation wavelength was set at 432 nm. All ligands were prepared in acetonitrile, maintaining a concentration of 10−6 M. Metal salt solutions were prepared by diluting the solutions with distilled water. The samples to be analyzed were prepared from a mixture of acetonitrile/water (1:1) at a concentration of 1 × 10−6 M for compound 2 and at a concentration of 1 × 10−4 M for the ions studied.
Salts used were as follows:
CrCl2 6H2O, CoCl2 6H2O, CuCl2, AlCl3 6H2O, NiCl2, FeCl2, FeCl2 4H2O, CaCl2, ZnCl2, FeCl3, HgCl2, BaCl2 2H2O.
Fe2(SO4)3 9H2O, CuSO4, CuSO4 5H2O, MnSO4, BaSO4, ZnSO4, NiSO4, MgSO4 H2O, HgSO4.
3. Results and Discussion
3.1. Synthesis of 2-Amino-3-cyanopyridine Derivatives from Alkenes
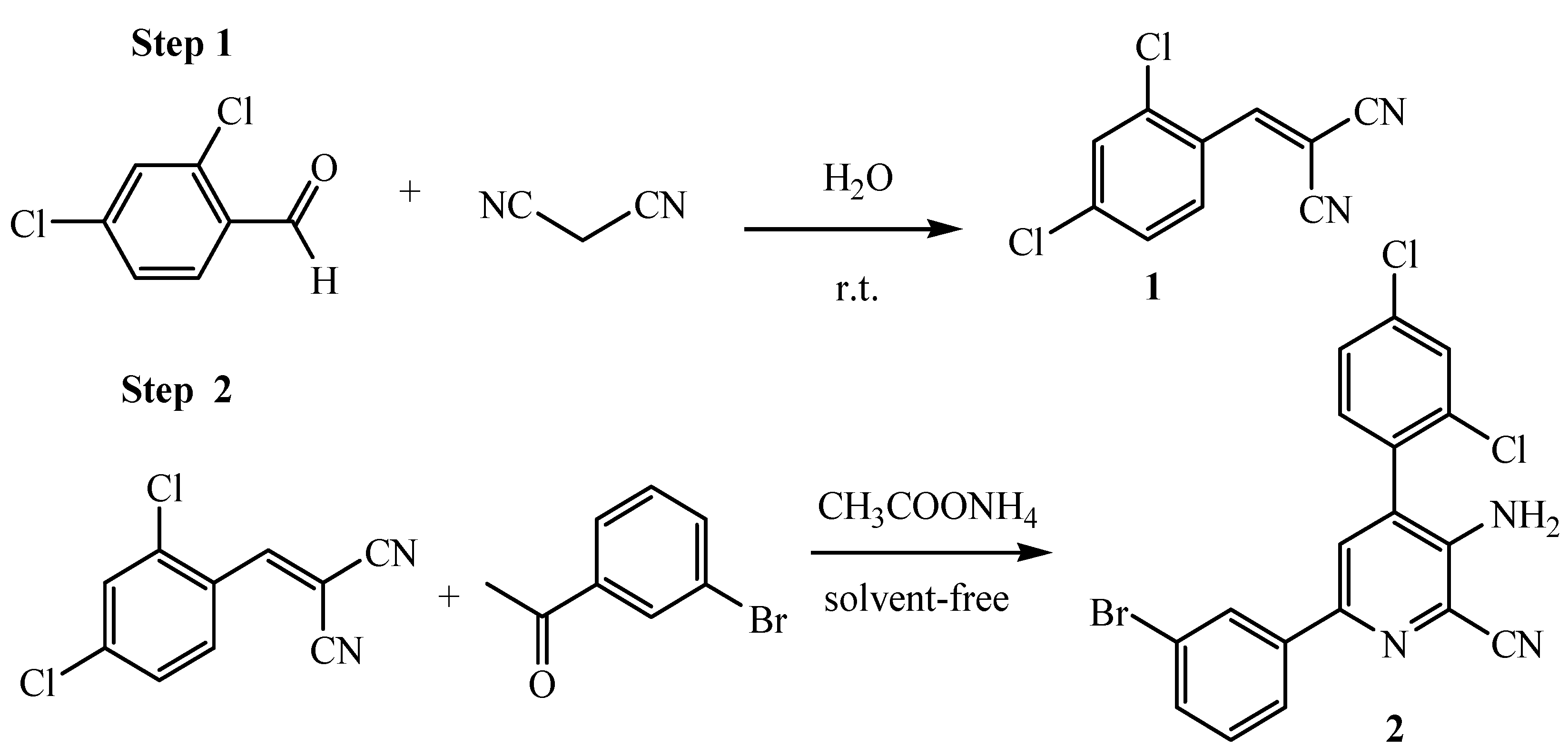
The first route involves the preparation of alkene
1 from Knoevenagel condensation between aromatic aldehyde and malononitrile in water at room temperature. Next, 2-amino-3-cyanopyridine derivative
2 was obtained by reacting alkene, acetophenone derivative and ammonium acetate. The reaction took place by conventional solvent-free heating (
Scheme 1). The reaction mixtures were subsequently washed with diethyl ether and a small amount of ethanol. The crude product was purified by recrystallization in ethanol to give the product in good yield.
Compound 2 was evaluated as a fluorometric chemosensor, and we evaluated its sensitivity and selectivity in the presence of several metals: Hg2+, Mg2+, Ba2+, Ca2+, Mn2+, Cu2+, Cr2+, Co2+, Ni2+, Zn2+, Fe2+, Fe3+ and Al3+.
3.2. Fluorometry Analysis
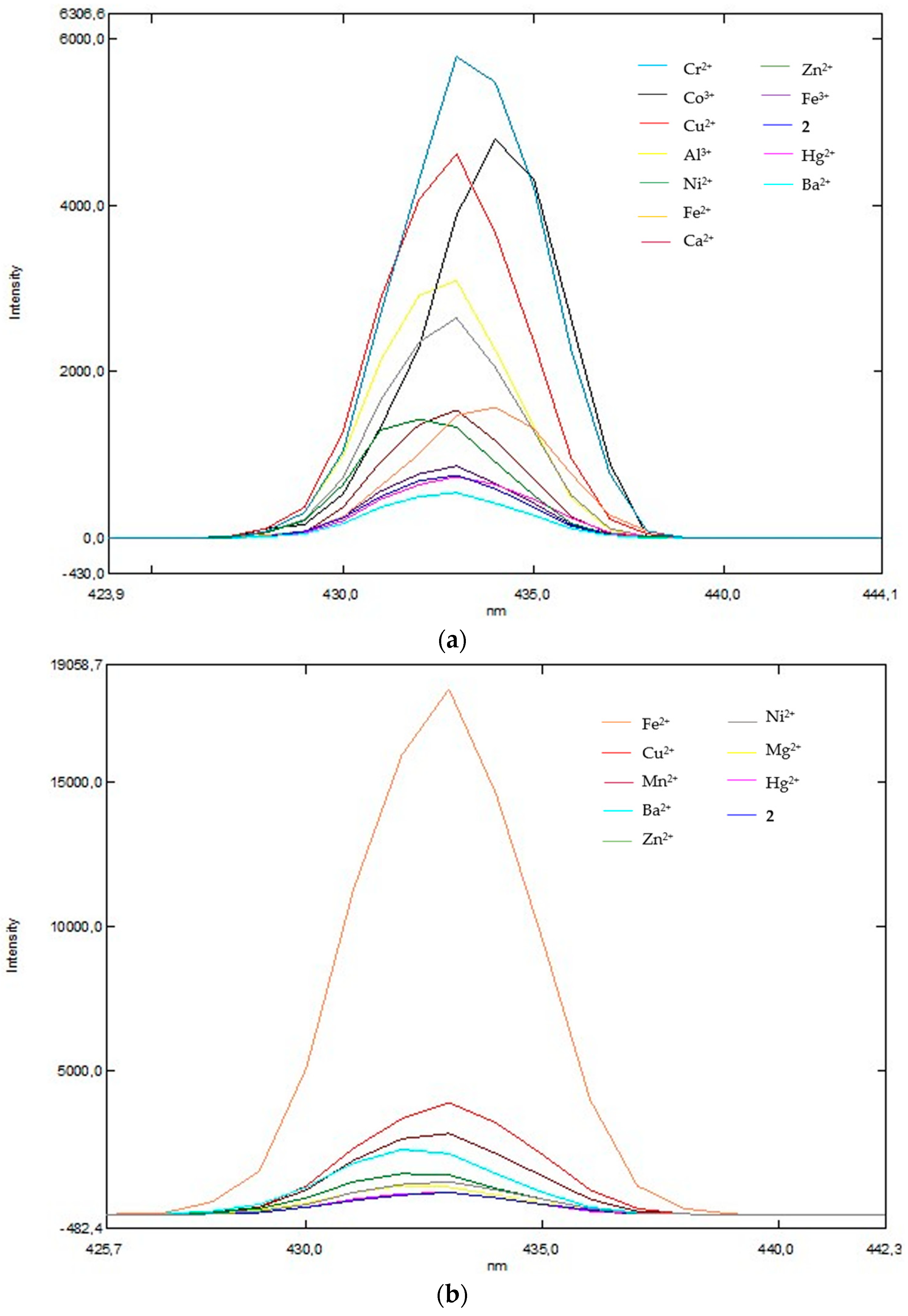
The results obtained imply that compound
2 does indeed act as a chemoselective agent for Cr
2+ ions in the presence of a chloride ion (
Figure 1a). As expected, our chemosensors had different fluorometric responses towards the different cations studied: compound
2 interacted with Cr
2+, Co
2+ and Cu
2+, revealing remarkable fluorescence intensity at 5796.4, 4626.2 and 4324.4 a.u., respectively, while compound
2 interacting with Ba
2+ revealed fluorescence quenching (
Figure 1a). In addition, we studied the fluorescence behavior associated with the detection of iron with different degrees of oxidation (Fe(II) and Fe(III)). Similarly, compound
2 revealed sensitivity and selectivity for these two ions, with a fluorescence intensity of 1578.7 a.u. for Fe
2+ and 862.5 a.u. for Fe
3+ in the presence of a chloride ion (
Figure 1a). In contrast, for the same compound, the addition of Fe
3+ resulted in a significant deviation, reaching 18.208 a.u. in the presence of sulfate as a counter ion (
Figure 1b). Compound
2 in the presence of cations such as Cu
2+, Mn
2+, Ba
2+, Zn
2+, Ni
2+ and Mg
2+ showed an increase in fluorescence intensity compared to the ligand. No decrease in fluorescence intensity was observed in the presence of sulfate ions (
Figure 1b).
4. Conclusions
Compound 2 revealed good selectivity and sensitivity for the detection of Cr2+, Co2+ and Cu2+ compared with the other metals studied; this ligand has chelating power with Fe3+, and it gave rise to a significant deviation in fluorescence intensity with sulfate as a counter ion. From this study, we conclude that product 2 can be considered an original fluoro-ionophore due to its good selectivity and sensitivity to the cations studied as well as to their appropriate counter ions.
Relevant fluorometric responses of compound 2 were observed for the detection of transition metals in acetonitrile/water (1:1) mixtures. However, given the importance of water-soluble probes, the development of this selective sensor will enable many real challenges in the recognition of toxic trace metals in different biological and environmental samples to be solved quickly and easily.
Binding to metal ions is probably attributed to the nitrogen atoms of pyridine moieties as donors. The size and charge of metal ions also affect fluorescence absorption.
Enhancing or reducing fluorescence absorption makes it easier to detect a particular metal ion than many others.
Author Contributions
Conceptualization; methodology; formal analysis, I.B.-A.; software, F.N.; R.H. and I.B.-A.; validation, I.B.-A., R.H. and F.N.; data curation, I.B.-A. and R.H.; writing—original draft preparation, I.B.-A.; writing—review and editing, I.B.-A.; visualization; supervision, Z.K.; N.C.-B., J.A.S. and M.P.V.-T.; project administration, N.C.-B., Z.K. and I.B.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors wish to thank the General Directorate for Scientific Research and Technological Development (DGRSDT), and the University of Tlemcen, the University of Ain Témouchent.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dutta, M.; Das, D. Recent developments in fluorescent sensors for trace-level determination of toxic-metal ions. Trends Anal. Chem. 2012, 32, 113–132. [Google Scholar] [CrossRef]
- Poomalai, S.; Kannan, A.; Sivaraj, P.; Selvan, T.; Mosae, G.; Paulraj, S. A simply synthesized biphenyl substituted piperidin-4-one for the fluorescence chemosensing of Cd2+. Luminescence 2017, 32, 1405–1410. [Google Scholar] [CrossRef] [PubMed]
- Koner, R.R.; Sinha, S.; Kumar, S.; Nandi, C.K.; Ghosh, S. 2-Aminopyridine derivative as fluorescence “On-Off” molecular switch for selective detection of Fe3+/Hg2+. Tetrahedron Lett. 2012, 53, 2302–2307. [Google Scholar] [CrossRef]
- Cheon, J.D.; Mutai, T.; Araki, K. Tuning of fluorescence properties of aminoterpyridine fluorophores by N-substitution. Org. Biomol. Chem. 2007, 5, 2762–2766. [Google Scholar] [CrossRef] [PubMed]
- Khalifeh, R.; Ghamari, M. A Multicomponent Synthesis of 2-Amino-3-cyanopyridine Derivatives Catalyzed by Heterogeneous and Recyclable Copper Nanoparticles on Charcoal. J. Braz. Chem. Soc. 2016, 27, 759–768. [Google Scholar] [CrossRef]
- Wu, D.; Chen, L.; Lee, W.; Ko, G.; Yin, J.; Yoon, J. Recent progress in the development of organic dye based near-infrared fluorescence probes for metal ions. Coord. Chem. Rev. 2017, 354, 74–97. [Google Scholar] [CrossRef]
- Rasheed, T.; Bilal, M.; Nabeel, F.; Iqbal, M.N.; Li, C.; Zhou, Y. Science of the Total Environment Fluorescent sensor based models for the detection of environmentally-related toxic heavy metals. Sci. Total Environ. 2018, 615, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Prabpal, J.; Vilaivan, T.; Praneenararat, T. Paper-Based Heavy Metal Sensors from the Concise Synthesis of an Anionic Porphyrin: A Practical Application of Organic Synthesis to Environmental Chemistry. J. Chem. Educ. 2017, 94, 1137–1142. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).