1. Introduction
Bone tissue disorders reflect a serious health condition with a strong impact on the quality of life, especially in elderly patients. Currently, although there are various ways to repair bone defects, most of the time their resolution involves a bone graft. However, these grafts present a series of limitations such as: reduced sources and complications following the removal of bone tissue, in the case of autograft, or problems related to immunogenicity and risk of disease transmission, in the case of allograft [
1,
2].
To overcome these limitations, attention has been directed to new alternatives for repairing bone defects, such as synthetic bone substitutes. Synthetic bone substitutes are part of ceramic materials class whose chemical structure is similar to the mineral phase of natural bone, among which the most used are: calcium sulfate, calcium phosphate (hydroxyapatite, tricalcium phosphate), bioglass or their combinations [
3,
4].
Calcium silicate is a ceramic material that has attracted attention in the field of bone tissue engineering, due to its excellent bioactivity and biocompatibility properties. Another important property, especially in the case of a porous scaffold type material, is bioresorbability [
5].
The disadvantages of calcium silicate have relatively poor mechanical properties, especially fracture resistance, and rapid degradation rate when presented as a scaffold. This rapid degradation rate can cause the 3D porous scaffold to collapse before the extracellular matrix (ECM) of the bone tissue is formed. Moreover, excessive degradation products can increase the pH of the environment, which can affect cell integrity. Studies have shown that magnesium ion doping can improve these inconveniences. Moreover, magnesium can promote osteogenesis as it is the fourth most abundant element in bone structure, [
6,
7].
Therefore, the aim of this work was to obtain a biomaterial (scaffold type) based on calcium silicate doped with magnesium ions, with superior properties, for bone regeneration with potential applications in tissue engineering, as an alternative to classical methods of bone regeneration.
2. Materials and Methods
2.1. Materials
The raw materials used to prepare scaffolds based on calcium silicate doped with magnesium ions were TEOS—tertaethylorthosilicate (C8H20O4Si, purity 98%), calcium nitrate tetrahydrate (Ca(NO3)2×4H2O, purity 99–103%), magnesium nitrate hexahydrate (Mg(NO3)2×6H2O, purity 99–102%), nitric acid (HNO3, 65% in H2O), purchased from Sigma-Aldrich Chemie GmbH (Eschenstrasse 5, D-82024 Taufkirchen, Deutschland). Furthermore, a commercial polyurethane sponge was selected to obtain scaffolds by replicating a polymer sponge.
2.2. Methods
In order to obtain scaffolds based on simple CaSiO3 and doped with magnesium, a combined method was used, sol-gel and the polymeric sponge replication method. In the first step, the sol-gel method was approached, in which the TEOS was added to distilled water under continuous stirring (600 rpm), at room temperature. Afterwards, nitric acid (HNO3) was added to adjust the pH of the mixture to about 1–2. After approximately 1 h, during which the hydrolysis process took place, calcium nitrate tetrahydrate (Ca(NO3)2×4H2O) and magnesium nitrate (Mg(NO3)2×6H2O) dissolved in the previously formed mixture, resulting a soil.
In the second step, the polyurethane sponges were immersed in the obtained soil, gently drained to remove the excess and dried in an oven at 80 °C for about 50 min. This process was repeated 6 (CS6, CS6_Mg5, CS6_Mg10) and 8 (CS8, CS8_Mg5, CS8_Mg10) times. A final important step in approaching this synthesis method consisted in burning the polymer sponge at a temperature of 400 °C and sintering the structure at a temperature of 1200 °C. Three types of scaffolds were obtained, simple and doped with 5 and 10% Mg
2+, respectively. In
Table 1, the sample codes and their composition are specified.
2.3. Characterization Techniques
The composition and crystallinity of the structures obtained after heat treatment from 1200 °C were evaluated by X-ray diffraction (XRD), performed with a Shimadzu XRD 6000 diffractometer (Shimadzu, Kyoto, Japan), with CuKα (1.5406 Å) radiation filtered with Ni, 2 theta in the range 10–60° with a scan step of 0.02° and a count time of 0.6 s/step.
The microstructure of the scaffolds was evaluated by scanning electron microscopy (SEM) using a FEG Quanta Inspect F50 electron microscope with a resolution of 1.2 nm (Thermo Fisher, Eindhoven, The Netherlands); the scaffolds being covered with a thin layer of gold.
3. Results and Discussion
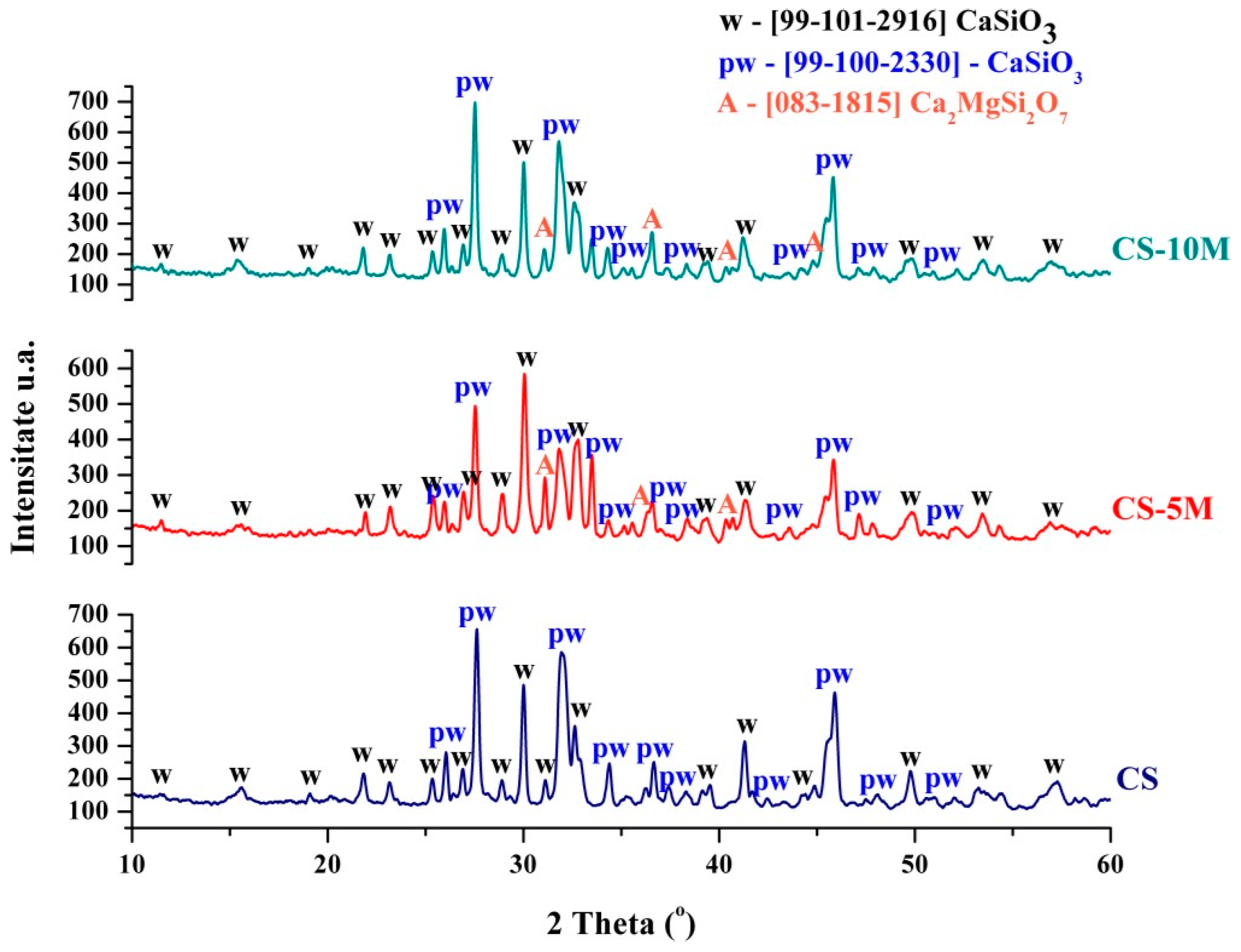
Figure 1 shows the diffractograms made on the scaffolds obtained from calcium silicate and calcium silicate doped with 5 and 10 molar % magnesium ions, respectively. As can be seen, the application of thermal treatment led to the crystallization of several mineralogical phases in the form of calcium silicates. In all three diffractograms, the presence of two polymorphic forms of calcium silicate is identified—CaSiO
3 (99-101-2916) with wollastonite polymorphic structure and CaSiO
3 (99-100-2330) with pseudo-wollastonite polymorphic structure, according to the ASTM sheet. In the samples doped with 5 and 10 molar magnesium ions, in addition to the two polymorphic forms of calcium silicate, a third compound is also identified, akermanite Ca
2MgSi
2O
7, according to the ASTM sheet (083-1815). Thus, we can conclude that through the approached synthesis and the appropriate thermal treatment, calcium silicate with the two polymorphic forms (wollastonite and pseudo-wollastonite) and also the doping of calcium silicate with magnesium ions was successfully obtained, aspects proven by the formation of akermanite.
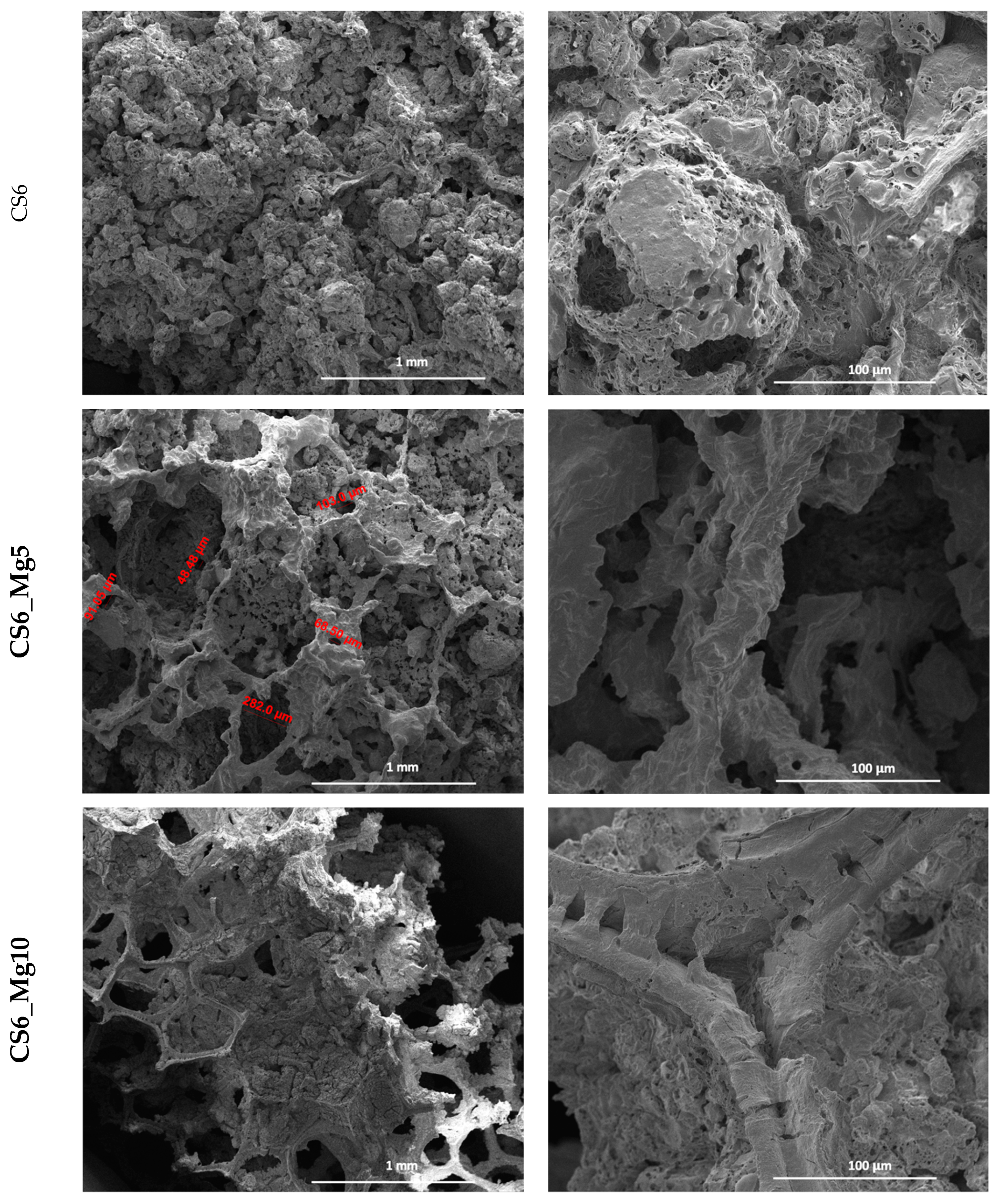
SEM micrographs taken on the scaffolds based on simple CaSiO
3 and doped with 5 and 10% Mg
2+ (
Figure 2 and
Figure 3) show a porous structure of the scaffolds, interconnected, with quasi-spherical pore morphology and variable sizes.
In
Figure 2, of the 6-fold impregnated simple calcium silicate scaffold (CS6), the pore size can vary between 76 and 141 µm. It should also be mentioned that at lower magnifications, pores with sizes of up to 50 µm can also be observed; they are distributed on the scaffold pore bridges.
In the case of scaffolds based on calcium silicate doped with 5% Mg2+, impregnated six times (CS6_Mg5), the pores are better defined, interconnected, and their size can vary between 48 and 202 µm. The pore size increased in this case.
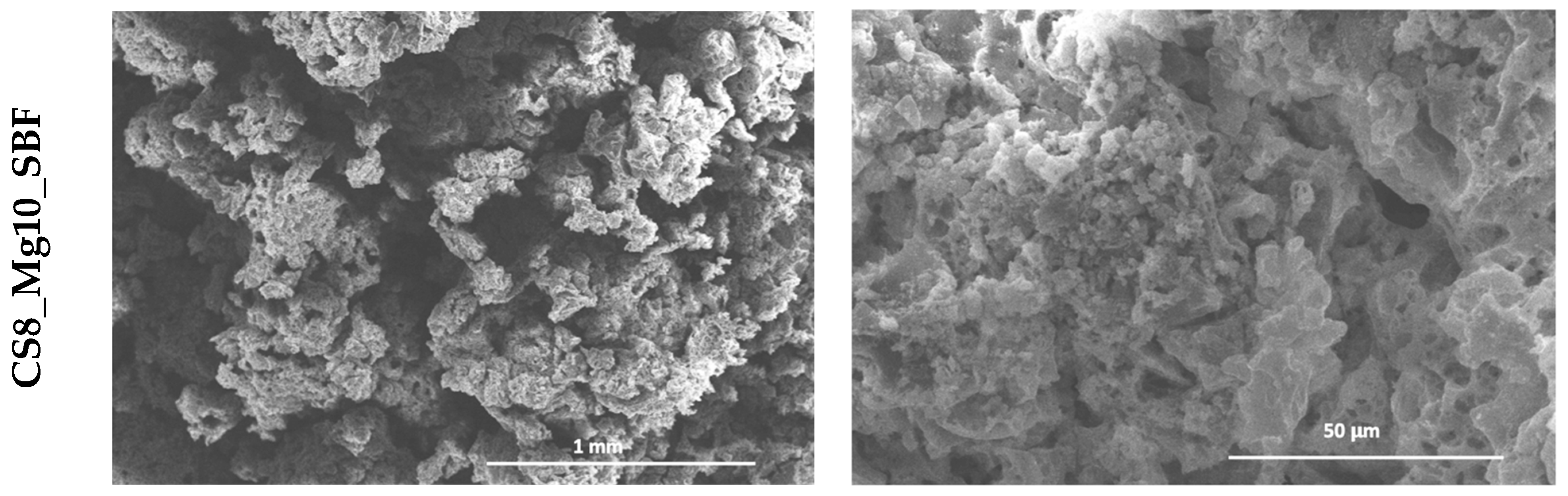
In the scaffolds based on calcium silicate doped with 10% Mg2+, impregnated six times (CS6_Mg10), the pores are also well highlighted, equally well defined and interconnected, with varying sizes between 250 and 500 µm. The pore size has also increased in this case.
We can conclude that Mg2+ doping improved the pore morphology and led to structures with a slightly larger pore size.
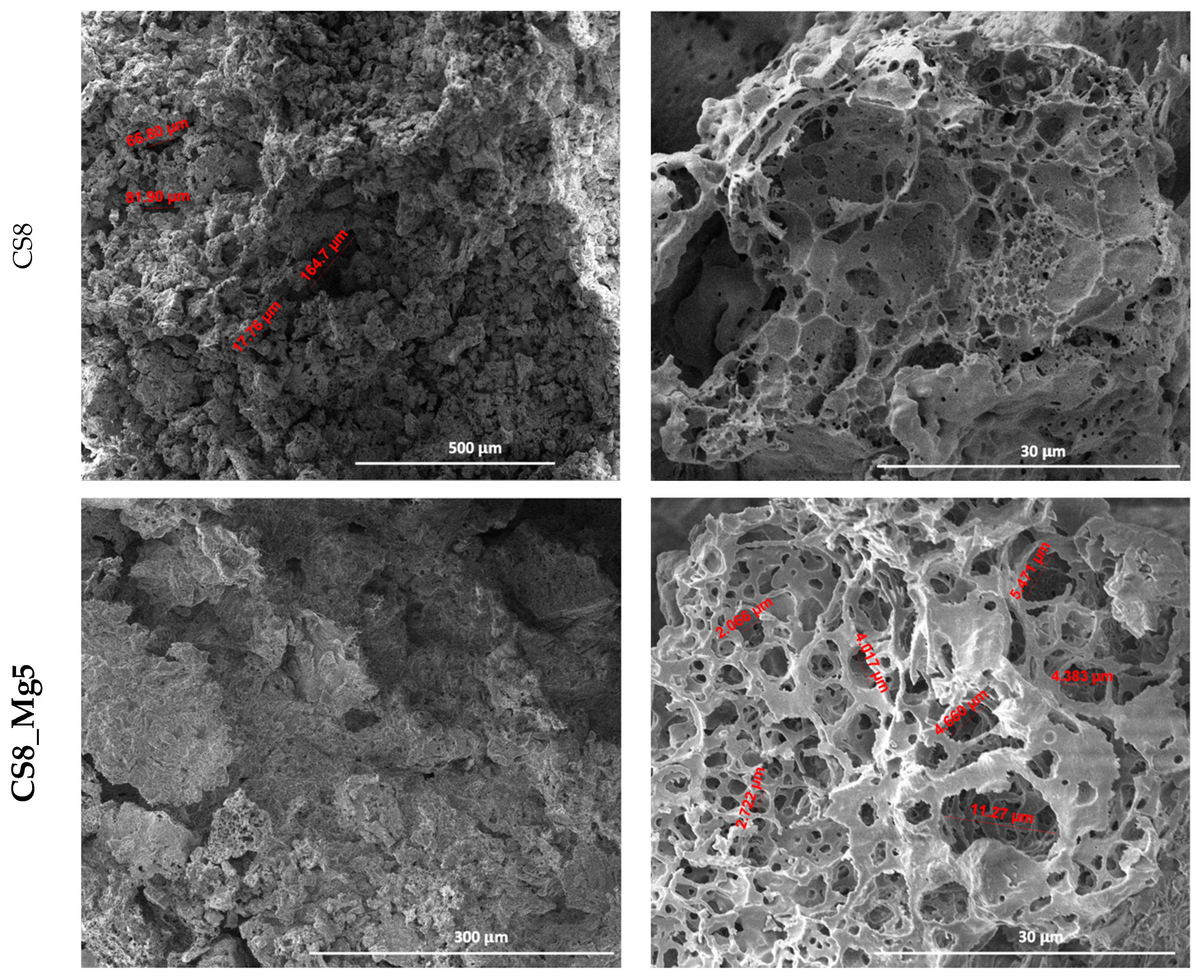
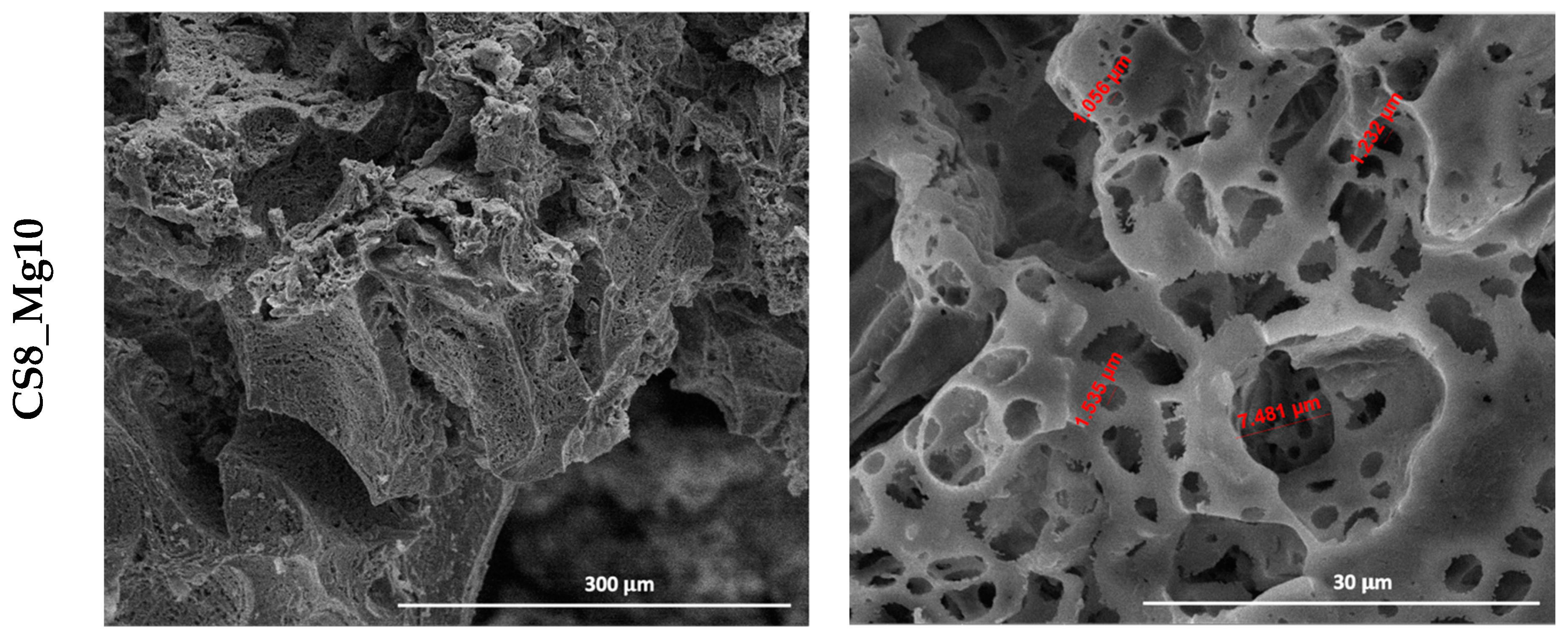
In the scaffolds based on calcium silicate doped with 5 and 10% Mg
2+, impregnated eight times (CS8_Mg5, CS8_Mg10) (
Figure 3), porous microstructures similar to bone tissue can be observed, with sizes between 2 and 11 µm, in case of CS8_Mg5, and, respectively, 1 and 7 µm CS8_Mg10.
We can say that the scaffolds based on simple CaSiO3 and doped with 5 and 10% Mg2+ and impregnated eight times, compared to the scaffolds based on simple CaSiO3 and doped with 5 and 10% Mg2+ and impregnated six times, have a microstructure more similar to that of bone tissue and an adequate pore size, which is an advantage considering the intended application.
Furthermore, as expected, the higher the number of immersed layers, the lower the pore size. This can be concluded by the fact that through the technique used, porous structures can be obtained, in which we can control the pore sizes to make these types of scaffolds suitable for bone regeneration processes, depending on the area that requires a regeneration process.
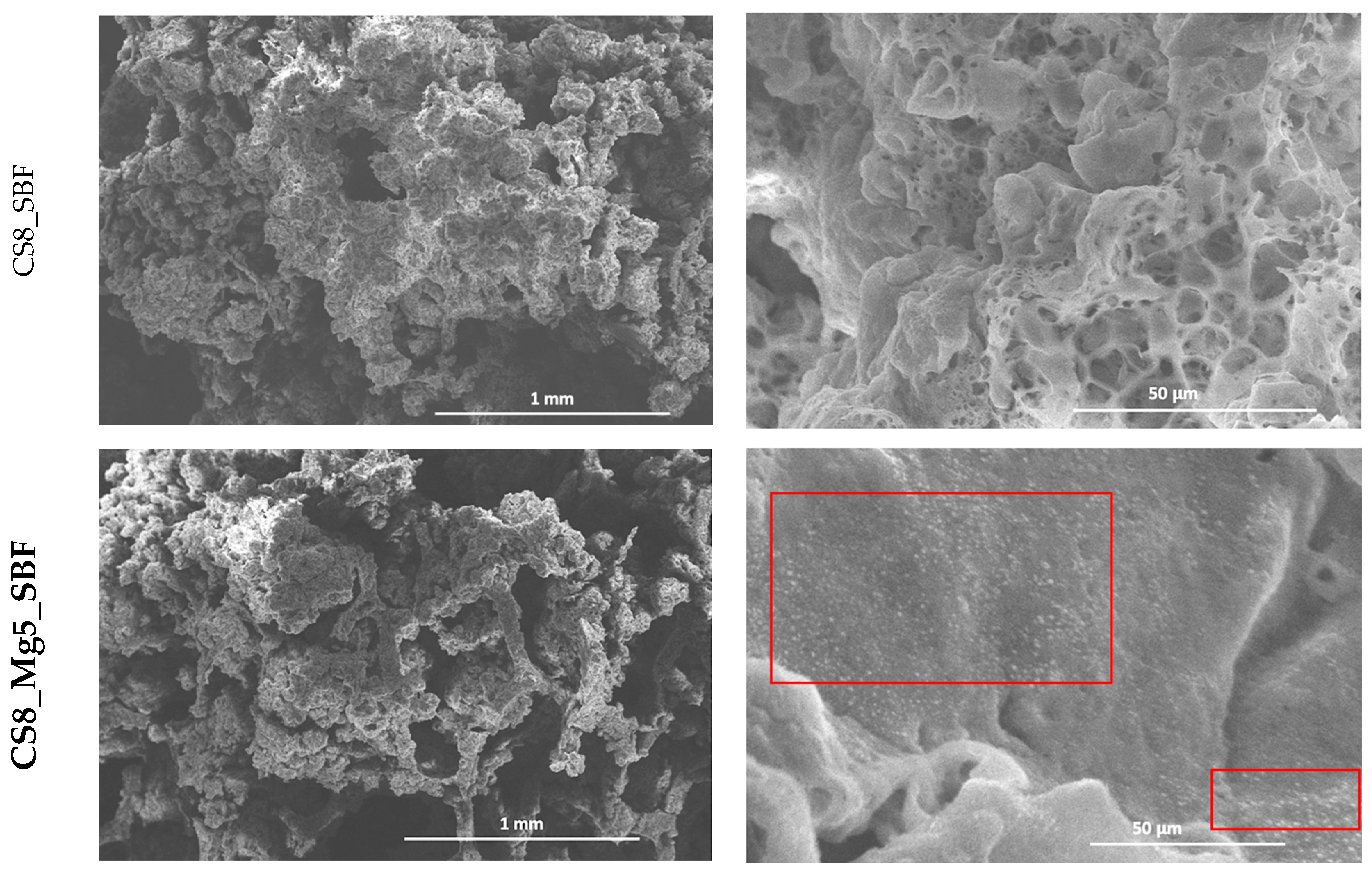
Figure 4 shows the SEM micrographs of scaffolds based on simple CaSiO
3 and doped with 5 and 10% Mg
2+, immersed 8×, after 7 days of immersion in simulated physiological serum (SBF). Therefore, after the immersion in SBF, the scanning electron microscopy images show a change in the microstructure of the scaffolds: carbonate hydroxyapatite deposits have formed on the surface of the structure. The most visible results are in the case of scaffolds based on CaSiO
3 doped with 5 and 10% Mg
2+, where the formation of the apatite is noticed on the surface and inside these types of scaffolds. The morphology of this apatite layer is granular and agglomerated.
4. Conclusions
The current study focused on obtaining scaffolds by the method of replicating a polymeric sponge, using soil and starting from raw materials, in which polyurethane sponges were impregnated, in the solutions formed, six and eight times, respectively, for the successive deposition of some layers and the consolidation of the final structure. The dopant ion concentration was 0, 5 and 10 mol%. For the obtained ceramic scaffolds, a series of characterization methods were carried out, such as X-ray diffraction to highlight the composition and crystallinity of the structures, scanning electron microscopy to observe the morphology and microstructure of the scaffolds, and evaluation of the bioactivity of the scaffolds by immersion in simulated biological serum at different time periods.
Following X-ray diffraction, the presence of several mineralogical phases in the form of calcium silicates was noted. In all three cases of doping, the presence of two polymorphic forms of calcium silicate is identified—CaSiO3 with wollastonite polymorph structure and CaSiO3 with pseudo-wollastonite polymorph structure.
Scanning electron microscopy showed the interconnected porosity of the scaffolds with quasi-spherical pore morphology and variable sizes. Scanning electron microscopy images taken after immersing the scaffolds in SBF showed a change in the microstructure of the scaffolds due to the formation of a carbonate hydroxyapatite deposit on the surface of the structure.
The most visible results were in the scaffolds based on CaSiO3 doped with 5 and 10% Mg2+, where the formation of apatite was noticed on the surface and inside these structures.