New Catalytic Systems Used for Wastewater Treatment †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Catalyst Preparation
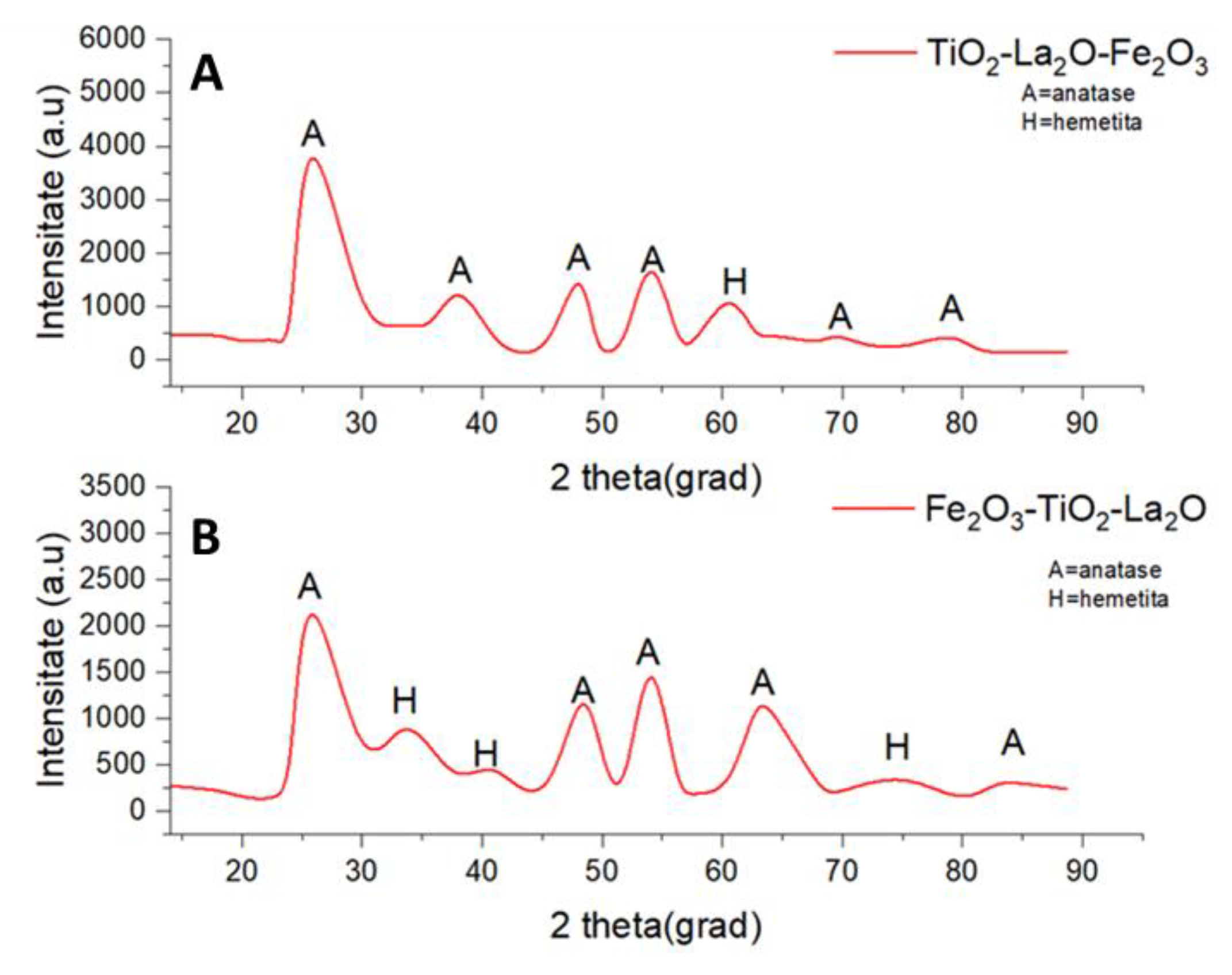
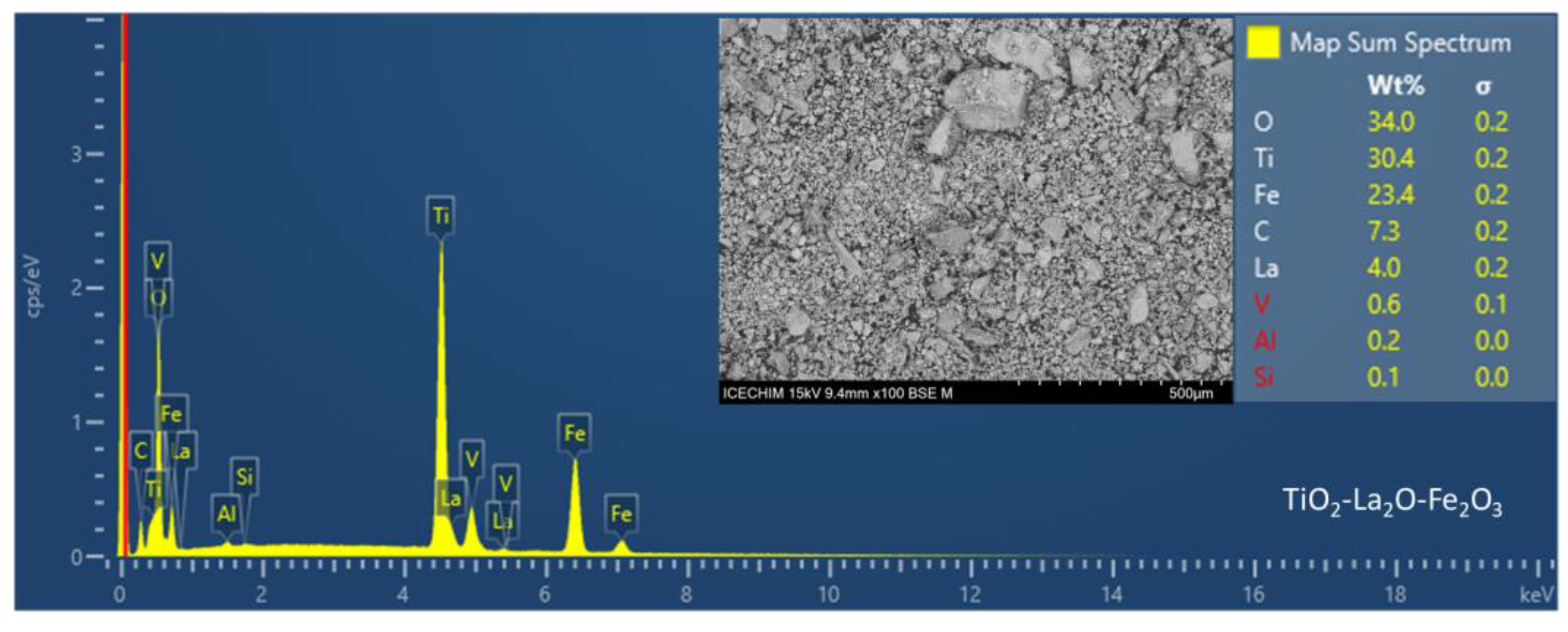
2.3. Catalyst Characterization
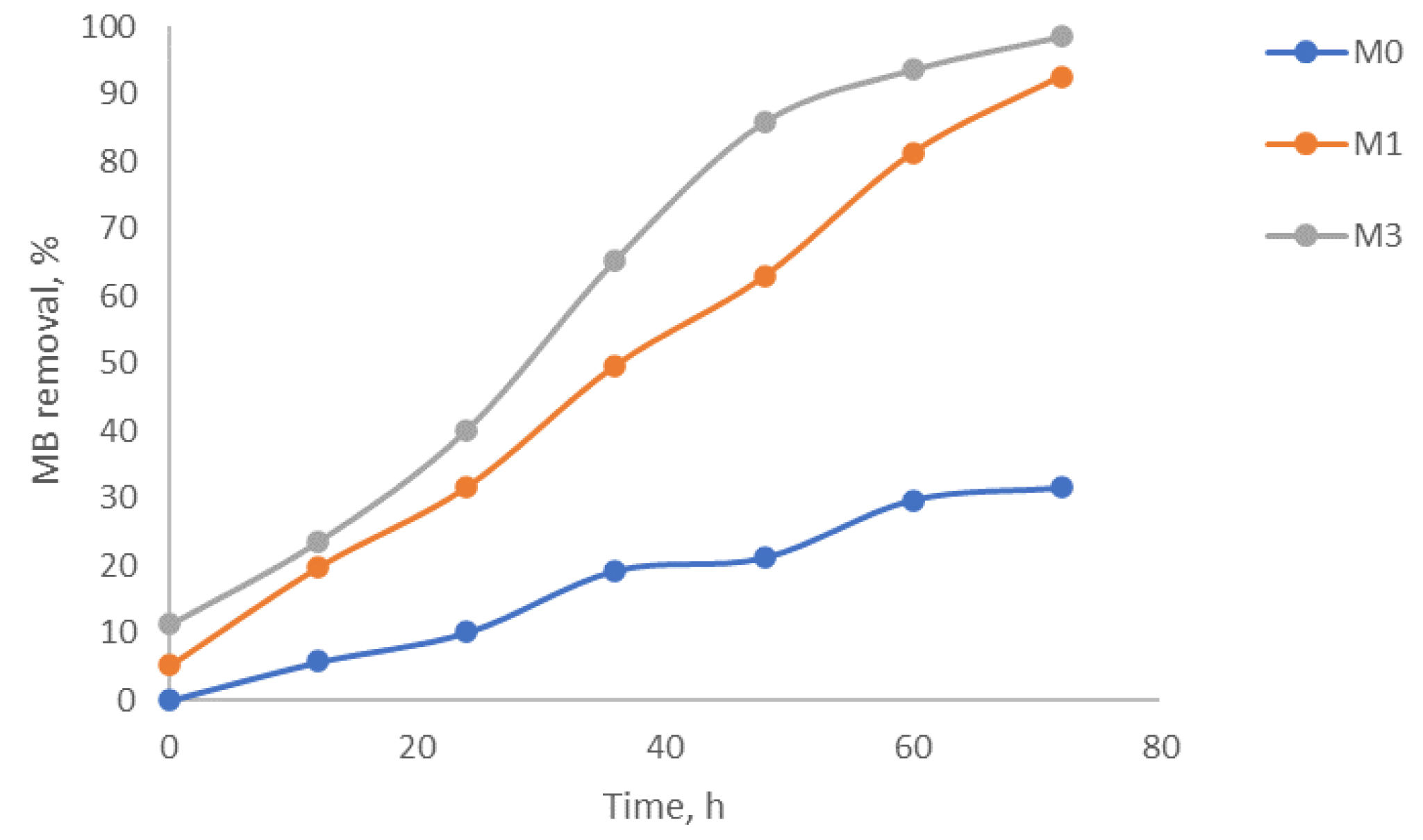
2.4. Photocatalytic Degradation Preliminary Tests
3. Results and Discussion
Catalyst Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, S.; Wang, J.; Zhang, Y.; Ma, J.; Huang, L.; Yu, S.; Chen, L.; Song, G.; Qiu, M.; Wang, X. Applications of water-stable metal-organic frameworks in the removal of water pollutants: A review. Environ. Pollut. 2021, 291, 118076. [Google Scholar] [CrossRef] [PubMed]
- Chandak, S.; Ghosh, P.K.; Gogate, P. Treatment of real pharmaceutical wastewater using different processes based on ultrasound in combination with oxidants. Process Saf. Environ. Prot. 2020, 137, 149–157. [Google Scholar] [CrossRef]
- Meili, L.; Soletti, J. Electrochemical process and Fenton reaction followed bylamellar settler to oil/surfactant effluent degradation. J. Water Process. Eng. 2019, 31, 100841. [Google Scholar]
- Sudhaik, A.; Raizada, P.; Khan, A.A.P.; Singh, A.; Singh, P. Graphitic carbon nitride-based upconversion photocatalyst for hydrogen production and water purification. Nanofabrication 2022, 7, 280–313. [Google Scholar] [CrossRef]
- Kumar, A.; Raizada, P.; Singh, P.; Saini, R.V.; Saini, A.K.; Hosseini-Bandegharaei, A. Perspective and status of polymeric graphitic carbon nitride-based Z-scheme photocatalytic systems for sustainable photocatalytic water purification. Chem. Eng. J. 2020, 391, 123496. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Macak, J.M.; Zlamal, M.; Krysa, J.; Schmuki, P. Self-organized TiO2 nanotube layers as highly efficient photocatalysts. Small 2007, 2, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Tiple, A.; Sinhmar, P.S.; Gogate, P.R. Improved direct synthesis of TiO2 catalyst using sonication and its application for the desulfurization of thiophene. Ultrason. Sonochem. 2021, 73, 105547. [Google Scholar] [CrossRef] [PubMed]
- Pinjari, D.V.; Prasad, K.; Gogate, P.R.; Mhaske, S.T.; Pandit, A.B. Synthesis of titanium dioxide by ultrasound assisted sol–gel technique: Effect of calcination and sonication time. Ultrason. Sonochem. 2015, 23, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Enascuta, C.-E.; Sirbu, E.-E.; Fierascu, R.C.; Ganciarov, M.; Psenovschi, G.; Vlaicu, A. Catalytic System with the Structure of Metal Oxides for the Treatment of Traces of Waste Water Residues. Patent Application no. A0339, 29 June 2023. [Google Scholar]
- Guijarro, C. Reforming of Tar Model Compounds Using Iron Catalysts. Degree Project in Chemical Engineering. Master’s Thesis, KTH Royal Institute of Technology, Stockholm, Sweden, 2020. [Google Scholar]
- Sudhagar, S.; Kumar, S.S.; Premkumar, I.I.; Vijayan, V.; Venkatesh, R.; Rajkumar, S.; Singh, M. UV- and visible-light-driven TiO2/La2O3 and TiO2/Al2O3 nanocatalysts: Synthesis and enhanced photocatalytic activity. Appl. Phys. A 2022, 128, 282. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enascuta, C.-E.; Sirbu, E.-E.; Fierascu, R.C.; Pasarin, D.; Ghizdareanu, A.-I.; Psenovschi, G.; Ganciarov, M. New Catalytic Systems Used for Wastewater Treatment. Chem. Proc. 2023, 13, 19. https://doi.org/10.3390/chemproc2023013019
Enascuta C-E, Sirbu E-E, Fierascu RC, Pasarin D, Ghizdareanu A-I, Psenovschi G, Ganciarov M. New Catalytic Systems Used for Wastewater Treatment. Chemistry Proceedings. 2023; 13(1):19. https://doi.org/10.3390/chemproc2023013019
Chicago/Turabian StyleEnascuta, Cristina-Emanuela, Elena-Emilia Sirbu, Radu Claudiu Fierascu, Diana Pasarin, Andra-Ionela Ghizdareanu, Grigore Psenovschi, and Mihaela Ganciarov. 2023. "New Catalytic Systems Used for Wastewater Treatment" Chemistry Proceedings 13, no. 1: 19. https://doi.org/10.3390/chemproc2023013019
APA StyleEnascuta, C.-E., Sirbu, E.-E., Fierascu, R. C., Pasarin, D., Ghizdareanu, A.-I., Psenovschi, G., & Ganciarov, M. (2023). New Catalytic Systems Used for Wastewater Treatment. Chemistry Proceedings, 13(1), 19. https://doi.org/10.3390/chemproc2023013019






