In Silico Comparison of Drug-Likeness of Phytochemicals from Nine Herbal Plants against Asthma †
Abstract
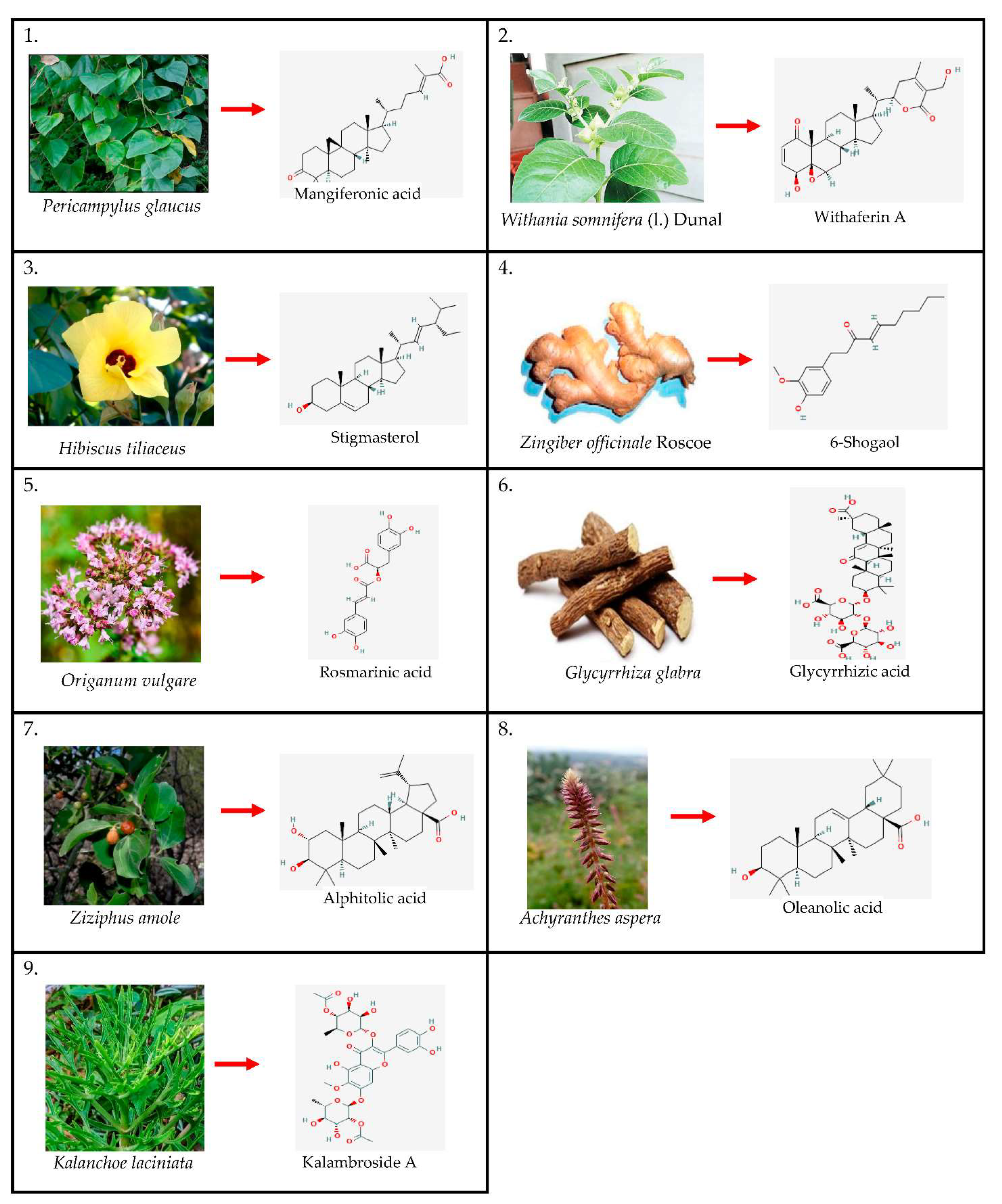
:1. Introduction
2. Methodology
3. Results and Discussion
3.1. Molinspiration
3.2. pKCSM Tool
3.2.1. Absorption
3.2.2. Distribution
3.2.3. Metabolism
3.2.4. Excretion
3.2.5. Toxicity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ye, Q.; He, X.-O.; D’Urzo, A. A Review on the Safety and Efficacy of Inhaled Corticosteroids in the Management of Asthma. Pulm. Ther. 2017, 3, 1–18. [Google Scholar] [CrossRef]
- Dash, G.; Mohanty, K.T.; Sahoo, D.; Mahalik, G.; Parida, S. Traditional medicinal plants used for the treatment of asthma in Bhubaneswar, Odisha. Int. J. Herb. Med. 2018, 6, 57–60. [Google Scholar]
- Prasad, R.; Lawania, R.D.; Manvi; Gupta, R. Role of Herbal plants in the Management of Asthma. Pharmacogn. Rev. 2009, 3, 247–258. [Google Scholar]
- Veenstra, J.P.; Johnson, J.J. Oregano (Origanum vulgare) extract for food preservation and improvement in gastrointestinal health. Int. J. Nutr. 2019, 3, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Amaral-Machado, L.; Oliveira, W.N.; Moreira-Oliveira, S.S.; Pereira, D.T.; Alencar, É.N.; Tsapis, N.; Egito, E.S.T. Use of Natural Products in Asthma Treatment. Evid. -Based Complementary Altern. Med. 2020, 2020, 1021258. [Google Scholar] [CrossRef]
- Gunawardana, S.L.A.; Jayasuriya, W.J.A.B.N. Medicinally Important Herbal Flowers in Sri Lanka. Evid. -Based Complementary Altern. Med. 2019, 2019, 2321961. [Google Scholar] [CrossRef]
- Fernandes, J.M.; Cunha, L.M.; Azevedo, E.P.; Lourenço, E.M.G.; Fernandes-Pedrosa, M.F.; Zucolotto, S.M. Kalanchoe laciniata and Bryophyllum pinnatum: An updated review about ethnopharmacology, phytochemistry, pharmacology and toxicology. Rev. Bras. Farmacogn. 2019, 29, 529–558. [Google Scholar] [CrossRef]
- Paul, S.; Chakraborty, S.; Anand, U.; Dey, S.; Nandy, S.; Ghorai, M.; Saha, S.C.; Patil, M.T.; Kandimalla, R.; Proćków, J. Withania somnifera (L.) Dunal (Ashwagandha): A comprehensive review on ethnopharmacology, pharmacotherapeutics, biomedicinal and toxicological aspects. Biomed. Pharmacother. 2021, 143, 112175. [Google Scholar] [CrossRef]
- Pubchem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 10 June 2022).
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Valerio, L.G. Application of advanced in silico methods for predictive modeling and information integration. Expert Opin. Drug Metab. Toxicol. 2012, 8, 395–398. [Google Scholar] [CrossRef]
- Madden, J.C.; Enoch, S.J.; Paini, A.; Cronin, M.T.D. A Review of In Silico Tools as Alternatives to Animal Testing: Principles, Resources and Applications. Altern. Lab. Anim. 2020, 48, 146–172. [Google Scholar] [CrossRef] [PubMed]
- Molinspiration Chemoinformatics Software. Available online: https://www.molinspiration.com/ (accessed on 5 March 2022).
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting Small-Molecule Pharmacokinetic and Toxicity Properties Using Graph-Based Signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef]
- Falade, V.A.; Adelusi, T.I.; Adedotun, I.O.; Abdul-Hammed, M.; Lawal, T.A.; Agboluaje, S.A. In silico investigation of saponins and tannins as potential inhibitors of SARS- CoV-2 main protease (Mpro). Silico Pharmacol. 2021, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Almi, I.; Belaidi, S.; Melkemi, N.; Bouzidi, D. Chemical Reactivity, Drug-Likeness and Structure Activity/Property Relationship Studies of 2,1,3-Benzoxadiazole Derivatives as Anti-Cancer Activity. J. Bionanoscience 2018, 12, 49–57. [Google Scholar] [CrossRef]
- Vlad, I.M.; Nuta, D.C.; Chirita, C.; Caproiu, M.T.; Draghici, C.; Dumitrascu, F.; Bleotu, C.; Avram, S.; Udrea, A.M.; Missir, A.V.; et al. In Silico and In Vitro Experimental Studies of New Dibenz[b,e]oxepin-11(6H)one O- (arylcarbamoyl)-oximes Designed as Potential Antimicrobial Agents. Molecules 2020, 25, 321. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, D.; Gupta, P. Nuclear Receptors in Asthma: Empowering Classical Molecules Against a Contemporary Ailment. Front. Immunol. 2021, 11, 594433. [Google Scholar] [CrossRef]
- Krishnamoorthy, G.; Banu, S.S.; Ismail, A.M.; Senthamarai, R. Design of Newer Analog of Imatinib as Anticancer Drug Targeting Protein Kinase Receptor. Int. J. Pharm. Sci. Res. 2016, 7, 3001–3011. [Google Scholar]
- Ujan, R.; Saeed, A.; Channar, P.; Larik, F.; Abbas, Q.; Alajmi, M.; El-Seedi, H.; Rind, M.; Hassan, M.; Raza, H.; et al. ‘Drug-1,3,4-Thiadiazole Conjugates as Novel Mixed-Type Inhibitors of Acetylcholinesterase: Synthesis, Molecular Docking, Pharmacokinetics, and ADMET Evaluation. Molecules 2019, 24, 860. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, J.; Hu, C.Q.; Zhang, X.; Ma, B.; Zhang, P. In silico ADME and Toxicity Prediction of Ceftazidime and Its Impurities. Front. Pharmacol. 2019, 10, 434. [Google Scholar] [CrossRef]
- Vilar, S.; Chakrabarti, M.; Costanzi, S. Prediction of passive blood–brain partitioning: Straightforward and effective classification models based on in silico derived physicochemical descriptors. J. Mol. Graph. Model. 2010, 28, 899–903. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, H.; Zhao, Q. In vitro inhibitory effects of Friedelin on human liver cytochrome P450 enzymes. Pharm. Biol. 2018, 56, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Samiei, M.; Asgary, S.; Farajzadeh, M.; Bargahi, N.; Abdolrahimi, M.; Kananizadeh, U.; Dastmalchi, S. Investigating the mutagenic effects of three commonly used pulpotomy agents using the Ames test. Adv. Pharm. Bull. 2015, 5, 121–125. [Google Scholar] [PubMed]
| Physiochemical Parameters | Chemical Compound | ||||
|---|---|---|---|---|---|
| Mangiferonic Acid | Withaferin A | Stigmasterol | 6-Shogaol | Rosmarinic Acid | |
| miLogP TPSA MW nON nOHNH nviolations nrotb | 6.69 54.37 454.69 3 1 1 5 | 3.86 96.36 470.61 6 2 0 3 | 7.87 20.23 412.70 1 1 1 5 | 4.35 46.53 276.38 3 1 0 9 | 1.63 144.52 360.32 8 5 0 7 |
| Physiochemical Parameters | Chemical Compound | ||||
| Glycyrrhizin | Alphitolic Acid | Oleanic Acid | Kalambroside A | Fluticasone Propionate (Control) | |
| miLogP TPSA MW nON nOHNH nviolations nrotb | 1.97 267.04 822.94 16 8 3 7 | 6.13 77.75 472.71 4 3 1 2 | 6.72 57.53 456.71 3 2 1 1 | 0.84 270.59 708.62 18 7 3 10 | 4.61 80.67 500.58 5 1 1 6 |
| Chemical Compounds | Nuclear Receptor Ligand |
|---|---|
| Rosmarinic acid | 0.57 |
| 6-shogaol | 0.20 |
| Glycyrrhizin | −2.36 |
| Fluticasone propionate | 1.83 |
| Kalambroside A | −1.11 |
| Oleanic acid | 0.77 |
| Stigmasterol | 0.74 |
| Withaferin A | 0.76 |
| Mangiferonic acid | 0.88 |
| Alphitolic acid | 0.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weerakoon, T.; Nadarajah, N.; Rizwan, R.; Ranathunga, R.; Vithanage, J. In Silico Comparison of Drug-Likeness of Phytochemicals from Nine Herbal Plants against Asthma. Chem. Proc. 2022, 12, 93. https://doi.org/10.3390/ecsoc-26-13527
Weerakoon T, Nadarajah N, Rizwan R, Ranathunga R, Vithanage J. In Silico Comparison of Drug-Likeness of Phytochemicals from Nine Herbal Plants against Asthma. Chemistry Proceedings. 2022; 12(1):93. https://doi.org/10.3390/ecsoc-26-13527
Chicago/Turabian StyleWeerakoon, Tharindra, Nisshaptha Nadarajah, Ramlah Rizwan, Rithmi Ranathunga, and Janani Vithanage. 2022. "In Silico Comparison of Drug-Likeness of Phytochemicals from Nine Herbal Plants against Asthma" Chemistry Proceedings 12, no. 1: 93. https://doi.org/10.3390/ecsoc-26-13527
APA StyleWeerakoon, T., Nadarajah, N., Rizwan, R., Ranathunga, R., & Vithanage, J. (2022). In Silico Comparison of Drug-Likeness of Phytochemicals from Nine Herbal Plants against Asthma. Chemistry Proceedings, 12(1), 93. https://doi.org/10.3390/ecsoc-26-13527






