Abstract
The present study aimed, for the first time, to evaluate the production and application of NaCl plant extracts in a coagulation–flocculation–decantation process (CFD process) for the optimization of the microfiltration process (MF process) for the treatment of winery wastewater (WW). To evaluate the efficiency of the NaCl extracts, aluminum sulfate (10%) was applied as a comparison. The CFD process was optimized by varying the WW pH, coagulant dosage, agitation, type and dosage of flocculants before the microfiltration process. The application of Chelidonium majus L. (seeds) achieved 29.7, 99.7 and 95.3% total organic carbon, turbidity and total suspended solids removal, respectively, with 108 mg of filter consumption. In conclusion, NaCl plant extracts are a promising technology for WW treatment.
1. Introduction
Portugal is a typical Mediterranean wine producer, with around 195,000 ha of vineyards and a wine production of about 6.7 MhL in 2019 [1]. It is the 11th largest producer worldwide and the 9th largest amongst global wine exporters [2]. This high amount of wine production leads to the high generation of winery wastewaters (WW) during the different activities carried out for wine elaboration, mainly originating from the washing and rinsing operations of fermentation tanks, barrels, and other items [3]. The coagulation–flocculation–decantation process (or CFD process) has been used by several authors for the treatment of winery wastewater; however, most of these authors employ iron and aluminum sulfates, which in excess can be responsible for several problems, due to the production of large volumes of metal hydroxide (toxic) sludge, which will create a disposal problem and an increase in metal (e.g., aluminum) concentration in the treated water that may have human health implications [4]. To avoid these consequences, plant-based coagulants have been extensively investigated at the laboratory scale, aiming to exploit them in wastewater treatment [5], to which there is little information. Due to the characteristics of the winery wastewater, a membrane-based process, such as microfiltration, can be employed as a complement treatment to the CFD process. Microfiltration membranes can include polymeric or ceramic in their constitution, with a pore size ranging from 50 to 500 nm, which are capable of segregation of suspended solids and bacteria by the mechanism of convective pore flow conforming to Darcy’s law [6]. To our knowledge, the combined CFD-microfiltration processes have never been applied to winery wastewater treatment and its effects in organic carbon, turbidity, total suspended solids and phenolic compounds reduction are unknown. Due to the lack of information regarding the production and application of NaCl plant extracts in CFD process and the application of microfiltration in winery wastewater treatment, the objectives of this work are as follows: (1) to characterize the plants, (2) to evaluate the production and application of NaCl plant extracts in CFD process, (3) to evaluate the effect of microfiltration in WW treatment; (4) to study the effect of the CFD process in microfiltration enhancement and cost reduction.
2. Material and Methods
2.1. Reagents and Winery Wastewater Sampling
Aluminum sulfate 18-hydrate (10% w/w, Al2(SO4)3·18H2O) was acquired by Scharlau, Barcelona, Spain, polyvinylpyrrolidone (10% w/w, PVPP) and potassium caseinate were acquired by A. Freitas Vilar, Lisboa, Portugal, activated sodium bentonite was supplied by Angelo Coimbra & Ca., Lda, Maia, Portugal, and activated charcoal by SAI Enology, Paredes, Portugal. For pH adjustment, sodium hydroxide (NaOH) from Labkem, Barcelona, Spain and sulfuric acid (H2SO4, 95%) from Scharlau, Barcelona, Spain were used. Deionized water was used to prepare the respective solutions.
The winery wastewater was collected from a cellar located in the Douro region (Northern Portugal). The wastewater samples were placed in plastic containers to be transported to the laboratory and they were stored at −40 °C. This work was performed at the University of Trás-os-Montes and Alto Douro, located in Vila Real, Portugal, latitude 41°17′9.18″ N and longitude 7°44′21.45″ W.
2.2. Analytical Determinations
Different physical-chemical parameters were determined in order to characterize the WW, including turbidity, total suspended solids (TSS), chemical oxygen demand (COD), biological oxygen demand (BOD5), total organic carbon (TOC) and total polyphenols. The main wastewater characteristics are shown in Table 1.

Table 1.
Winery wastewater characterization.
2.3. Plant Extract Preparation
All the vegetable parts (Table 2) collected were washed and dried in an oven at 70 °C for 24 h. Then, they were grounded into powder using a groundnut miller. The grounded powder was sieved to a mesh size of 150 μm to obtain the powder. Finally, the powder was once more dried in an oven at 70 °C for 30 min to remove the moisture. The powder was then left to cool and stored in a tightly closed plastic jar. The extraction process was carried out in the following manner: a 1 M NaCl solution was prepared and 5 g of plant powder was added to 100 mL of NaCl solution (the stock solution was, thus, considered to be 5% w/w). The NaCl solution with powder was vigorously stirred at pH 7 and room temperature for 30 min with magnetic stirring. The extract was then filtered twice, including once through commercial filter paper in a Büchner funnel and once again through a fine filtering Millipore system (0.45 µm glass fiber). The result is a clear white liquid.

Table 2.
Plant identification with description of species, sub-species, part collected and herbarium number.
2.4. Coagulation–Flocculation–Decantation/Microfiltration Experimental Setup
Coagulation–flocculation–decantation (CFD) experiments were performed in a conventional model jar-test apparatus (ISCO JF-4, Louisville, KY, USA), using 500 mL of effluent in 1000 mL beakers. The microfiltration experiments were performed by a VACUUBRAND GMBH + CO pump (Germany) with a flow rate of 1.9 m3/h, coupled with a magnetic filter funnel (Gelman Sciences). The wastewater samples were filtered by glass microfiber filters (Prat Dumas) with a thickness of 270 µm, a micrometric retention of 1.2 µm and a weight of 108.39 mg. Several trials were performed in order to optimize coagulation–flocculation–decantation before the microfiltration processes, which were as follows:
(1) A total of 0.1, 0.2, 0.5 and 1.0 g/L of NaCl extracts and aluminum sulfate were added to 500 mL of the wastewater sample, and the pH was varied to 3.0, 5.0, 7.0, 9.0 and 11.0;
(2) Pre-determined optimum values (pH and dosage) of NaCl extracts and aluminum sulfate obtained in (1) were added to the WW sample. Then, the stirring process (rpm/min) was varied under different fast mix (rpm/min)–slow mix (rpm/min) conditions (120/1–20/30; 150/3–20/20; 150/2–50/30; 180/3–40/17; 200/2–60/30);
(3) Under the best conditions obtained in (1) and (2), four different types of flocculants (potassium caseinate, polyvinylpyrrolidone, activated sodium bentonite and activated charcoal) with a concentration of 0.5 g/L were added;
(4) The pre-determined optimum values (coagulant dosage, pH, mixing conditions, type of flocculant) obtained in (1), (2) and (3) were added to the WW sample. Different flocculant concentrations (5, 50, 100, or 500 mg/L), were added and the liquid was mixed in accordance with the optimal conditions obtained in (2). After the withdrawal of the supernatant, the volume of wet sludge produced was determined by an Imhoff cone, from the sludge level on the bottom of the jar-test beakers. The WW was then filtered through a glass microfiber filters, and both filter consumption and filtered sludge were quantified.
2.5. Statistical Analysis
Differences among the means were determined by analysis of variance (ANOVA) using OriginLab 2019 software (Northampton, MA, USA) and Minitab Statistical Software 2018 (State College, PA, USA) and the Tukey’s test was used for the comparison of means, which were considered different when p < 0.05. The data are presented as mean and standard error (mean ± SE).
3. Results and Discussion
3.1. Characterization of Plant Powders
The Fourier-transform infrared spectroscopy (FTIR) analysis (Figure 1) showed a band at 3421.72 cm−1, which corresponds to the stretching vibrations of the OH groups (from water, alcohols, phenols, carbohydrates, peroxides), as well from amides [7]. The adsorption bands at 2920.23 and 2850.79 cm−1 correspond to C–H stretching vibrations specific to CH3 and CH2 from lipids, metoxy derivatives, C–H (aldehydes), including cis double bonds. The 1741.72 cm−1 adsorption band indicates the presence of glycerides. The 1639.49 cm−1 adsorption band corresponds to bending vibrations N–H (amino acids), C=O stretchings (aldehydes and cetones, esters), as well to free fatty acids [8,9]. The 1028.06 cm−1 absorption band was attributed to C–O stretching vibrations from the glucose ring vibration and the holocellulose and hemicellulose [10,11,12]. The 1200–1000 cm−1 absorption bands included the C–O–C symmetrically stretching vibration and the aromatic C–H in-plane bending vibrations [12].
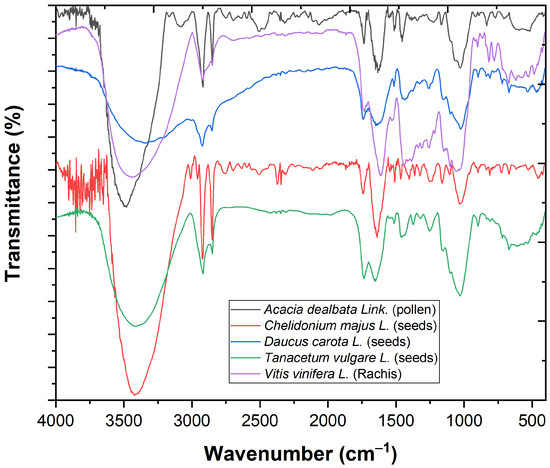
Figure 1.
The FTIR spectrum of Acacia dealbata Link. (pollen), Chelidonium majus L. (seeds), Daucus carota L. (seeds), Tanacetum vulgare L. (seeds) and Vitis vinifera L. (rachis).
Thirty-seven fatty acids were separated via capillary gas chromatography (GC) using a Shimadzu GC- 2010 Plus (Shimadzu, Kyoto, Japan), equipped with an autosampler and an automatic split/splitless injector. The results showed the presence of major concentrations of fatty acids, such as erucic (25.69%), arachidic (20.65%), cis-10-pentadecenoic (13.61%), cis-5,8,11,14,17-eicosapentaenoic (20.48%) and arachidic (38.82%), respectively, for Acacia dealbata Link. (pollen), Chelidonium majus L. (seeds), Daucus carota L. (seeds), Tanacetum vulgare L. (seeds) and Vitis vinifera L. (rachis).
The quantitative analysis of individual phenolic compounds was carried out on a Gilson (Villers-le-bel, France) high-performance liquid chromatography (HPLC) instrument consisting of an autosampler, binary pump, column compartment, and a Finnigan photodiode array detector (DAD 81401; Thermo Electron, San Jose, CA, USA). The results showed the presence of a major percentage of ellagic acid (55.84%), kaempferol (33.28%), caffeic acid (10.37%), ellagic acid (31.12%) and gallic acid (9.59%), respectively, for Acacia dealbata Link. (pollen), Chelidonium majus L. (seeds), Daucus carota L. (seeds), Tanacetum vulgare L. (seeds) and Vitis vinifera L. (rachis).
3.2. Coagulation–Flocculation–Decantation/Microfiltration Batch Treatment Experiments
With the optimization of the coagulation–flocculation–decantation–microfiltration process, the best operational conditions were achieved, which are presented in Table 3.

Table 3.
Best operational conditions of NaCl plant extract Acacia dealbata Link. (pollen), Chelidonium majus L. (seeds), Daucus carota L. (seeds), Tanacetum vulgare L. (seeds) and Vitis vinifera L. (rachis) and aluminum sulfate for CFD process, which is as follows: (TOC)0 = 400 mg C/L, turbidity = 296 NTU, TSS = 750 mg/L, temperature 298 K, sedimentation time 12 h, pump flow rate of 1.9 m3/h, glass microfiber filters, with micrometric retention of 1.2 µm.
By application of the best operational conditions (Table 3), a TOC removal of 8.4, 8.5, 29.7, 17.7, 40.8, 38.6 and 26.1%, a turbidity removal of 97.2, 97.1, 99.7, 98.2, 99.2, 98.4 and 99.7% and a TSS removal of 94.8, 94.7, 95.3, 94.8, 95.5, 95.0 and 95.6% were observed for raw WW (no coagulant), Acacia dealbata Link., Chelidonium majus L., Daucus carota L., Tanacetum vulgare L., Vitis vinifera L. and aluminum sulfate, respectively. These results indicated that the performance of pre-treatment with coagulation–flocculation–decantation enhanced TOC, turbidity and TSS removal after the microfiltration process, regarding raw WW. One of this work’s objectives is the reduction in microfiltration costs, which derives mainly from the consumption of filters; therefore, the cost in filter consumption was evaluated (in mg of filter consumed) after the microfiltration process. After the application of the best operational conditions, a filter consumption of 1301, 217, 108, 217, 325, 217 and 108 mg, respectively, for raw WW (no coagulant), Acacia dealbata Link., Chelidonium majus L., Daucus carota L., Tanacetum vulgare L., Vitis vinifera L. and aluminum sulfate was observed. Despite the immediate removal of turbidity and TSS from raw WW, the costs are very high. These results showed a positive correlation between sludge compaction by the coagulation–flocculation–decantation process, and filter consumption by the microfiltration process (y = 2.55x + 12.68, r2 = 0.949), which indicated that the CFD process had a direct effect on microfiltration cost reduction.
4. Conclusions
In this work, WW was treated by a combined CFD/MF treatment. It is concluded that (1) it is possible to produce NaCl plant extracts and apply them as coagulants, (2) the NaCl plant extracts enhance the microfiltration process and decrease the costs; (3) the application of the CFD/MF process is an economic and sustainable technology for WW treatment.
Supplementary Materials
The presentation material can be downloaded at: https://www.mdpi.com/article/10.3390/IOCAG2022-12331/s1.
Author Contributions
Conceptualization, N.J.; methodology, N.J. and P.G.; software, N.J. and P.G.; validation, N.J., A.R.T., L.M. and P.G.; formal analysis, N.J., M.S.L. and J.A.P.; investigation, N.J.; resources, P.G., M.S.L. and J.A.P.; data curation, M.S.L. and J.A.P.; writing—original draft preparation, N.J.; writing—review and editing, N.J., M.S.L. and J.A.P.; visualization, N.J., M.S.L. and J.A.P.; supervision, M.S.L. and J.A.P.; project administration, J.A.P. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful for the financial support of the Project AgriFood XXI, operation nº NORTE-01-0145-FEDER-000041, and to the Fundação para a Ciência e a Tecnologia (FCT) for the financial support provided to CQVR through UIDB/00616/2020. Ana R. Teixeira also thanks the FCT for the financial support provided through the doctoral scholarship UI/BD/150847/2020.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- OIV. State of the World Vitivinicultural Sector in 2019; OIV, International Organisation of Vine and Wine: Paris, France, 2019; pp. 1–15. [Google Scholar]
- Costa, J.M.; Oliveira, M.; Egipto, R.J.; Cid, J.F.; Fragoso, R.A.; Lopes, C.M.; Duarte, E.N. Water and Wastewater Management for Sustainable Viticulture and Oenology in South Portugal—A Review. Ciência Técnica Vitivinícola 2020, 35, 1–15. [Google Scholar] [CrossRef]
- Mosteo, R.; Sarasa, J.; Ormad, M.P.; Ovelleiro, J.L. Sequential Solar Photo-Fenton-Biological System for the Treatment of Winery Wastewaters. J. Agric. Food Chem. 2008, 56, 7333–7338. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.J.S.; Mccrohan, C.R.; White, K.N. Influence of Aqueous Aluminium on the Immune System of the Freshwater Crayfish Pacifasticus Leniusculus. Aquat. Toxicol. 2006, 77, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Heredia, J.B.; Martín, J.S. Improvement of Water Treatment Pilot Plant with Moringa Oleifera Extract as Flocculant Agent. Environ. Technol. 2009, 30, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Rezakazemi, M.; Khajeh, A.; Mesbah, M. Membrane Filtration of Wastewater from Gas and Oil Production. Environ. Chem. Lett. 2018, 16, 367–388. [Google Scholar] [CrossRef]
- Zavoi, S.; Fetea, F.; Ranga, F.; Pop, R.M.; Baciu, A.; Socaciu, C. Comparative Fingerprint and Extraction Yield of Medicinal Herb Phenolics with Hepatoprotective Potential, as Determined by UV-Vis and FT-MIR Spectroscopy. Not. Bot. Horti Agrobot. Cluj-Napoca 2011, 39, 82–89. [Google Scholar] [CrossRef] [Green Version]
- Dobrucka, R.; Dlugaszewska, J.; Kaczmarek, M. Cytotoxic and Antimicrobial Effects of Biosynthesized ZnO Nanoparticles Using of Chelidonium Majus Extract. Biomed. Microdevices 2018, 20, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciorîță, A.; Suciu, M.; Macavei, S.; Kacso, I.; Lung, I.; Soran, M.L.; Pârvu, M. Green Synthesis of Ag-MnO2 Nanoparticles Using Chelidonium Majus and Vinca Minor Extracts and Their In Vitro Cytotoxicity. Molecules 2020, 25, 819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lisperguer, J.; Saravia, Y.; Vergara, E. Structure and Thermal Behavior of Tannins from Acacia Dealbata Bark and Their Reactivity toward Formaldehyde. J. Chil. Chem. Soc. 2016, 61, 3188–3190. [Google Scholar] [CrossRef] [Green Version]
- Carlo, J.; Jiménez, J.; Sofía, A.; Abarca, V.; Bonilla, P.J.; Alpízar, H.B.; Mario, R.; Fallas, S. Extracción y Evaluación de Taninos Condensados a Partir de La Corteza de Once Especies Maderables de Costa Rica. Rev. Tecnol. En Marcha 2012, 25, 15–22. [Google Scholar] [CrossRef]
- Lee, W.; Lan, W. Properties of Resorcinol–Tannin–Formaldehyde Copolymer Resins Prepared from the Bark Extracts of Taiwan Acacia and China Fir. Bioresour. Technol. 2006, 97, 257–264. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).