Abstract
Background: Insulin resistance (IR) is a key pathophysiological mechanism linking obesity, type 2 diabetes, and cardiovascular disease. Office workers, due to prolonged sedentary behavior and suboptimal lifestyle patterns, may be particularly susceptible to IR. However, large-scale studies in this occupational group remain scarce. Objective: To evaluate the prevalence of elevated IR risk using non–insulin-based indices—TyG, METS-IR, and SPISE—and their associations with sociodemographic and lifestyle factors in a large sample of Spanish office workers. Methods: This cross-sectional study included 82,020 office workers from Spain (2021–2022). IR risk was assessed using the TyG index, METS-IR, and SPISE, all derived from fasting biochemical and anthropometric data. Sociodemographic and lifestyle variables were self-reported using validated questionnaires. Sex-stratified analyses and multivariate logistic regression models were performed. Results: Men showed significantly higher odds of elevated IR risk compared to women across all indices: TyG (OR = 2.48, 95% CI: 2.37–2.60), METS-IR (OR = 1.47, 95% CI: 1.38–1.57), and SPISE (OR = 1.88, 95% CI: 1.78–1.99). Smoking, physical inactivity, and low adherence to the Mediterranean diet were independently associated with elevated IR scores, regardless of sex or age. Conclusions: A substantial proportion of office workers exhibit elevated insulin resistance risk, particularly among men and those with unhealthy lifestyles. TyG, METS-IR, and SPISE are valuable, low-cost tools for early IR detection in occupational health settings. These findings support the implementation of preventive strategies targeting modifiable behaviors in sedentary working populations.
1. Introduction
Insulin resistance (IR) is central to the development of type 2 diabetes and cardiometabolic diseases [1,2]. The global burden of IR is increasing in parallel with the rising prevalence of obesity and sedentary behavior. In high-income countries, up to 25% of adults may present with IR without overt hyperglycemia [3]. In Europe, recent data estimate that more than one in five individuals exhibit some degree of IR, with significant differences across age, sex, and socioeconomic groups [4]. Beyond biological determinants, occupational sedentary patterns play a major role, especially among office workers.
The gold standard for assessing insulin sensitivity is the hyperinsulinemic-euglycemic clamp, which directly quantifies glucose disposal rates in response to insulin infusion [5]. Despite its accuracy, this method is invasive, time-consuming, and costly, making it unsuitable for routine clinical use or population-level screening. Alternative methods such as the frequently sampled intravenous glucose tolerance test (FSIVGTT) or oral glucose tolerance test (OGTT) offer more practical options but remain resource-intensive [6]. Consequently, surrogate indices based on fasting biomarkers have gained popularity.
Among insulin-based indices, the Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) and the Quantitative Insulin Sensitivity Check Index (QUICKI) are widely used and validated [7]. However, they require insulin measurements, which are not routinely available in all clinical settings and are subject to assay variability. This has led to the development and increasing use of non–insulin-based indices, which utilize routinely available parameters such as fasting glucose, triglycerides, and anthropometric measures.
The triglyceride-glucose index (TyG) is one such metric, calculated as the natural logarithm of fasting triglycerides multiplied by fasting glucose, divided by two. TyG has demonstrated robust correlations with insulin sensitivity measured by clamp techniques and is consistently associated with T2DM incidence, arterial stiffness, and atherosclerotic burden [8,9]. A recent meta-analysis reported pooled area under the curve (AUC) values for TyG above 0.85 in predicting IR and metabolic syndrome across diverse populations [10].
The Metabolic Score for Insulin Resistance (METS-IR) further incorporates HDL-cholesterol and BMI into the TyG framework, improving predictive performance for cardiometabolic risk and all-cause mortality [11]. METS-IR is particularly useful in stratifying risk among individuals with overlapping metabolic abnormalities and has shown strong associations with hepatic steatosis and subclinical atherosclerosis [12]. In contrast, the Single Point Insulin Sensitivity Estimator (SPISE), derived from BMI, triglycerides, and HDL-c, has been validated mainly in adolescents and young adults, offering an indirect but accessible estimate of insulin sensitivity [13]. Notably, SPISE has been shown to predict incident T2DM and cardiovascular outcomes in longitudinal cohorts [14].
Each of these indices offers unique advantages in terms of simplicity, accessibility, and applicability in large epidemiological studies or occupational health programs. Nevertheless, their diagnostic performance can be influenced by age, sex, ethnic background, and lifestyle factors such as diet and physical activity [15,16]. For instance, smoking, low adherence to the Mediterranean diet, and physical inactivity have been linked to higher TyG index and METS-IR values, independent of BMI [17,18]. Furthermore, significant sex-specific differences in the prevalence and pathophysiological expression of IR have been reported, likely related to hormonal, genetic, and behavioral factors [19].
While several studies have validated non–insulin-based indices in general populations, few large-scale analyses have focused specifically on office workers. This occupational group represents a high-risk population due to prolonged sedentary behavior [20], yet remains underrepresented in epidemiological studies of insulin resistance.
Given these considerations, workplace-based studies are of particular interest for IR surveillance. Sedentary occupations, such as office work, are associated with lower energy expenditure and higher cardiometabolic risk profiles compared to more physically active jobs [21]. Early identification of IR in these populations may allow timely implementation of preventive strategies and reduce long-term disease burden. Despite this, few large-scale studies have examined the prevalence of elevated IR risk scores in occupational cohorts, particularly with stratification by sex and lifestyle variables.
This study aims to address this gap by evaluating the prevalence of elevated insulin resistance risk scores (TyG, METS-IR, and SPISE) in a large cohort of over 80,000 office workers. Using sex-stratified and lifestyle-adjusted analyses, we explore how these indices vary across sociodemographic strata, providing insight into the metabolic health of sedentary working populations. Our findings may help to inform workplace health initiatives and support the integration of non-invasive IR screening tools into routine occupational health assessments.
2. Methods
2.1. Study Design and Population
This cross-sectional study analyzed data collected between January 2021 and December 2022 during periodic occupational health assessments performed by an accredited occupational risk prevention service in Spain. The target population included salaried workers from diverse sectors, with a focus on sedentary employees (office workers). Initially, 105,436 participants aged 18 to 69 years were evaluated.
2.1.1. Inclusion Criteria
- Age between 18 and 69 years.
- Active employment status during the study period.
- Occupation classified as sedentary (i.e., office-based).
- Availability of complete data on anthropometric, biochemical, sociodemographic, and lifestyle variables.
2.1.2. Exclusion Criteria
- Missing data on fasting glucose or triglycerides.
- Incomplete records for anthropometric measurements (e.g., weight, height, waist circumference).
- Missing responses on physical activity, smoking, or dietary questionnaires.
- Diagnosed type 1 or type 2 diabetes mellitus.
- Extreme values or biologically implausible measurements. Extreme values were defined as observations lying beyond ±4 standard deviations from the mean for continuous variables. These values were excluded as probable data entry or measurement errors.
- Individuals with diagnosed diabetes were excluded because their lifestyle behaviors may have already been modified by medical advice, which could confound associations. We acknowledge that this exclusion may lead to underestimation of the true prevalence of IR in office workers.
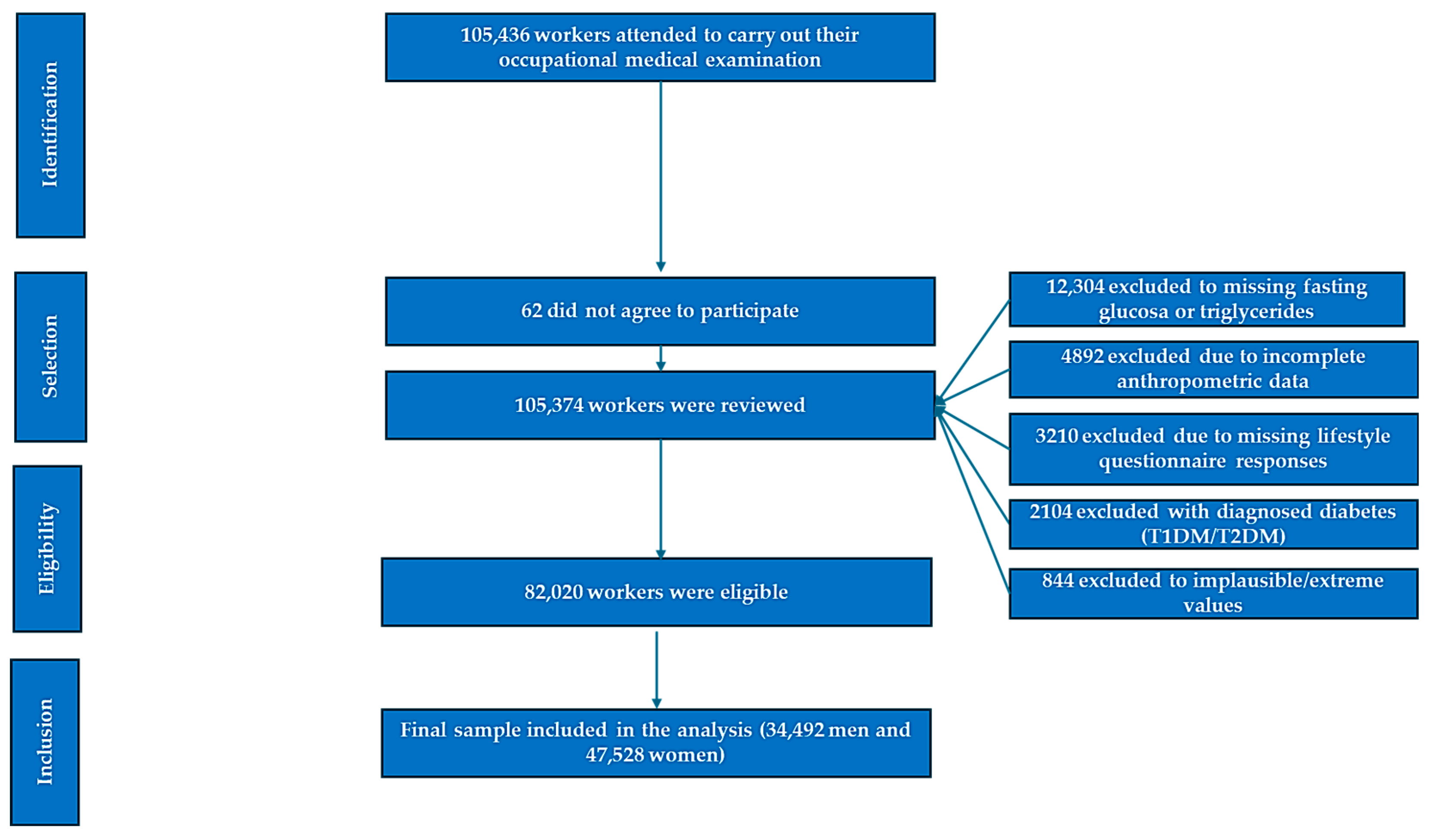
After applying these criteria, a total of 82,020 office workers were included in the final analytical sample (Figure 1).
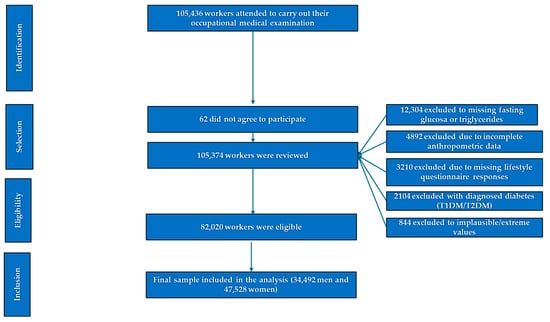
Figure 1.
Flowchart of participant selection.
2.2. Anthropometric and Clinical Measurements
Trained medical staff performed all measurements using standardized protocols. Height and weight were measured with calibrated stadiometers and scales, and body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). Waist circumference was measured at the midpoint between the iliac crest and the lower rib margin. Blood pressure was recorded in the sitting position after five minutes of rest using validated automated sphygmomanometers.
Fasting venous blood samples were collected after at least 8 h of fasting. Laboratory analyses included fasting plasma glucose, triglycerides, total cholesterol, HDL cholesterol, and calculated LDL cholesterol (via Friedewald formula). All laboratory procedures followed ISO 9001 quality standards.
2.3. Insulin Resistance Indices
Three non-insulin-based indices were calculated to estimate insulin resistance:
- TyG index = ln [fasting triglycerides (mg/dL) × fasting glucose (mg/dL)/2].
- METS-IR = ln [(2 × fasting glucose) + triglycerides] × BMI / ln [HDL cholesterol].
- SPISE as calculated as 600 × HDL-c^0.185/(triglycerides^0.2 × BMI^1.338). Lower SPISE values indicate higher insulin resistance. To facilitate interpretation, we additionally computed a derived variable (SPISE-IR = 10/SPISE), following previous methodological proposals [22,23].
These surrogate markers have demonstrated strong correlation with the hyperinsulinemic-euglycemic clamp method and are widely used in epidemiological and occupational studies [24,25,26]. Different publications have found good sensitivity and specificity for use in active populations [27,28,29,30].
Thresholds for elevated IR risk were defined according to previously validated cutoffs in Spanish and international cohorts. For the TyG index, cutoffs associated with impaired insulin sensitivity and higher cardiometabolic risk were established in validation studies against the euglycemic–hyperinsulinemic clamp and in large Spanish cohorts. For the METS-IR, thresholds predictive of visceral adiposity and incident type 2 diabetes have been consistently reported in both Latin American and Asian populations. Similarly, the SPISE index has been validated in European and pediatric cohorts, showing strong discriminatory capacity for impaired glucose regulation. All three indices demonstrated areas under the curve (AUC) greater than 0.80 compared with gold-standard methods, supporting their application in large-scale epidemiological and occupational health research [13,25,26,31].
2.4. Lifestyle and Sociodemographic Variables
Sociodemographic data included age, sex, education level, and employment sector. Lifestyle factors were evaluated using validated tools:
- Smoking status was categorized as current, former, or never smoker.
- Physical activity was assessed using the short version of the International Physical Activity Questionnaire (IPAQ-SF). Participants were classified as physically active if they reported ≥150 min/week of moderate activity, ≥75 min/week of vigorous activity, or an equivalent combination [32].
- Physical activity was assessed using the International Physical Activity Questionnaire-Short Form (IPAQ-SF), validated in Spanish adult populations [33]. Diet quality was evaluated with the 14-item Mediterranean Diet Adherence Screener (MEDAS), validated in Spanish cohorts [34]. Although not specifically tested in office workers, these instruments have shown reliability in occupational health contexts.
- Adherence to the Mediterranean diet was measured using a validated short screener; scores ≥ 9 indicated good adherence to the dietary pattern [35].
- Lifestyle factors were evaluated using validated tools: physical activity with the IPAQ-SF and dietary habits with the 14-item Mediterranean diet screener (MEDAS), both previously validated in Spanish populations. Smoking status was obtained through a standardized occupational health questionnaire. To minimize recall and social desirability biases, all questionnaires were administered during routine occupational health assessments under uniform conditions, with guarantees of anonymity and confidentiality to limit reporting bias.
2.5. Statistical Analysis
Quantitative variables were summarized as means and standard deviations, and categorical variables as percentages. Sex differences in continuous and categorical variables were assessed using independent t-tests and χ2 tests, respectively. Associations between sociodemographic and lifestyle factors and elevated insulin resistance risk (defined by each index) were examined using multivariate logistic regression models adjusted for age, education, diet, physical activity, and smoking status. Results were stratified by sex.
Normality of continuous variables was evaluated using Kolmogorov–Smirnov and Shapiro–Wilk tests, complemented by histogram and Q-Q plot inspection. Given the large sample size, small deviations reached statistical significance; however, most variables displayed approximately normal distributions. For skewed variables (notably triglycerides), both mean ± SD and median [IQR] are reported. Non-parametric tests (Mann–Whitney U) were performed as sensitivity analyses, yielding results consistent with the t-test findings.
We additionally performed sensitivity analyses stratifying by age ranges (18–29, 30–39, 40–49, 50–59, and 60–69 years) to evaluate whether associations between sociodemographic and lifestyle variables and IR risk indices remained consistent across age groups. Results were highly consistent with the overall models, supporting the robustness of our findings. Given that all participants were office workers, subgroup analyses by other occupational sectors were not applicable in this study.
Statistical analyses were performed using SPSS software, version 29.0 (IBM Corp., Armonk, NY, USA), and a p-value < 0.05 was considered statistically significant.
3. Results
Table 1 summarizes the baseline characteristics of the study population stratified by sex. Statistically significant differences (p < 0.001) are observed across all variables. Men showed higher mean values for weight, height, waist circumference, systolic and diastolic blood pressure, triglycerides, glucose, and LDL-cholesterol, whereas women exhibited higher HDL-cholesterol levels and a more favorable lifestyle profile, including greater adherence to the Mediterranean diet and higher physical activity rates.

Table 1.
Baseline anthropometric, clinical, and lifestyle characteristics by sex (n = 82,020).
These findings align with well-documented sex-specific differences in cardiometabolic risk and lifestyle behaviors. The lower HDL-c and higher triglyceride levels observed in men are of particular concern given their association with increased insulin resistance and cardiovascular disease. The markedly healthier lifestyle pattern in women, especially their higher engagement in physical activity and diet quality, may partially explain the observed metabolic disparities. The large sample size lends robustness to the observed differences and supports the relevance of sex-stratified prevention strategies in occupational health settings.
Table 2 displays the average values of three insulin resistance (IR) indices—Triglyceride-Glucose Index (TyG), Metabolic Score for Insulin Resistance (METS-IR), and Single Point Insulin Sensitivity Estimator (SPISE)—stratified by sex, age group, smoking status, diet, and physical activity.

Table 2.
Mean values of TyG, METS-IR, and SPISE-IR indices by age, smoking status, mediterranean diet adherence, and physical activity, stratified by sex.
A clear age-related increase in TyG and METS-IR is observed in both men and women, indicating a progressive decline in insulin sensitivity with aging. Conversely, SPISE values, which inversely correlate with insulin resistance, decrease with age, further supporting the same trend.
Smoking, lack of physical activity, and non-adherence to the Mediterranean diet are consistently associated with higher TyG and METS-IR scores and lower SPISE values across sexes. These findings reinforce the strong influence of modifiable lifestyle factors on insulin sensitivity. Notably, the impact of healthy behaviors such as regular physical activity and Mediterranean dietary adherence appears substantial, with these groups showing markedly more favorable IR profiles regardless of age or sex.
The sex-stratified analysis highlights that women, overall, present lower TyG and METS-IR and higher SPISE values, suggesting better metabolic health compared to men. These results underscore the utility of non-insulin-based indices in large epidemiological and occupational settings and the need for lifestyle-targeted interventions to mitigate IR-related risk.
Table 3 presents the proportion of individuals with high insulin resistance risk, defined by elevated values of TyG, METS-IR, and SPISE indices, stratified by sex and key demographic and lifestyle variables.

Table 3.
Prevalence of elevated TyG, METS-IR, and SPISE index values by age, smoking status, mediterranean diet adherence, and physical activity, stratified by sex.
An age-related gradient is evident in both sexes, particularly for the TyG index. The prevalence of high TyG values increases substantially with age, reaching 39.0% in men and 31.2% in women aged 60–69. This trend is similarly observed for METS-IR and SPISE, though with slightly lower prevalence rates. These findings confirm the progressive metabolic deterioration associated with aging.
The lifestyle variables show strong associations with IR risk. Non-adherence to the Mediterranean diet and physical inactivity are markedly associated with higher proportions of elevated IR scores across all indices. For example, in non-physically active men, the prevalence of high TyG reaches 43.2%, compared to only 1.9% in their active counterparts. Similar patterns are observed for METS-IR and SPISE, reinforcing the crucial role of preventive lifestyle interventions.
Women consistently show lower prevalence rates of elevated IR indices across categories, supporting previously observed sex-based metabolic advantages. However, lifestyle behaviors appear to significantly modify this advantage, particularly among women with poor dietary or physical activity profiles.
This table provides compelling evidence for targeted screening strategies using simple IR indices and highlights the modifiable nature of many IR risk factors, which is critical in occupational health settings.
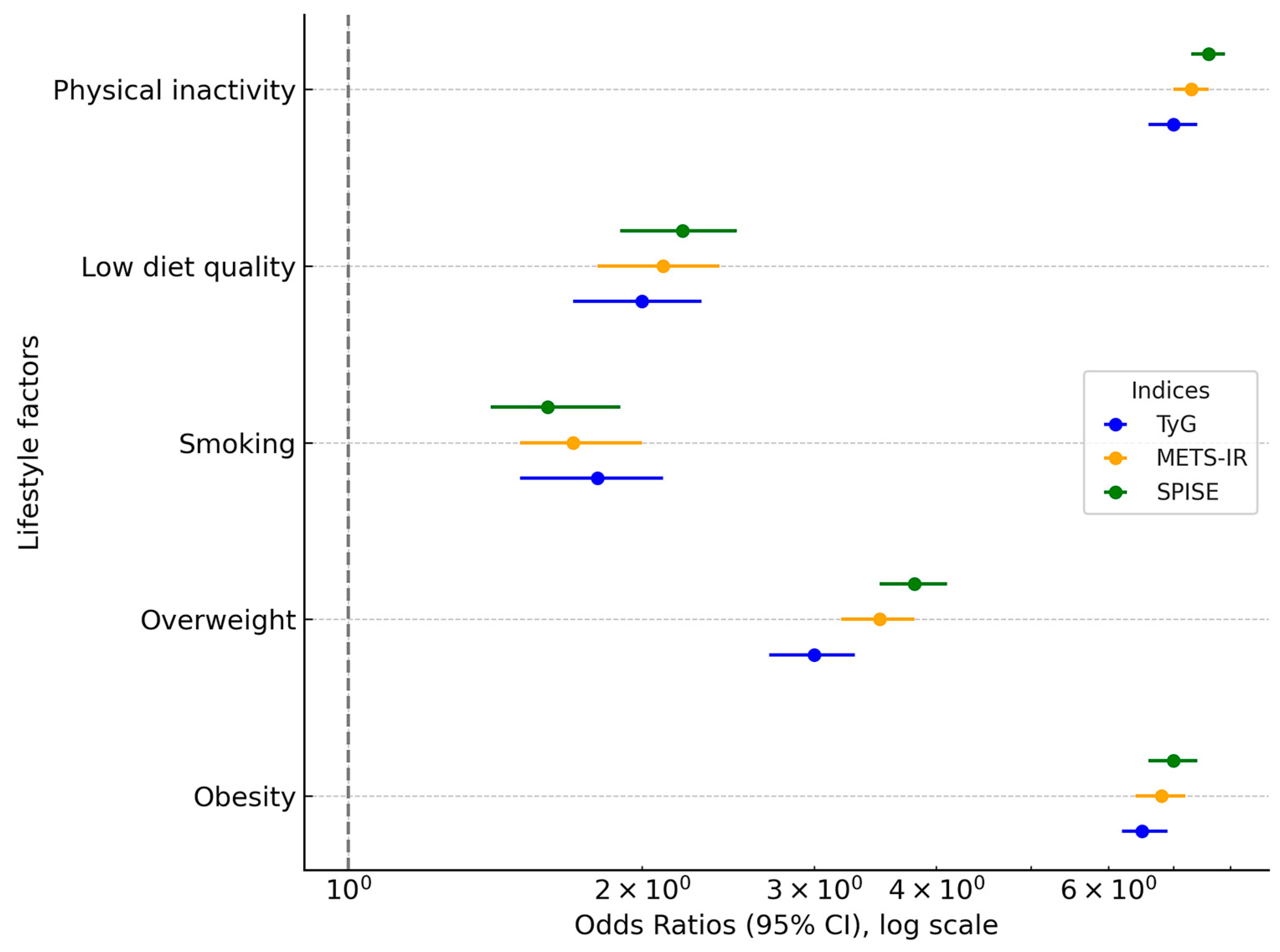
Table 4 and Figure 2 present the results of the multivariate logistic regression analysis assessing the association between sex, age, and lifestyle factors with the likelihood of elevated values of the three insulin resistance (IR) indices: TyG, METS-IR, and SPISE. Women served as the reference category in all models.

Table 4.
Adjusted Odds Ratios (OR) and Absolute Risk Differences (ARD, in %) for elevated TyG, METS-IR, and SPISE indices: multivariate logistic regression analysis.
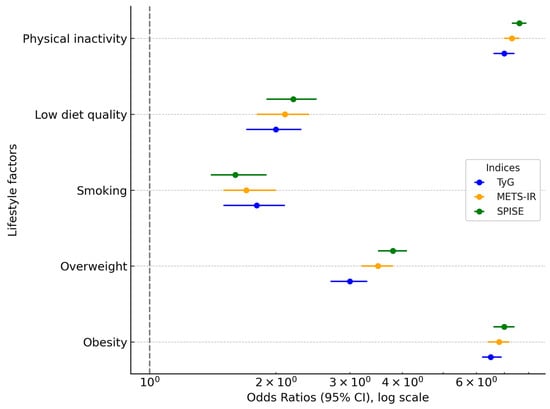
Figure 2.
Forest plot of odds ratios for insulin resistance indices (TyG, METS-IR, SPISE) by sex, lifestyle, and sociodemographic variables. The x-axis is log-transformed for clarity, and the x = 0 reference point is explicitly marked.
As expected, men showed significantly higher odds of exhibiting elevated IR risk across all indices. Specifically, men had an odds ratio (OR) of 2.48 (95% CI: 2.37–2.60) for high TyG, 1.47 (95% CI: 1.38–1.57) for high METS-IR, and 1.88 (95% CI: 1.78–1.99) for low SPISE values (indicating greater insulin resistance). Importantly, when expressed as absolute risk differences (ARDs), these associations translate into an excess risk of +11.6% for high TyG, +3.1% for high METS-IR, and +5.7% for high SPISE-IR in men compared with women.
Similar gradients were observed across age groups, smoking status, diet, and physical activity. For example, physical inactivity was associated with an OR of 4.59 (95% CI: 3.98–5.19) for high TyG, corresponding to an ARD of +19.7% compared with active individuals. Non-adherence to the Mediterranean diet was associated with an OR of 2.35 (95% CI: 2.10–2.61) for high TyG and an ARD of +12.3%. These complementary ARD estimates improve clinical interpretability by quantifying the actual excess probability of elevated IR scores attributable to sociodemographic and lifestyle risk factors.
Sensitivity analyses excluding participants with borderline IR index values and stratifying by BMI categories yielded similar results, supporting the robustness of our findings (Supplementary Tables S1 and S2). Additional sensitivity analyses stratified by age ranges confirmed the robustness of the associations, with consistent patterns observed across all age groups (Supplementary Table S3).
Model fit was further evaluated using the Akaike Information Criterion (AIC). TyG demonstrated the lowest AIC, indicating the best model fit, followed by SPISE and METS-IR. These results were consistent across sex-stratified analyses, reinforcing the robustness of TyG as the most predictive index in this cohort.
4. Discussion
4.1. Comparison with Previous Studies
This study contributes to a growing body of literature highlighting the relevance of surrogate insulin resistance (IR) indices in occupational health. Our findings address a critical research gap by focusing on office workers, a population where sedentary behavior is predominant but has been rarely studied at this scale in relation to insulin resistance risk.
A recent study published evaluated cardiometabolic risk in more than 43,000 Spanish office employees and found that older age, male sex, smoking, and low physical activity were independently associated with increased risk of metabolic disorders [36]. Although their approach was based on general cardiometabolic indices, and not specific IR scores, our findings corroborate their conclusion by applying validated surrogate indices such as TyG, METS-IR, and SPISE. Importantly, we provide a more granular risk stratification, including sex-specific logistic regression models and lifestyle-adjusted analyses. Although these indices are surrogate markers, meta-analyses and occupational studies have shown AUC > 0.80 compared to gold-standard measures, supporting their validity in epidemiological surveillance.
A systematic review and meta-analysis reported that, based on current evidence from observational studies, a high TyG index may be significantly associated with the incidence of stable coronary artery disease, myocardial infarction, and overall cardiovascular disease [37]. However, no association was observed with cardiovascular or all-cause mortality in the general population. Furthermore, findings suggest a possible positive linear relationship between TyG index and stable coronary artery disease, as well as with the combined incidence of cardiovascular disease [37]. In a Korean study, the TyG index was significantly associated with coronary artery calcification (CAC), even after adjustment for conventional factors. It also showed a stronger relationship with coronary atherosclerosis than HOMA-IR in healthy adults. These findings suggest that the TyG index may serve as a useful marker of cardiovascular risk, though further prospective studies are needed [38].
Other occupational studies offer relevant comparisons. For example, a national study on Spanish healthcare workers (n = 44,939) observed elevated IR risk scores—particularly among smokers, individuals with low Mediterranean diet adherence, and those reporting physical inactivity [39]. These results are consistent with our findings and reinforce the notion that professional environments marked by psychosocial stress and sedentary routines—such as hospitals or offices—can amplify metabolic vulnerability [40,41,42,43,44].
Beyond Spain, a cross-sectional study of bank employees in Brazil highlighted the association between IR and central obesity, reporting a strong predictive role for waist circumference and BMI [45]. Similar findings were reported in another study conducted in India [46]. Similar associations between lifestyle behaviors and IR risk have been observed in occupational cohorts from Asia [47,48] and North America [49,50], underscoring the generalizability of our findings beyond Spain [51,52].
Another recent investigation involving over 700,000 Spanish workers confirmed the utility of TyG, METS-IR, and SPISE for identifying high-risk individuals, supporting their use in population-based screening programs [53]. Nevertheless, few of these studies have focused exclusively on office workers—a gap that our study addresses with one of the largest samples to date in this population.
Our findings also agree with international literature. A Chinese study found that METS-IR was a similar predictor of prediabetes and metabolic syndrome than HOMA-IR in non-diabetic adults [54]. SPISE was shown to predict insulin sensitivity in adolescents, especially in low-income, underserved populations [55]. These observations confirm the cross-cultural and age-group validity of these indices, and support their adaptation to occupational health settings where insulin assays are impractical [56,57].
The large prevalence contrast (43.2% vs. 1.9%) likely reflects true subgroup differences in lifestyle and metabolic risk. However, it may also be influenced by residual confounding or index-based misclassification, and thus requires cautious interpretation.
The strong associations observed in our study, particularly the odds ratio exceeding 7 for physical inactivity, highlight the central role of lifestyle behaviors in the development of insulin resistance. However, effect sizes of this magnitude should be interpreted with caution. Such associations may be partially explained by residual confounding due to unmeasured factors such as alcohol consumption, sleep duration, and psychosocial stress, which were not available in our dataset. In addition, the reliance on self-reported lifestyle measures may have introduced exposure misclassification, potentially exaggerating observed associations. Interaction effects across demographic subgroups (e.g., age, sex, BMI categories) could also contribute to the magnitude of these findings. Previous large-scale epidemiological studies have similarly reported strong links between physical inactivity and insulin resistance, though typically with more moderate effect sizes [58,59,60]. Therefore, while our results emphasize the importance of promoting physical activity in occupational settings, they should be interpreted within the context of potential biases, and replication in longitudinal studies with comprehensive adjustment for lifestyle and biological covariates is warranted.
The sex differences observed in insulin resistance risk are consistent with prior evidence and may be explained by a combination of biological and behavioral mechanisms. Estrogen appears to confer protective effects on glucose metabolism and insulin sensitivity by modulating adipose tissue distribution, enhancing insulin signaling, and improving vascular function [61,62]. These protective effects diminish during the menopausal transition, contributing to the rise in IR risk among women in midlife [63]. In contrast, men are more prone to visceral adiposity and ectopic fat deposition at earlier ages, which accelerates the development of insulin resistance [64]. Beyond hormonal regulation, sex-specific genetic factors, differences in adipokine secretion, and variations in inflammatory response may also play a role [65]. Moreover, behavioral patterns—including disparities in physical activity, dietary habits, and smoking prevalence—may interact with biological mechanisms to further shape sex-related differences in IR risk [66]. Recognizing these mechanisms is important for contextualizing our findings and may support the development of tailored prevention strategies for men and women across different life stages.
Our findings also have important implications for workplace health promotion. Evidence from occupational health programs suggests that structured wellness initiatives can effectively reduce cardiometabolic risk factors when integrated into routine workplace practices. Interventions may include scheduled physical activity breaks, promotion of active commuting, and the implementation of healthy canteen policies offering balanced dietary options. Smoking cessation initiatives and stress management workshops have also demonstrated beneficial effects on metabolic outcomes [67,68,69]. However, the scalability and sustainability of such programs depend on organizational commitment, resource allocation, and employee engagement. Tailoring interventions to the specific needs of office workers, while considering age, sex, and baseline risk profiles, may enhance their effectiveness. Furthermore, integration of digital health tools, such as mobile applications and wearable devices, could provide additional opportunities for monitoring and supporting lifestyle modification in occupational settings. Future research should evaluate not only the clinical effectiveness of these interventions but also their cost-effectiveness and feasibility in diverse occupational environments.
4.2. Main Contributions of This Study
This study offers several novel contributions:
- This study represents one of the largest analyses to date focusing exclusively on office workers, adding valuable evidence to the field.
- Use of validated, non-insulin-based indices: By employing TyG, METS-IR, and SPISE, we provide a feasible, cost-effective alternative to direct insulin measurements, which are rarely included in occupational screening protocols.
- Comprehensive lifestyle analysis: Our inclusion of physical activity (measured by IPAQ), smoking status, and adherence to the Mediterranean diet allows for a more nuanced understanding of behavioral determinants of IR.
- Analytical depth: We present detailed multivariate logistic regression models stratified by sex, adjusting for key sociodemographic and lifestyle factors, enhancing the robustness and clinical relevance of our findings.
- Furthermore, the robustness of our results was supported by sensitivity analyses stratified by age ranges, which yielded consistent associations across all subgroups. This reinforces the reliability of our findings and suggests that the observed patterns are not driven by specific age categories within the office-working population.
4.3. Strengths and Limitations
Strengths of this study include:
- Large, homogeneous sample size of over 82,000 office workers, which enhances statistical power and generalizability to similar occupational environments.
- Standardized data collection by trained personnel following rigorous clinical protocols.
- Application of multiple IR indices, allowing comparison of their predictive performance in a real-world occupational setting.
- Stratification by sex and lifestyle, which reduces confounding and highlights high-risk subgroups for targeted interventions.
However, the study is not without limitations:
- Given the cross-sectional design, temporality cannot be established, and reverse causation (e.g., individuals with elevated IR risk being less active) cannot be excluded. Longitudinal studies are needed to confirm causal pathways.
- Although validated, the surrogate indices used (TyG, METS-IR, SPISE) may not fully reflect dynamic metabolic processes captured by gold-standard techniques such as the euglycemic clamp.
- Although validated questionnaires and standardized protocols were applied, self-reported lifestyle factors are inherently prone to recall and social desirability bias, which may have led to some degree of misclassification.
- Additionally, individuals with diagnosed diabetes were excluded from the analyses. This decision was made to avoid potential bias arising from lifestyle modifications following medical advice, which could distort associations with IR indices. However, this exclusion may also result in underestimation of the true prevalence of insulin resistance among office workers.
- Our sample was limited to Spanish office workers, predominantly from a homogeneous European background. Extrapolation to more ethnically diverse populations, those with different occupational structures, or workers in lower-income settings should be done cautiously.
- Although the thresholds used to classify elevated IR risk are based on widely cited validation studies [13,25,26,31], it is important to acknowledge that specific validation in occupational cohorts remains limited. The use of these indices should therefore be interpreted as a pragmatic approach to estimate IR risk in large populations of office workers rather than as definitive diagnostic criteria. Future research should focus on establishing tailored cutoffs that account for occupational and demographic characteristics.
- Another limitation of our study is the absence of data on certain lifestyle and psychosocial factors that may act as potential confounders. Specifically, alcohol consumption, psychological stress, and sleep duration/quality were not available in our dataset. Each of these factors has been consistently associated with insulin resistance and metabolic dysfunction in previous studies [70]. For example, moderate-to-high alcohol intake has been linked to impaired insulin signaling and increased risk of type 2 diabetes, while chronic stress may contribute to hypercortisolism and systemic inflammation, both of which exacerbate insulin resistance. Similarly, short or disrupted sleep has been identified as an independent determinant of insulin resistance through mechanisms involving altered glucose metabolism, sympathetic activation, and hormonal dysregulation. The absence of these variables in our analyses may therefore contribute to residual confounding and partially explain the strength of some associations observed. Future studies in occupational cohorts should incorporate comprehensive assessments of alcohol intake, stress, and sleep to more accurately capture the multifactorial determinants of insulin resistance.
- Finally, a potential healthy worker bias should be acknowledged, as employed individuals undergoing occupational health assessments may represent a healthier subset compared to the general population, possibly underestimating true IR prevalence.
4.4. Implications and Future Directions
Our findings suggest the importance of incorporating IR risk assessment into occupational health strategies. However, rather than a paradigm shift, these results should be seen as supporting evidence within the broader preventive health framework.
However, our sample was limited to Spanish office workers, predominantly from a homogeneous European background. Extrapolation to more ethnically diverse populations, those with different occupational structures, or workers in lower-income settings should be done cautiously.
Future research should aim to:
- Validate these indices prospectively, tracking their ability to predict incident diabetes, cardiovascular disease, and mortality in diverse occupational cohorts.
- Investigate interventional strategies targeting modifiable risk factors (diet, smoking, physical inactivity) to assess whether improvements in TyG, METS-IR, and SPISE translate to clinical benefit.
- Explore the integration of digital health tools (e.g., wearables, mobile apps) for continuous lifestyle tracking and personalized feedback.
- Conduct cost-effectiveness analyses comparing these indices with traditional diagnostic approaches in occupational medicine settings.
Ultimately, this research supports a paradigm shift in workplace health, from disease management to early metabolic risk identification and prevention, with implications for policy, employer wellness strategies, and clinical guidelines.
5. Conclusions
This study highlights a high prevalence of elevated insulin resistance (IR) risk scores among office workers, a population typically exposed to prolonged sedentary behavior and suboptimal lifestyle patterns. Using validated non-insulin-based indices—TyG, METS-IR, and SPISE—we identified substantial sex-based disparities and significant associations with modifiable lifestyle factors such as physical activity, smoking, and adherence to the Mediterranean diet.
Our results highlight strong associations between lifestyle factors and insulin resistance risk in office workers, underscoring the value of non–insulin-based indices in occupational health surveillance. Given the cross-sectional design, causal inferences cannot be drawn.
Our findings support a more targeted rather than universal approach to screening for insulin resistance in occupational settings. Specifically, the data indicate that high-risk subgroups—such as older men, physically inactive workers, individuals with central adiposity, and those reporting poor dietary habits—are more likely to benefit from early identification. Focused screening in these populations may optimize resource allocation and increase the yield of preventive interventions. At the same time, broader implementation of screening strategies could be considered in occupational health systems with sufficient resources, particularly given the high overall prevalence of insulin resistance markers in office workers. Ultimately, integrating risk stratification into routine occupational health assessments may help balance feasibility, cost-effectiveness, and equity in preventive care delivery.
Given the global rise in sedentary employment, these results underscore the importance of integrating metabolic risk assessment into routine occupational health protocols. Future longitudinal research is warranted to confirm the predictive value of these indices and to evaluate the impact of lifestyle interventions on improving IR status and preventing progression to diabetes and cardiovascular disease.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/diabetology6110137/s1, Table S1: Sensitivity analysis excluding borderline IR index values*; Table S2: Sensitivity analysis stratified by BMI categories; Table S3: Sensitivity analyses stratified by age ranges.
Author Contributions
Conceptualization: A.R.G. and Á.A.L.-G.; Data collection and analysis: P.J.T.L., I.C.C. and C.B.-C.; Data curation: C.B.-C. and P.J.T.L. Methodology: M.G.S. and I.C.C. Validation: J.I.R.M.; Formal analysis: A.R.G.; Investigation C.B.-C.; Draft: P.J.T.L., I.C.C., J.I.R.M. and M.G.S.; Revision: Á.A.L.-G., A.R.G. and J.I.R.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study was conducted entirely independently, without any financial support, institutional funding, or sponsorship from external sources.
Institutional Review Board Statement
The study protocol was reviewed and approved by the Ethics Committee of the Balearic Islands (Comité de Ética de la Investigación de las Islas Baleares, CEI-IB) under reference number IB 4383/20 (dated 26 November 2020). All identifying data were anonymized using encrypted codes accessible only to the principal investigator, thereby ensuring strict con-fidentiality. No personal or identifiable data will be disclosed or published.
Informed Consent Statement
Written informed consent was obtained from all participants after they were fully briefed on the nature and purpose of the study.
Data Availability Statement
The research data are securely stored at the ADEMA University School and are managed in accordance with applicable data protection legislation. Oversight is provided by the institution’s designated Data Protection Officer, Ángel Arturo López González.
Conflicts of Interest
The authors declare no actual or potential conflicts of interest with respect to the design, conduct, analysis, or publication of this research.
Ethical Approval and Regulatory Compliance
All research procedures strictly adhered to national and international ethical standards for biomedical research, following the principles set forth in the Declaration of Helsinki. The study design ensured full protection of participant rights, privacy, and anonymity. Prior to inclusion, all participants received comprehensive verbal and written information regarding the objectives, procedures, and scope of the study. Participation was voluntary, and written informed consent was obtained from every participant prior to data collection. The study protocol was reviewed and approved by the Ethics Committee of the Balearic Islands (Comité de Ética de la Investigación de las Islas Baleares, CEI-IB) under reference number IB 4383/20 (dated 26 November 2020). All identifying data were anonymized using encrypted codes accessible only to the principal investigator, thereby ensuring strict confidentiality. No personal or identifiable data will be disclosed or published. In full compliance with Spain’s Organic Law 3/2018 on the Protection of Personal Data and Guarantee of Digital Rights, as well as with the European Union’s General Data Protection Regulation (EU Regulation 2016/679), all participants were informed of their rights to access, rectify, delete, or object to the processing of their personal data.
References
- Kosmas, C.E.; Bousvarou, M.D.; Kostara, C.E.; Papakonstantinou, E.J.; Salamou, E.; Guzman, E. Insulin resistance and cardiovascular disease. J. Int. Med. Res. 2023, 51, 03000605231164548. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Adams-Huet, B.; Zubirán, R.; Remaley, A.T.; Jialal, I. The triglyceride-glucose index is superior to homeostasis model assessment of insulin resistance in predicting metabolic syndrome in an adult population in the United States. J. Clin. Lipidol. 2024, 18, e518–e524. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Ke, Y.; Nianogo, R.A. Trends in Hyperinsulinemia and Insulin Resistance Among Nondiabetic US Adults, NHANES, 1999–2018. J. Clin. Med. 2025, 14, 3215. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aguiló Juanola, M.C.; López-González, A.A.; Tomás-Gil, P.; Paublini, H.; Tárraga-López, P.J.; Ramírez-Manent, J.I. Influence of tobacco consumption on the values of different insulin resistance risk scales and non-alcoholic fatty liver disease and hepatic fibrosis scales in 418,343 spanish people. Acad. J. Health Sci. 2024, 39, 9–15. [Google Scholar] [CrossRef]
- Freeman, A.M.; Acevedo, L.A.; Pennings, N. Insulin Resistance. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Gubbi, S.; Muniyappa, R.; Sharma, S.T.; Grewal, S.; McGlotten, R.; Nieman, L.K. Mifepristone Improves Adipose Tissue Insulin Sensitivity in Insulin Resistant Individuals. J. Clin. Endocrinol. Metab. 2021, 106, 1501–1515. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Paracha, A.I.; Haroon, Z.H.; Aamir, M.; Bibi, A. Diagnostic Accuracy of Markers of Insulin Resistance (HOMA-IR) and Insulin Sensitivity (QUICKI) in Gestational Diabetes. J. Coll. Physicians Surg. Pak. 2021, 31, 1015–1019. [Google Scholar] [CrossRef] [PubMed]
- Sastre-Alzamora, T.; Tomás-Gil, P.; Paublini, H.; Pallarés, L.; Ramírez-Manent, J.I.; López-González, A.A. Relationship between heart age and insulin resistance risk scales in 139634 Spanish workers. Acad. J. Health Sci. 2024, 39, 16–22. [Google Scholar] [CrossRef]
- Ramdas Nayak, V.K.; Satheesh, P.; Shenoy, M.T.; Kalra, S. Triglyceride Glucose (TyG) Index: A surrogate biomarker of insulin resistance. J. Pak. Med. Assoc. 2022, 72, 986–988. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.S.; Kuriyakose, D.; Polisetty, L.D.; Patil, A.A.; Ameen, D.; Bonu, R.; Shetty, S.P.; Biswas, P.; Ulrich, M.T.; Letafatkar, N.; et al. Diagnostic and prognostic value of triglyceride glucose index: A comprehensive evaluation of meta-analysis. Cardiovasc. Diabetol. 2024, 23, 310. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vicente-Herrero, M.T.; Egea-Sancho, M.; Ramírez Iñiguez de la Torre, M.V.; López-González, A.A. Relación de los índices de adiposidad visceral (VAI) y adiposidad disfuncional (DAI) con las escalas de riesgo de resistencia a la insulina y prediabetes. Acad. J. Health Sci. 2024, 39, 25–31. [Google Scholar] [CrossRef]
- Peng, H.; Xiang, J.; Pan, L.; Zhao, M.; Chen, B.; Huang, S.; Yao, Z.; Liu, J.; Lv, W. METS-IR/HOMA-IR and MAFLD in U.S. adults: Dose–response correlation and the effect mediated by physical activity. BMC Endocr. Disord. 2024, 24, 132. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tantari, G.; Bassi, M.; Pistorio, A.; Minuto, N.; Napoli, F.; Piccolo, G.; La Valle, A.; Spacco, G.; Cervello, C.; D’annunzio, G.; et al. SPISE INDEX (Single point insulin sensitivity estimator): Indicator of insulin resistance in children and adolescents with overweight and obesity. Front. Endocrinol. 2024, 15, 1439901. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vicente-Herrero, M.T.; Gordito Soler, M.; García Agudo, S.; Vallejos, D.; López-González, A.A.; Ramírez-Manent, J.I. Cardiometabolic risk level in 1136 Spanish professional musicians. Acad. J. Health Sci. 2024, 39, 59–66. [Google Scholar] [CrossRef]
- Zhou, H.; Ding, X.; Lan, Y.; Chen, S.; Wu, S.; Wu, D. Multi-trajectories of triglyceride-glucose index and lifestyle with Cardiovascular Disease: A cohort study. Cardiovasc. Diabetol. 2023, 22, 341. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Teymoori, F.; Jahromi, M.K.; Ahmadirad, H.; Daftari, G.; Mokhtari, E.; Farhadnejad, H.; Mirmiran, P.; Azizi, F. The association of dietary and lifestyle indices for insulin resistance with the risk of cardiometabolic diseases among Iranian adults. Sci. Rep. 2023, 13, 6224. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tian, X.; Zuo, Y.; Chen, S.; Meng, X.; Chen, P.; Wang, Y.; Wu, S.; Luo, Y.; Wang, A. Distinct triglyceride-glucose trajectories are associated with different risks of incident cardiovascular disease in normal-weight adults. Am. Heart J. 2022, 248, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Sangouni, A.A.; Hosseinzadeh, M.; Parastouei, K. Effect of dietary approaches to stop hypertension diet on insulin resistance and lipid accumulation product in subjects with metabolic syndrome. Sci. Rep. 2025, 15, 17025. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ciarambino, T.; Crispino, P.; Guarisco, G.; Giordano, M. Gender Differences in Insulin Resistance: New Knowledge and Perspectives. Curr. Issues Mol. Biol. 2023, 45, 7845–7861. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ryde, G.C.; Brown, H.E.; Gilson, N.D.; Brown, W.J. Are we chained to our desks? Describing desk-based sitting using a novel measure of occupational sitting. J. Phys. Act. Health 2014, 11, 1318–1323. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Recasens, M.; Alfaro-Barrio, S.; Tarro, L.; Llauradó, E.; Solà, R. Occupational Physical Activity and Cardiometabolic Risk Factors: A Cross-Sectional Study. Nutrients 2023, 15, 1421. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cederholm, J.; Zethelius, B. SPISE and other fasting indexes of insulin resistance: Risks of coronary heart disease or type 2 diabetes. Comparative cross-sectional and longitudinal aspects. Upsala J. Med. Sci. 2019, 124, 265–272. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Correa-Burrows, P.; Matamoros, M.; de Toro, V.; Zepeda, D.; Arriaza, M.; Burrows, R. A Single-Point Insulin Sensitivity Estimator (SPISE) of 5.4 is a good predictor of both metabolic syndrome and insulin resistance in adolescents with obesity. Front. Endocrinol. 2023, 14, 1078949. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Manzanero, R.Z.; López-González, A.A.; Tomás-Gil, P.; Paublini, H.; Martínez-Jover, A.; Ramírez-Manent, J.I. Cardiometabolic risk assessment in 28300 spanish waiters. Acad. J. Health Sci. 2023, 39, 16–24. [Google Scholar] [CrossRef]
- Guerrero-Romero, F.; Simental-Mendía, L.E.; Gonzalez-Ortiz, M.; Martínez-Abundis, E.; Ramos-Zavala, M.G.; Hernandez-Gonzalez, S.O.; Jacques-Camarena, O.; Rodríguez-Moran, M. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J. Clin. Endocrinol. Metab. 2010, 95, 3347–3351. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Lee, J.W.; Kwon, Y.J. Comparison of the triglyceride glucose (TyG) index, triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio, and metabolic score for insulin resistance (METS-IR) associated with periodontitis in Korean adults. Ther. Adv. Chronic Dis. 2022, 13, 20406223221122671. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- D’Elia, L.; Rendina, D.; Iacone, R.; Strazzullo, P.; Galletti, F. Triglyceride–Glucose Index and New-Onset Type 2 Diabetes Mellitus in Middle-Aged Men. Metabolites 2025, 15, 537. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Paulmichl, K.; Hatunic, M.; Højlund, K.; Jotic, A.; Krebs, M.; Mitrakou, A.; Porcellati, F.; Tura, A.; Bergsten, P.; Forslund, A.; et al. Modification and Validation of the Triglyceride-to-HDL Cholesterol Ratio as a Surrogate of Insulin Sensitivity in White Juveniles and Adults without Diabetes Mellitus: The Single Point Insulin Sensitivity Estimator (SPISE). Clin. Chem. 2016, 62, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Bello-Chavolla, O.Y.; Almeda-Valdes, P.; Gomez-Velasco, D.; Viveros-Ruiz, T.; Cruz-Bautista, I.; Romo-Romo, A.; Sánchez-Lázaro, D.; Meza-Oviedo, D.; Vargas-Vázquez, A.; Campos, O.A.; et al. METS-IR, a novel score to evaluate insulin sensitivity, is predictive of visceral adiposity and incident type 2 diabetes. Eur. J. Endocrinol. 2018, 178, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hui, X.; Huang, X.; Li, J.; Liu, N. Relationship between a novel non-insulin-based metabolic score for insulin resistance (METS-IR) and coronary artery calcification. BMC Endocr. Disord. 2022, 22, 274. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sánchez-Íñigo, L.; Navarro-González, D.; Fernández-Montero, A.; Pastrana-Delgado, J.; Martínez, J.A. The TyG index may predict the development of cardiovascular events. Eur. J. Clin. Investig. 2016, 46, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Meh, K.; Jurak, G.; Sorić, M.; Rocha, P.; Sember, V. Validity and Reliability of IPAQ-SF and GPAQ for Assessing Sedentary Behaviour in Adults in the European Union: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 4602. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Román Viñas, B.; Ribas Barba, L.; Ngo, J.; Serra Majem, L. Validación en población catalana del cuestionario internacional de actividad física [Validity of the international physical activity questionnaire in the Catalan population (Spain)]. Gac. Sanit. 2013, 27, 254–257. (In Spanish) [Google Scholar] [CrossRef] [PubMed]
- Schröder, H.; Fitó, M.; Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventós, R.; Ros, E.; Salaverría, I.; Fiol, M.; et al. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Mestre Font, M.; Busquets-Cortés, C.; Ramírez-Manent, J.I.; Vallejos, D.; Sastre Alzamora, T.; López-González, A.A. Influence of sociodemographic variables and healthy habits on the values of cardiometabolic risk scales in 386.924 spanish workers. Acad. J. Health Sci. 2024, 39, 112–121. [Google Scholar] [CrossRef]
- Tosoratto, J.; Carriedo, B.; Cantón, C. Cardiometabolic risk level in 43.074 Spanish office workers: Associated variables. Acad. J. Health Sci. 2024, 39, 48–56. [Google Scholar] [CrossRef]
- Liu, X.; Tan, Z.; Huang, Y.; Zhao, H.; Liu, M.; Yu, P.; Ma, J.; Zhao, Y.; Zhu, W.; Wang, J. Relationship between the triglyceride-glucose index and risk of cardiovascular diseases and mortality in the general population: A systematic review and meta-analysis. Cardiovasc. Diabetol. 2022, 21, 124. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, M.K.; Ahn, C.W.; Kang, S.; Nam, J.S.; Kim, K.R.; Park, J.S. Relationship between the triglyceride glucose index and coronary artery calcification in Korean adults. Cardiovasc. Diabetol. 2017, 16, 108. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tárraga Marcos, P.J.; López-González, Á.A.; Martínez-Almoyna Rifá, E.; Paublini Oliveira, H.; Martorell Sánchez, C.; Tárraga López, P.J.; Ramírez-Manent, J.I. Risk of Insulin Resistance in 44,939 Spanish Healthcare Workers: Association with Sociodemographic Variables and Healthy Habits. Diseases 2025, 13, 33. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hackett, R.A.; Steptoe, A. Type 2 diabetes mellitus and psychological stress—A modifiable risk factor. Nat. Rev. Endocrinol. 2017, 13, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Foguet-Boreu, Q.; Ayerbe García-Morzon, L. Estrés psicosocial, hipertensión arterial y riesgo cardiovascular [Psychosocial stress, high blood pressure and cardiovascular risk]. Hipertens. Riesgo Vasc. 2021, 38, 83–90. (In Spanish) [Google Scholar] [CrossRef] [PubMed]
- Wirtz, P.H.; von Känel, R. Psychological Stress, Inflammation, and Coronary Heart Disease. Curr. Cardiol. Rep. 2017, 19, 111. [Google Scholar] [CrossRef] [PubMed]
- Osborne, M.T.; Shin, L.M.; Mehta, N.N.; Pitman, R.K.; Fayad, Z.A.; Tawakol, A. Disentangling the Links Between Psychosocial Stress and Cardiovascular Disease. Circ. Cardiovasc. Imaging 2020, 13, e010931. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vaccarino, V.; Bremner, J.D. Stress and cardiovascular disease: An update. Nat. Rev. Cardiol. 2024, 21, 603–616. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Salaroli, L.B.; Cattafesta, M.; Molina, M.D.C.B.; Zandonade, E.; Bissoli, N.S. Insulin resistance and associated factors: A cross-sectional study of bank employees. Clinics 2017, 72, 224–230. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Prajapati, A.K.; Prajapati, R. Prevalence of metabolic syndrome and its risk factors among the government bank’s employees of district Bijnor, Uttar Pradesh: A cross-sectional study. J. Fam. Med. Prim. Care 2024, 13, 5825–5832. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Park, K.; Ahn, C.W.; Lee, S.B.; Kang, S.; Nam, J.S.; Lee, B.K.; Kim, J.H.; Park, J.S. Elevated TyG Index Predicts Progression of Coronary Artery Calcification. Diabetes Care 2019, 42, 1569–1573. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Yang, Q.; Xu, H.; Wu, S.; Wang, W.; Zhou, R.; Yang, Y.; Yu, Q. Association between triglyceride-glucose index and the risk of type 2 diabetes mellitus. Arch. Endocrinol. Metab. 2025, 69, e230493. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moffatt, S.M.; Stewart, D.F.; Jack, K.; Dudar, M.D.; Bode, E.D.; Mathias, K.C.; Smith, D.L. Cardiometabolic health among United States firefighters by age. Prev. Med. Rep. 2021, 23, 101492. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shan, Z.; Li, Y.; Zong, G.; Guo, Y.; Li, J.; Manson, J.E.; Hu, F.B.; Willett, W.C.; Schernhammer, E.S.; Bhupathiraju, S.N. Rotating night shift work and adherence to unhealthy lifestyle in predicting risk of type 2 diabetes: Results from two large US cohorts of female nurses. BMJ 2018, 363, k4641. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kurniawan, L.B. Triglyceride-Glucose Index As A Biomarker Of Insulin Resistance, Diabetes Mellitus, Metabolic Syndrome, And Cardiovascular Disease: A Review. Ejifcc 2024, 35, 44–51. [Google Scholar] [PubMed] [PubMed Central]
- Sánchez-García, A.; Rodríguez-Gutiérrez, R.; Mancillas-Adame, L.; González-Nava, V.; Díaz González-Colmenero, A.; Solis, R.C.; Álvarez-Villalobos, N.A.; González-González, J.G. Diagnostic Accuracy of the Triglyceride and Glucose Index for Insulin Resistance: A Systematic Review. Int. J. Endocrinol. 2020, 2020, 4678526. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Camero, A.; Muriel, J.L.; Morell, N.; Lurquin, M.; López González Ángel, A.; Serra-Capó, A.; Villaroel, G. Risk of insulin resistance applying 3 different scales in 703,472 spanish workers: Associated variables. J. Clin. Trials Exp. Investig. 2024, 3, 125–135. [Google Scholar]
- Qiu, J.; He, S.; Yu, C.; Yang, R.; Kuang, M.; Sheng, G.; Zou, Y. Assessing the validity of METS-IR for predicting the future onset of diabetes: An analysis using time-dependent receiver operating characteristics. BMC Endocr. Disord. 2024, 24, 238. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barchetta, I.; Dule, S.; Bertoccini, L.; Cimini, F.A.; Sentinelli, F.; Bailetti, D.; Marini, G.; Barbonetti, A.; Loche, S.; Cossu, E.; et al. The single-point insulin sensitivity estimator (SPISE) index is a strong predictor of abnormal glucose metabolism in overweight/obese children: A long-term follow-up study. J. Endocrinol. Investig. 2022, 45, 43–51. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Duan, M.; Zhao, X.; Li, S.; Miao, G.; Bai, L.; Zhang, Q.; Yang, W.; Zhao, X. Metabolic score for insulin resistance (METS-IR) predicts all-cause and cardiovascular mortality in the general population: Evidence from NHANES 2001–2018. Cardiovasc. Diabetol. 2024, 23, 243. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Balkau, B.; Mhamdi, L.; Oppert, J.M.; Nolan, J.; Golay, A.; Porcellati, F.; Laakso, M.; Ferrannini, E.; on behalf of the EGIR-RISC Study Group. Physical activity and insulin sensitivity: The RISC study. Diabetes 2008, 57, 2613–2618. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hu, F.B.; Li, T.Y.; Colditz, G.A.; Willett, W.C.; Manson, J.E. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA 2003, 289, 1785–1791. [Google Scholar] [CrossRef] [PubMed]
- Helmrich, S.P.; Ragland, D.R.; Leung, R.W.; Paffenbarger, R.S., Jr. Physical activity and reduced occurrence of non-insulin-dependent diabetes mellitus. N. Engl. J. Med. 1991, 325, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Mauvais-Jarvis, F. Gender differences in glucose homeostasis and diabetes. Physiol. Behav. 2018, 187, 20–23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barros, R.P.; Gustafsson, J.Å. Estrogen receptors and the metabolic network. Cell Metab. 2011, 14, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Lizcano, F.; Guzmán, G. Estrogen Deficiency and the Origin of Obesity during Menopause. BioMed Res. Int. 2014, 2014, 757461. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karastergiou, K.; Smith, S.R.; Greenberg, A.S.; Fried, S.K. Sex differences in human adipose tissues—The biology of pear shape. Biol. Sex Differ. 2012, 3, 13. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Link, J.C.; Reue, K. Genetic Basis for Sex Differences in Obesity and Lipid Metabolism. Annu. Rev. Nutr. 2017, 37, 225–245. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Regitz-Zagrosek, V.; Kararigas, G. Mechanistic Pathways of Sex Differences in Cardiovascular Disease. Physiol. Rev. 2017, 97, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Cancelliere, C.; Cassidy, J.D.; Ammendolia, C.; Côté, P. Are workplace health promotion programs effective at improving presenteeism in workers? A systematic review and best evidence synthesis of the literature. BMC Public Health 2011, 11, 395. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Proper, K.I.; van Oostrom, S.H. The effectiveness of workplace health promotion interventions on physical and mental health outcomes—A systematic review of reviews. Scand. J. Work Environ. Health 2019, 45, 546–559. [Google Scholar] [CrossRef] [PubMed]
- Conn, V.S.; Hafdahl, A.R.; Cooper, P.S.; Brown, L.M.; Lusk, S.L. Meta-analysis of workplace physical activity interventions. Am. J. Prev. Med. 2009, 37, 330–339. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Knott, C.; Bell, S.; Britton, A. Alcohol Consumption and the Risk of Type 2 Diabetes: A Systematic Review and Dose-Response Meta-analysis of More Than 1.9 Million Individuals From 38 Observational Studies. Diabetes Care 2015, 38, 1804–1812. [Google Scholar] [CrossRef] [PubMed]
- Reutrakul, S.; Van Cauter, E. Sleep influences on obesity, insulin resistance, and risk of type 2 diabetes. Metabolism 2018, 84, 56–66. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).