Abstract
1,4-Butane-sultone functionalized graphitic carbon nitride nanosheets (g-C3N4@Bu-SO3H) was prepared and applied as an efficient heterogeneous catalyst for the synthesis of various quinazolines derivatives with high yield. In next step, the structure and morphology of catalyst was characterized by different analyses such as, FT-IR, EDS, XRD and FE-SEM. On the other side, considering the noticeable features of g-C3N4@Bu- SO3H such as high stability, easy to synthesize, non-toxicity, excellent reusability, and so on, the synthesis of 2,3-dihydroquinazolines derivatives with numerous advantages such as short reaction time reaction condition, easy separation and etc were realized.
1. Introduction
Quinazolines and their derivatives are as a significant class of nitrogen-containing heterocyclic scaffolds that the structure of these compounds have been formed from six-membered fused rings [1]. Accordingly, these quinazolines derivatives have numerous biological activities, including anticancer, antimalaria, antimicrobial, antiviral, anti-HIV, anti-inflammatory, antifungal, acaricidal, weedicide, antidepressant, anticonvulsant, muscle relaxant, and so on [2,3]. On the other side, because of various biological values, they are utilized for synthesis of considerable drugs such as prazosin (treatment of benign prostatic obstruction) [4], geftinib (antitumor therapeutic agents) [5,6], erlotinib (EGFR inhibitor) [7], lapatinib (tyrosine kinase inhibitor) [8], alfuzosin (anticancer) [9], febrifugine (antimalaria) [10], and etc. (Scheme 1).
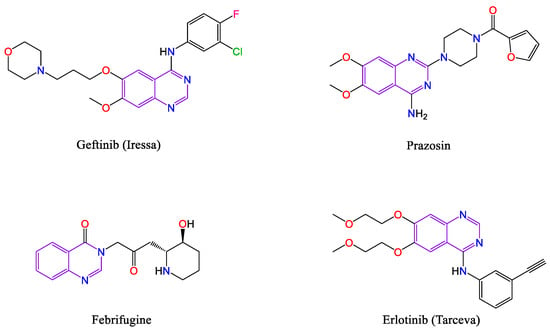
Scheme 1.
Some of the pharmaceutical active compounds containing quinazoline structures.
Recently, the preparation of 2,3-dihydroquinazolines derivatives has been heeded as the basic structure of the most bioactive medicines [11]. Therefore, to apply an effective and excellent catalyst is a noticeable approach to develop the synthesis of them with high yield. Because of various advantages such as, photocatalytic activity, wastewater treatment, organic transformation, disinfection, healthcare, environmental, electrochemical biosensor, CO2 reduction, and H2 generation [12,13]. Graphitic carbon nitride (g-C3N4) is considered as catalytic support for synthesis of different heterogeneous catalyst. In addition, Among the various catalytic methods, the use of metal-free heterogeneous catalysts is one of the best methods due to its green nature, easy synthesis and separation [14]. Although, there are different types of metal and metal-free catalysts such as PBDS-SCMNPs ionic liquid [15], Wang-OSO3H [16], Silica sulfuric acid [17], titanium silicon oxide [18], montmorillonite-KSF [19], SrCl2.6H2O [20], and Y(NO3)3.6H2O [21] that have been used for the synthesis of these heterocyclic derivatives but These catalysts have disadvantages and limitations such as difficult and long synthesis steps, expensive reagents, high reaction temperature, and low stability that lead to importance of the synthesis and characterization of suitable metal-free catalyst that can be beneficial for eliminating these disadvantages.
In this work, a high efficient metal-free heterogeneous catalyst (g-C3N4@Bu-SO3H) was prepared and applied for synthesis of 2,3-dihydroquinazoline and its derivatives with excellent advantages consisting of short reaction time, inexpensive and available raw materials, no oxidant, and high selectivity (Scheme 2 and Table 1).
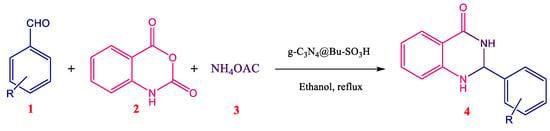
Scheme 2.
Multi-component reaction for the synthesis of 2,3-dihydroquinazolines derivatives.

Table 1.
Synthesis of 2,3-dihydroquinazoline derivatives using g-C3N4@Bu-SO3H metal-free catalyst.
2. Experimental
2.1. Material
All chemicals were purchased from the Merck (NJ, USA) and Sigma-Aldrich (Burlington, MA, USA) Co. Fourier Transform Infrared (FT-IR) spectra were recorded on Tensor 27. Nuclear Magnetic Resonance (NMR) data were acquired on a Varian-Inova 500 MHz. X-Ray Diffraction (XRD) patterns were obtained using Dron-8 diffractometer. Energy-dispersive X-ray (EDX) spectrum was recorded on Numerix DXP-X10P. Field Emission Scanning Electron Microscopy (FE-SEM) images were recorder with TESCAN-MIRA III.
2.2. Preparation of Bulk g-C3N4 and Nanosheets
First, the melamine powder was heated at 550˚C in furnace in air atmosphere at the heating rate 2.5 °C/min for 4 h. Then, the obtained yellow powder was well ground with a mortar to obtain a blended solid powder. In next step, for the synthesis of g-C3N4 nanosheets, bulk g-C3N4 (1.0 g) was stirred with H2SO4 (20.0 mL) at 90 °C for 5 h. The resulting mixture was stirred by (200 mL) ethanol in room temperature at 2 h and it remained constant until all the material was settled. After 2 days, the resulting mixture was dispersed by ultrasonic probe at 300 W for 1.5 h. Eventually, the formed suspension was washed three times by ethanol and seven times by distilled water. After that, white product was dried in oven at 60 °C.
2.3. Preparation of Graphitic Carbon Nitride Nanosheets Functionalized with 1,4-Butane-Sultone (g-C3N4@Bu-SO3H)
First, the g-C3N4 nanosheets (1.0 g) were dispersed in toluene (25 mL), after that 1,4-butane-sultone (3.0 g) was added the reaction mixture and was refluxed under nitrogen atmosphere for 6 h. Finally, the resulting product got cold in room temperature, then it was centrifuged and washed with chloroform and ethyl ether solvents, and dried in oven at 60 °C.
2.4. Selected Spectral Data
2-phenyl-2, 3-dihydro-4(1H)-quinazolinone (4a)
FT-IR (KBr, cm−1): 3300, 3176, 2981, 1651, 1610, 1507, 1440, 1385, 745 cm−1. 1H NMR (500 MHz, DMSO): δ H (ppm) = 5.75 (s, 1H, CH), 6.67 (t, 1H, Ar-H), 6.74 (d, 1H, Ar-H), 7.1 (s, 1H, NH), 7.23 (t, 1H, Ar-H), 7.34 (t, 1H, Ar-H), 7.38 (t, 1H, Ar-H), 7.49 (d, 1H, Ar-H), 7.60 (d, 1H, Ar-H), 8.27 (s, 1H, CONH) (Figure 1 and Figure 2).
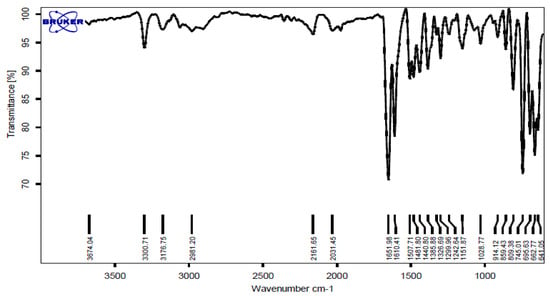
Figure 1.
FT-IR spectrum of the 2-phenyl-2,3-dihydro-4(1H)-quinazolinone.
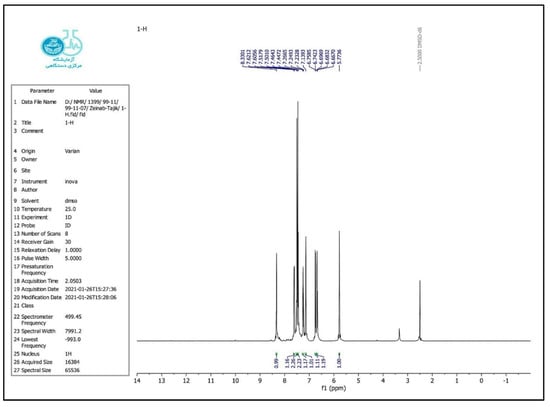
Figure 2.
1H NMR spectrum of the 2-phenyl-2,3-dihydro-4(1H)-quinazolinone.
2-(4-chloro-phenyl)-2, 3-dihydro-1H-quinazoline-4-one (4b)
FT-IR (KBr, cm−1): 3305, 3184, 3062, 1654, 1606, 1431, 1090, 749 cm−1. 1H NMR (500 MHz, DMSO): δ H (ppm) = 5.77 (s, 1H, CH), 6.68 (t, 1H, Ar-H), 6.74 (d, 1H, Ar-H), 7.1 (s, 1H, NH), 7.24 (t, 1H, Ar-H), 7.45 (d, 1H, Ar-H), 7.50 (d, 1H, Ar-H), 7.61 (d, 1H, Ar-H), 8.27 (s, 1H, CONH) (Figure 3 and Figure 4).
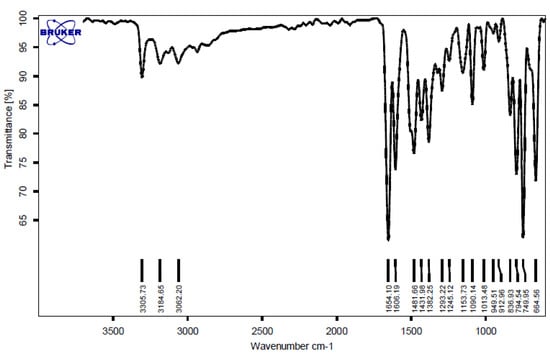
Figure 3.
FT-IR spectrum of the 2-(4-chloro-phenyl)-2,3-dihydro-1H-quinazoline-4-one.
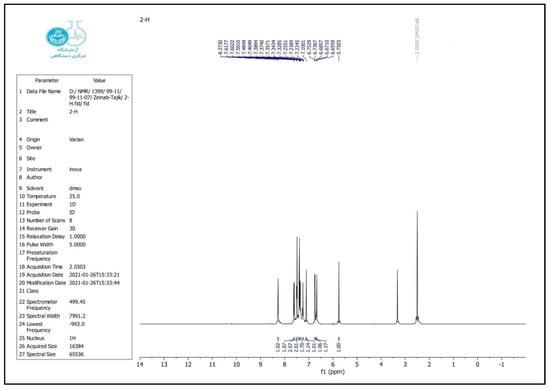
Figure 4.
1H NMR spectrum of the 2-(4-chloro-phenyl)-2,3-dihydro-1H-quinazoline-4-one.
3. Results and Discussion
The g-C3N4@Bu-SO3H heterogeneous catalyst was synthesized in just three steps (Scheme 3). In the first step, bulk g-C3N4 was prepared by polymerization of melamine. In the second step, the morphology of bulk g-C3N4 was changed to g-C3N4 nanoparticle. Finally, g-C3N4 nanoparticle was functionalized with 1,4-butane-sultone. This catalyst was proved by different analyses such as Fourier Transform Infrared (FT-IR) Spectroscopy, Energy Dispersive Spectrometer (EDS), Field Emission Scanning Electron Microscopy (FE-SEM), and X-ray diffraction analysis (XRD) [22].
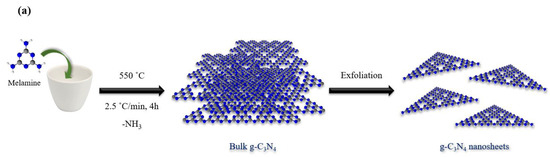
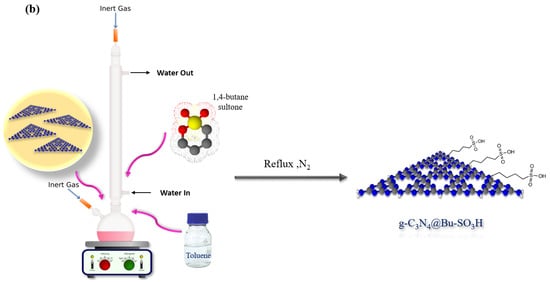
Scheme 3.
Synthesis of g-C3N4 nanosheets (a), g-C3N4@Bu-SO3H (b).
The FT-IR spectra of g-C3N4 nanosheets (a) and g-C3N4@Bu-SO3H (b) have been showed in Figure 5. A relatively strong peak in the range of 3000 to 3300 cm−1 is related to stretching vibration of N–H bonds, the 1602 cm−1 peak is related to C=N stretching vibration modes. The absorption peak of C–N bonds observed in rang of 1303 and 1082 cm−1 that can be attributed to C–N bonds between triazine and N–H groups. On the other hand, the characteristic peaks at 1448 and 1379 cm−1 are related to C–N ring bonds and finally, the peak at 784 cm−1 may be related to tri-s-triazine units (Figure 5a).
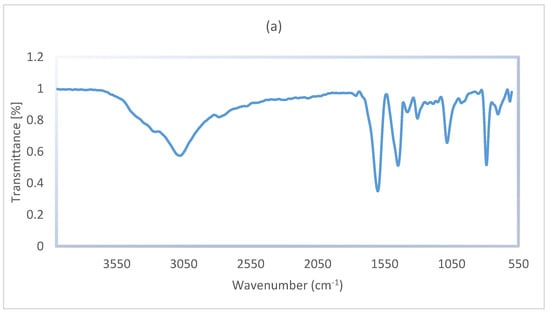
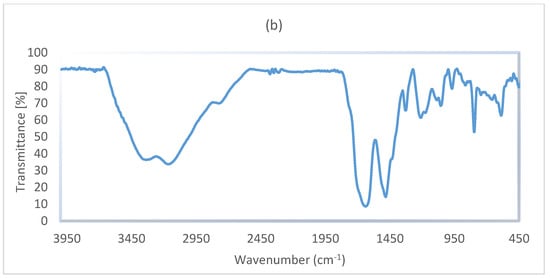
Figure 5.
The FT-IR spectra of g-C3N4 nanosheets (a), g-C3N4@Bu-SO3H catalyst (b).
In the spectrum of g-C3N4@Bu-SO3H, which is shown in Figure 5b, the two peaks 2781 and 2758 cm−1 are related to C–H groups in 1,4-butane-sultone. The symmetric and asymmetric stretching vibration modes of SO2 have appeared in the regions 1220 and 1348 cm−1, the characteristic peaks at 1176 and 1207 cm−1 are related to S–OH bonds.
In the Figure 6a, the presence of carbon and nitrogen atoms in structure of g-C3N4 nanosheets was confirmed by the EDS analysis. As shown in the Figure 6b, the presence of oxygen and sulfur elements proves synthesis of desired catalyst (g-C3N4-Bu-SO3H).
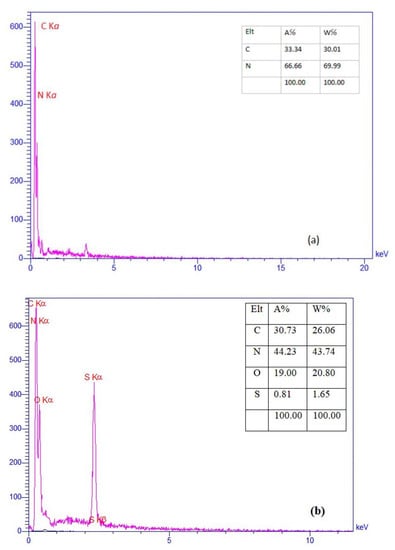
Figure 6.
EDS spectra of g-C3N4 nanosheets (a), g-C3N4@Bu-SO3H (b).
The morphology of g-C3N4 nanosheets and g-C3N4@Bu-SO3H were shown by the FE-SEM images. In the Figure 7a, the g-C3N4 nanosheets have a relatively flat surface, while in the Figure 7b, image of g-C3N4@Bu-SO3H is partly different and irregular. Therefore, this variance can verified the deposition of sultone on the g-C3N4 nanosheets.
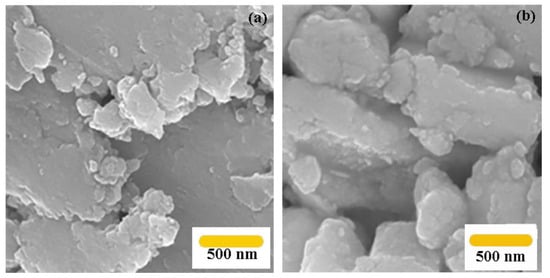
Figure 7.
FE-SEM image of g-C3N4 nanosheets (a), g-C3N4@Bu-SO3H (b).
The XRD pattern of g-C3N4 nanosheets and g-C3N4@Bu-SO3H can be seen in Figure 8. Diffraction peaks at 2θ: 27.35° (002) and 13.04° (100) are related g-C3N4 nanosheets (Figure 8a). Also diffraction peaks at 2θ: 27.4° (002), 2θ = 17.9°, and 14.8° (100) are related to the g-C3N4@Bu-SO3H that approve synthesis of this catalyst (Figure 8b).
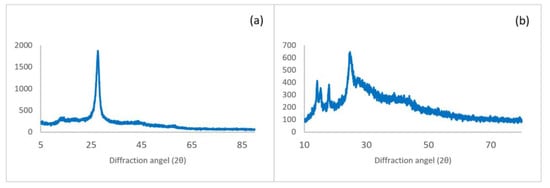
Figure 8.
XRD spectra of g-C3N4 nanosheets (a), g-C3N4@Bu-SO3H (b).
4. Reusability
Reusability of g-C3N4@Bu-SO3H catalyst was examined for synthesis of 2,3-dihydroquinazolines derivatives in four runs, the considering Figure 9, reaction yield was decreased considerably after third run.
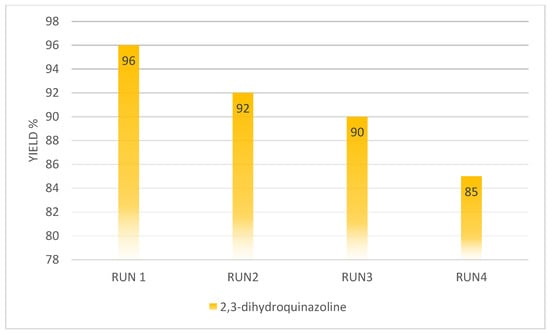
Figure 9.
Reusability of g-C3N4@Bu-SO3H catalyst for synthesis of 2,3-dihydroquinazolines derivatives.
5. Conclusions
In summary, an efficient heterogeneous catalyst (g-C3N4@Bu-SO3H) was synthesized and utilized for production of 2,3-dihydroquinazolines derivatives with highly advantages such as short reaction time, mild condition, and easy separation. On the other side, this catalyst can be used and recycled for five times with high yield.
Author Contributions
Conceptualization, supervision, validation, H.G.; investigation, Z.T.; methodology, Z.N. and Z.T.; writing—review and editing, writing—original draft preparation, H.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, J.; Chang, D.; Xiao, F.; Deng, G.-J. Four-component quinazoline synthesis from simple anilines, aromatic aldehydes and ammonium iodide under metal-free conditions. Green Chem. 2018, 20, 5459–5463. [Google Scholar] [CrossRef]
- Asif, M. Chemical characteristics, synthetic methods, and biological potential of quinazoline and quinazolinone derivatives. Int. J. Med. Chem. 2014, 2014, 395637. [Google Scholar] [CrossRef] [PubMed]
- Gupta, T.; Rohilla, A.; Pathak, A.; Akhtar, J.; Haider, R.; Yar, M.S. Current perspectives on quinazolines with potent biological activities: A review. Synth. Commun. 2018, 48, 1099–1127. [Google Scholar] [CrossRef]
- Akduman, B.; Crawford, E. Terazosin, doxazosin, and prazosin: Current clinical experience. Urology 2001, 58, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.M.; Ahmad, I.; Pawara, R.; Shaikh, M.; Surana, S. In silico search of triple mutant T790M/C797S allosteric inhibitors to conquer acquired resistance problem in non-small cell lung cancer (NSCLC): A combined approach of structure-based virtual screening and molecular dynamics simulation. J. Biomol. Struct. Dyn. 2021, 39, 1491–1505. [Google Scholar] [CrossRef]
- Kassem, M.G.; Motiur Rahman, A.F.M.; Korashy, H.M. Profiles of Drug Substances, Excipients and Related Methodology; Elsevier: Amsterdam, The Netherlands, 2014; p. 239. [Google Scholar]
- Cheng, W.; Wang, S.; Yang, Z.; Tain, X.; Hu, Y. Design, synthesis, and biological study of 4-[(2-nitroimidazole-1H-alkyloxyl) aniline]-quinazolines as EGFR inhibitors exerting cytotoxicities both under normoxia and hypoxia. DrugDes. Dev. Ther. 2019, 13, 3079. [Google Scholar] [CrossRef]
- Geyer, C.E.; Forster, J.; Lindquist, D.; Chan, S.; Romieu, C.G.; Pienkowski, T.; Jagiello-Gruszfeld, A.; Crown, J.; Chan, A.; Kaufman, B.; et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N. Engl. J. Med. 2006, 355, 2733–2743. [Google Scholar] [CrossRef]
- Mari, A.; Antonelli, A.; Cindolo, L.; Fusco, F.; Minervini, A.; De Nunzio, C. Alfuzosin for the medical treatment of benign prostatic hyperplasia and lower urinary tract symptoms: A systematic review of the literature and narrative synthesis. Ther. Adv. Urol. 2021, 13, 1756287221993283. [Google Scholar] [CrossRef]
- Kikuchi, H.; Horoiwa, S.; Kasahara, R.; Hariguchi, N.; Matsumoto, M.; Oshima, Y. Synthesis of febrifugine derivatives and development of an effective and safe tetrahydroquinazoline-type antimalarial. Eur. J. Med. Chem. 2014, 76, 10–19. [Google Scholar] [CrossRef]
- Khan, I.; Ibrar, A.; Ahmed, W.; Saeed, A. Synthetic approaches, functionalization and therapeutic potential of quinazoline and quinazolinone skeletons: The advances continue. Eur. J. Med. Chem. 2015, 90, 124–169. [Google Scholar] [CrossRef]
- Vinoth, S.; Devi, K.S.; Pandikumar, A. A comprehensive review on graphitic carbon nitride based electrochemical and biosensors for environmental and healthcare applications. TrAC Trends Anal. Chem. 2021, 140, 116274. [Google Scholar] [CrossRef]
- Akhundi, A.; Badiei, A.; Ziarani, G.M.; Habibi-Yangjeh, A.; Muñoz-Batista, M.J.; Luque, R. Graphitic carbon nitride-based photocatalysts: Toward efficient organic transformation for value-added chemicals production. Mol. Catal. 2020, 488, 110902. [Google Scholar] [CrossRef]
- Azhdari, A.; Azizi, N.; Sanaeishoar, H.; Tahanpesar, E. Amidosulfonic acid supported on graphitic carbon nitride: Novel and straightforward catalyst for Paal–Knorr pyrrole reaction under mild conditions. Mon. Für Chem.-Chem. Mon. 2021, 152, 625–634. [Google Scholar] [CrossRef]
- Fatehi, A.; Ghorbani-Vaghei, R.; Alavinia, S.; Mahmoodi, J. Synthesis of Quinazoline Derivatives Catalyzed by a New Efficient Reusable Nanomagnetic Catalyst Supported with Functionalized Piperidinium Benzene-1, 3-Disulfonate Ionic Liquid. ChemistrySelect 2020, 5, 944–951. [Google Scholar] [CrossRef]
- Rao, A.D.; Vykunteswararao, B.; Bhaskarkumar, T.; Jogdand, N.R.; Kalita, D.; Lilakar, J.K.D.; Siddaiah, V.; Sanasi, P.D.; Raghunadh, A. Sulfonic acid functionalized Wang resin (Wang-OSO3H) as polymeric acidic catalyst for the eco-friendly synthesis of 2, 3-dihydroquinazolin-4 (1H)-ones. Tetrahedron Lett. 2015, 56, 4714–4717. [Google Scholar]
- Dabiri, M.; Salehi, P.; Baghbanzadeh, M.; Zolfigol, M.A.; Agheb, M.; Heydari, S. Silica sulfuric acid: An efficient reusable heterogeneous catalyst for the synthesis of 2, 3-dihydroquinazolin-4 (1H)-ones in water and under solvent-free conditions. Catal. Commun. 2008, 9, 785–788. [Google Scholar] [CrossRef]
- Mekala, R.; Madhubabu, M.V.; Dhanunjaya, G.; Regati, S.; Chandrasekhar, K.B.; Sarva, J. Efficient synthesis of 2, 3-dihydroquinazolin-4 (1H)-ones catalyzed by titanium silicon oxide nanopowder in aqueous media. Synth. Commun. 2017, 47, 121–130. [Google Scholar] [CrossRef]
- Tekale, S.U.; Munde, S.B.; Kauthale, S.S.; Pawar, R.P. An efficient, convenient, and solvent-free synthesis of 2, 3-dihydroquinazolin-4 (1 H)-ones using montmorillonite-KSF clay as a heterogeneous catalyst. Org. Prep. Proced. Int. 2018, 50, 314–322. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, T.T.; Liang, Y.; Gao, J.J. Strontium chloride-catalyzed one-pot synthesis of 2, 3-dihydroquinazolin-4 (1H)-ones in protic media. Chin. Chem. Lett. 2011, 22, 1423–1426. [Google Scholar] [CrossRef]
- Khan, A.A.; Mitra, K.; Mandal, A.; Baildya, N.; Mondal, M.A. Yttrium nitrate catalyzed synthesis, photophysical study, and TD-DFT calculation of 2, 3-dihydroquinazolin-4 (1H)-ones. Heteroat. Chem. 2017, 28, e21379. [Google Scholar] [CrossRef]
- Rahmati, M.; Ghafuri, H.; Ghanbari, N.; Tajik, Z. 1, 4 Butanesultone functionalized graphitic carbon nitride: Efficient catalysts for the one-pot synthesis of 1, 4-dihydropyridine and polyhydroquinoline derivative through hantzsch reaction. Polycycl. Aromat. Compd. 2022, 42, 3019–3035. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
