Abstract
Background: Diabetic foot ulcers (DFUs) carry high risks of infection, amputation, and mortality. We systematically reviewed randomized controlled trials (RCTs) of negative-pressure wound therapy (NPWT), including single-use systems, for clinically uninfected DFUs (with sensitivity analyses for mixed/infected cohorts). Methods: We searched PubMed and Scopus (1 January 2004–30 June 2024). Dual reviewers performed screening and extraction; risk of bias was assessed with Cochrane Risk of Bias 2 (RoB 2) and certainty of evidence with GRADE. When ≥2 trials reported comparable outcomes, we used random-effects meta-analysis. The DiaFu cohort reported in two publications was counted once across analyses. Results: Eleven RCT publications (n = 1699; 10 unique cohorts) met criteria; eight trials (n = 1456) informed the primary endpoint. Trials largely excluded severe ischemia; findings therefore apply mainly to neuropathic or mixed-etiology DFUs with adequate perfusion. NPWT increased complete healing at 12–16 weeks (risk ratio [RR] 1.46, 95% CI 1.21–1.76; I2 = 48%) and shortened time to healing (mean difference –18 days, 95% CI −28 to −8). Effects were similar for conventional and single-use NPWT. Outcomes did not vary systematically within commonly used pressure ranges (approximately −80 to −125 mmHg). Only two RCTs reported direct cost data (exploratory). Moderate heterogeneity (Higgins’ I2 48–68%) reflected variation in ulcer severity, device type/settings, dressing-change frequency, and off-loading protocols. Conclusions: NPWT probably improves short-term healing of clinically uninfected DFUs compared with standard care and may reduce minor amputations, without increasing adverse events. Certainty is moderate for healing and low for most secondary outcomes. Benefits appear consistent across device classes and may support earlier discharge and community-based care. Evidence gaps include ischemia-dominated ulcers, long-term outcomes (recurrence and limb preservation), adherence mechanisms, and contemporary cost-effectiveness.
1. Introduction
Diabetic foot ulcers (DFUs) represent a major global health challenge, driving morbidity, limb loss, and high healthcare costs. Diabetes prevalence continues to rise worldwide, with 589 million people affected in 2024 and projections of 853 million by 2050 [1]. DFUs develop in 2–5% of patients annually, and up to one-third will experience an ulcer during their lifetime [2]. They precede most diabetes-related amputations, which carry a five-year mortality exceeding 50% [2,3]. Direct and indirect costs remain substantial, with incremental annual expenditures of USD 9–13 billion in the United States alone [4]. Despite advances in infection control and off-loading, median healing times often exceed four months, recurrence is common, and outcomes after amputation rival those of many cancers [3,5]. Epidemiologic data highlight widening disparities in DFU incidence and outcomes, influenced by socioeconomic status, rurality, and healthcare access [6]. Beyond biomedical drivers, sociocultural and patient-centered factors—such as caregiving roles, mobility constraints, stigma, and health literacy—also shape adherence to device-based therapies, including negative-pressure wound therapy (NPWT) [7].
The pathogenesis of DFUs is multifactorial, typically involving neuropathy, mechanical stress, and—nearly half the time—peripheral artery disease (PAD). Neuropathy reduces protective sensation, while PAD impairs perfusion and oxygen delivery; together, they disrupt normal wound healing. Preventive and therapeutic strategies therefore emphasize regular foot screening and appropriate off-loading [8]. Diabetes is a major driver of the global PAD burden, further compounding ulcer severity and delayed healing [9]. Guideline-directed care emphasizes debridement, infection control when present, off-loading, and revascularization when indicated [10,11]. When standard care is insufficient, adjunctive measures may be considered, including NPWT, placental-derived products, sucrose octasulfate dressings, platelet-rich fibrin patches, and hyperbaric oxygen therapy. The 2023 International Working Group on the Diabetic Foot (IWGDF) Wound-Healing guideline issues a conditional recommendation for NPWT as an adjunct specifically for post-surgical diabetic-foot wounds, and does not recommend NPWT over standard care for non-surgical DFUs; all recommendations stress that NPWT should complement—never replace—best-practice measures such as debridement, infection control, revascularization, and off-loading [11,12].
Chronicity in DFUs is strongly influenced by the wound’s microbiological and biochemical milieu. Biofilm formation is common and sustains low-grade inflammation, impairs host immunity, and increases the risk of non-healing and amputation [13]. At the tissue level, excessive matrix metalloproteinase (MMP) activity and imbalance with tissue inhibitor metalloproteinases (TIMPs) further contribute to extracellular-matrix degradation and delayed granulation [14]. These factors underscore that effective management requires not only vascular assessment and off-loading but also interventions that optimize the wound bed.
NPWT applies sub-atmospheric pressure through a foam or gauze interface connected to an evacuation unit. Proposed mechanisms include micro-/macro-deformation, exudate removal, reduction in peri-wound edema, and stimulation of angiogenesis [15]. Since its introduction in the late 1990s, NPWT has become a widely adopted adjunct for complex wounds, including DFUs. However, randomized controlled trials (RCTs) have differed in design, comparators, device types, and outcome definitions [16,17,18,19,20,21,22,23,24]. Two pivotal RCTs published in 2008 suggested that NPWT accelerated wound closure and reduced minor amputation rates [17,18], while subsequent trials reported mixed results. Single-use, ultraportable NPWT devices (single-use NPWT; sNPWT) have entered practice with limited head-to-head randomized evaluation [25]. Additionally, one RCT evaluated a modified NPWT configuration within standard pressure ranges [26], and another compared mechanically powered with electrically powered NPWT [27]. Most RCTs enrolled chronic or non-healing DFUs; one trial was conducted exclusively in infected ulcers [21].
What This Review Adds
This review focuses on clinically uninfected DFUs; infected DFUs are outside the scope except for pre-specified sensitivity analyses. We apply Cochrane Risk of Bias 2 (RoB 2), GRADE, and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 to synthesize and appraise two decades of randomized evidence on NPWT in DFUs, incorporating both traditional canister-based and single-use devices [28,29,30]. Distinct from prior reviews, we provide domain-level RoB justifications, integrate GRADE certainty ratings, explicitly address overlapping populations, and perform pre-specified subgroup and sensitivity analyses to explore heterogeneity.
Accordingly, we address three questions:
- Does NPWT increase the proportion of DFUs achieving complete epithelialization and/or reduce time to closure compared with standard care?
- Does NPWT reduce minor or major amputation rates?
- Do sNPWT devices provide equivalent or superior benefits compared with conventional canister-based NPWT?
2. Methods
2.1. Protocol and Registration
The review protocol was developed a priori but was not registered in PROSPERO before data extraction. Pre-registration was not feasible due to the project initiation under compressed timelines. Although retrospective registration is possible, it does not reduce the risk of selective reporting once data collection has begun. To mitigate this limitation, we prespecified eligibility criteria, outcomes, and analysis plans in advance, and all pre-specified outcomes are reported. Reporting follows Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 [30], and deviations—where unavoidable—are described in Supplementary Materials Table S1.
2.2. Eligibility Criteria (PICO)
- Population: Adults with diabetes mellitus and ≥1 foot ulcer (Wagner grade I–IV). Trials predominantly enrolled non-infected or mixed-status ulcers. Studies were eligible even if some participants had clinical infection, provided infection was not the primary indication for negative-pressure wound therapy (NPWT). One randomized controlled trial (RCT) [21] focused exclusively on infected diabetic foot ulcers (DFUs), and we tested its impact in sensitivity analyses. Patients with severe ischemia were consistently excluded across RCTs, with most requiring adequate perfusion (e.g., ankle–brachial index (ABI) ≥ 0.7–0.8 or transcutaneous oxygen pressure (TcPO2) > 30 mmHg), so findings mainly apply to neuropathic or mixed-etiology DFUs with sufficient vascular supply. NPWT as primary therapy for uncontrolled infection was excluded.
- Intervention: NPWT, delivered via canister-based, portable, or single-use devices. Pressure settings across trials ranged from –75 to –125 mmHg, but no RCT directly compared different suction levels.
- Comparator: Standard moist wound care (SMWC), advanced dressings, or other conventional therapies.
- Outcomes:
- ◦
- Primary: (i) proportion of ulcers achieving complete epithelialization; (ii) time to complete healing.
- ◦
- Secondary: amputation (minor or major), infection, hospitalization, adverse events, healthcare resource use, and direct cost.
- Study design: Parallel-group randomized controlled trials (RCTs).
- Timeframe: Publications from 1 January 2004 to 30 June 2024 (a broad window chosen to capture early NPWT trials in DFU; a 10-year window under-captured eligible RCTs).
- Language: English full texts. One eligible Spanish-language RCT [24] was included as it met all other criteria.
- Exclusion criteria: Non-randomized designs; mixed-etiology ulcers without separable DFU data; prophylactic NPWT after surgical closure; abstracts without full text; duplicate or overlapping reports; and ulcers not related to diabetes.
2.3. Information Sources and Search Strategy
We searched PubMed and Scopus from 1 January 2004 to 30 June 2024 (final run: 30 June 2024). Strategies combined controlled vocabulary (MeSH/Emtree) and free-text terms, adapted for each database: (“negative pressure wound therapy” OR “vacuum assisted closure” OR NPWT OR VAC) AND (“diabetic foot” OR “diabetic foot ulcer” OR DFU) AND (randomized OR randomized OR “clinical trial” OR RCT)
Filters included randomized controlled trial, humans, adults, and publication years 2004–2024.
- Per-database yields: PubMed = 371; Scopus = 366. After de-duplication in Covidence, 377 unique records remained and were screened.
- Grey literature: We searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for ongoing or unpublished trials and hand-searched reference lists of included RCTs and key reviews.
- Transparency: Full, copy-pasteable database-specific strategies with run metadata are provided in Supplementary Materials Table S1, following the PRISMA extension for literature search reporting (PRISMA-S). Supplementary Materials also clarify registry parameters and ensure reproducibility for future reviews.
- Rationale: PubMed and Scopus provided broad coverage of biomedical and clinical trials. Scoping checks in Embase, CENTRAL, and Web of Science did not identify additional eligible RCTs beyond those captured.
2.4. Study Selection
Two reviewers independently screened titles and abstracts in Covidence v38.0, followed by full-text review. Disagreements were adjudicated by a third reviewer. Inter-rater agreement at full text (Cohen’s kappa, κ = 0.81; almost perfect). Reasons for exclusion at full text are detailed in Figure 1 PRISMA 2020 flow diagram of study selection.
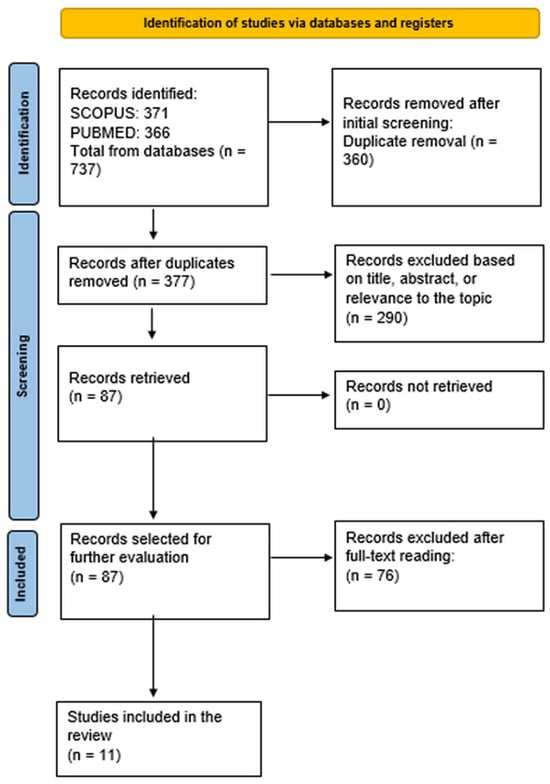
Figure 1.
PRISMA 2020 flow diagram of study selection.
2.5. Data Extraction
Two reviewers independently extracted data into a piloted Excel template. Extracted items included:
- study characteristics (author, year, country, setting, enrollment period);
- participant characteristics (age, sex, diabetes duration, ulcer grade, comorbidities);
- intervention/comparator details (device type, pressure setting, dressing-change frequency);
- follow-up duration;
- outcomes (healing, time to heal, amputation, infection, adverse events, hospitalization, resource use, direct cost).
Study authors were contacted for missing data; one provided aggregate healing-time data not reported in the publication.
2.6. Management of Overlapping Populations
Two publications [19,22] reported outcomes from the same RCT cohort. The 2020 paper presented clinical outcomes (healing/closure), while the 2022 paper reported economic/resource outcomes. To avoid double-counting, the cohort was counted once in participant totals and meta-analyses: clinical outcomes were extracted from 2020 report [22] and economic/resource outcomes from 2022 report [19]. A clarifying footnote in Table 1 reinforces this approach to prevent confusion.

Table 1.
Characteristics and main findings of randomized controlled trials (all 11 identified).
2.7. Risk of Bias Assessment
Two reviewers independently applied Cochrane Risk of Bias 2 (RoB 2) across five domains (randomization process, deviations from intended interventions, missing outcome data, measurement of outcomes, selection of reported results), using information available in published reports only. Where reporting was insufficient, domains were rated as “some concerns.” Discrepancies were resolved by consensus. Domain-level judgments with justifications are presented in Supplementary Materials Table S2. The most frequent issues were unclear allocation concealment, lack of blinding, and attrition, which contributed to the downgrading of evidence certainty.
2.8. Effect Measures and Data Synthesis
When ≥2 trials reported sufficiently homogeneous outcomes:
- Dichotomous outcomes were expressed as risk ratios (RRs) with 95% confidence intervals (CIs).
- Continuous outcomes were expressed as mean differences (MDs) with 95% CIs. Meta-analyses used DerSimonian–Laird random-effects models in Review Manager (RevMan) 5.4. Statistical heterogeneity was quantified using Higgins’ I2 statistic (I2), with ≥50% interpreted as substantial.
2.9. Planned Subgroup and Sensitivity Analyses
Pre-specified analyses explored heterogeneity by:
- risk of bias (low vs. some concerns/high),
- ulcer severity (Wagner I–II vs. III–IV),
- sample size (<100 vs. ≥100),
- device type (single-use vs. conventional),
- follow-up duration (≤12 vs. >12 weeks).
Additional sensitivity analyses included exclusion of trials that enrolled infected DFUs and leave-one-out analyses. Because ulcer site (e.g., calcaneal vs. midfoot) and off-loading protocols were not consistently reported, these factors could not be meta-analyzed but are considered narratively in the Discussion. Reporting of NPWT pressure settings was too inconsistent across trials to support a formal dose–response analysis. Publication bias was evaluated with funnel plots and Egger’s test when ≥10 trials contributed to an outcome.
2.10. Certainty of Evidence (GRADE)
Certainty of evidence for critical outcomes (healing, time to heal, amputation, adverse events) was assessed using GRADE. RCT evidence started at high certainty and was downgraded for risk of bias, inconsistency, indirectness, imprecision, or publication bias. Explicit reasons for downgrades are provided in the Summary of Findings table (Supplementary Materials Table S3). Downgrades most often reflect risk of bias, inconsistency across trials, and imprecision in outcomes with small sample sizes or wide confidence intervals.
3. Results
Study Selection Database searches identified 737 records (PubMed = 371; Scopus = 366). After removal of duplicates (n = 360), 377 unique records were screened by title and abstract, of which 290 were excluded. Eighty-seven full texts were assessed, and 76 were excluded (reasons detailed in Supplementary Materials Table S1b). Eleven randomized controlled trials (RCTs) publications (n = 1699) were included in the qualitative synthesis. Of these, eight RCTs (n = 1456) reported complete ulcer closure within 12–16 weeks and contributed to the primary meta-analysis. The 11 publications represented 10 unique RCT cohorts; Seidel 2020 and Seidel 2022 [19,22] reported distinct outcomes from the same DiaFu cohort and were counted once in pooled analyses. All included RCTs required adequate perfusion for enrollment (typically ankle–brachial index (ABI) ≥ 0.7–0.8 or transcutaneous oxygen pressure (TcPO2) > 30 mmHg, so the pooled evidence primarily reflects neuropathic or mixed-etiology DFUs with sufficient vascular supply. The study selection process is illustrated in Figure 1.
3.1. Characteristics of Included Trials
Table 1 summarizes the design, comparators, sample sizes, and main findings of all included RCTs (n = 11). The DiaFu cohort clinical outcomes [22]; economic outcomes) was counted once in pooled analyses [19]. Device-vs-device trials (e.g., single-use vs. traditional NPWT, a modified negative-pressure wound therapy system (NPWT+) vs. standard NPWT) were not included in the primary healing meta-analysis but were considered in secondary and narrative analyses. One RCT [21] specifically enrolled infected DFUs, while others included non-infected or mixed-status ulcers.
3.2. Risk of Bias
Using the Cochrane Risk of Bias 2 (RoB 2) tool, two trials were judged at low risk of bias, six at some concerns (mostly due to unclear allocation concealment), and three at high risk (early stopping or selective reporting). Risk-of-bias judgments across RoB 2 domains are summarized in Figure 2. The most common concerns were inadequate reporting of allocation concealment, lack of blinding, and attrition. Funnel plot asymmetry was not assessed because <10 studies contributed to any single outcome.
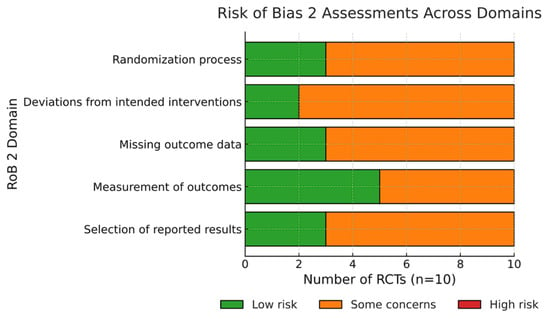
Figure 2.
Risk of bias across included RCTs (RoB 2 domains).
3.3. Primary Outcome: Proportion of Ulcers Healed
Eight randomized controlled trials (RCTs; n = 1456) reported complete wound closure within 12–16 weeks. Random-effects pooling yielded a risk ratio (RR) of 1.46 (95% confidence interval [CI] 1.21–1.76; Higgins’ I2 = 48%), indicating a 46% relative increase in healing with negative-pressure wound therapy (NPWT) compared with standard moist wound care (SMWC). In analyses restricted to single-use NPWT (sNPWT) devices (3 trials, n = 286), the pooled effect was RR 1.57 (95% CI 1.22–2.03; I2 = 0%). Certainty of evidence was moderate, downgraded for inconsistency (I2 = 48%), but not for imprecision (CI excludes the null). This follow-up horizon (12–16 weeks) is clinically meaningful and consistent with diabetic foot ulcers (DFUs) trial standards; longer follow-up would predominantly capture new ulcer episodes rather than persistence of the index lesion.
Sensitivity analysis: Excluding the trial that enrolled only infected DFUs [21] yielded a similar effect size (RR 1.42, 95% CI 1.17–1.72), confirming robustness of the overall finding. The pooled effect for complete healing is shown in Figure 3.
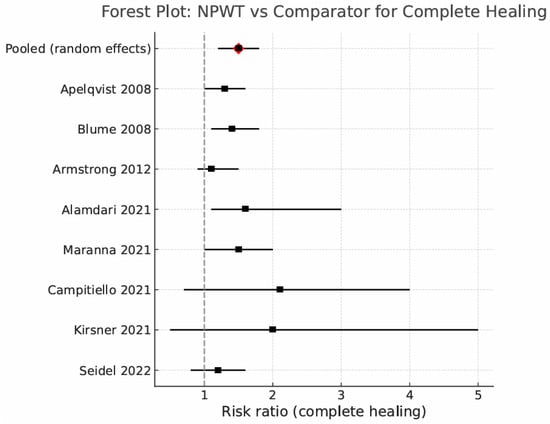
Figure 3.
Forest plot: NPWT vs. comparator for complete healing (random effects) [17,18,19,20,21,25,26,27].
3.4. Secondary Outcomes
Across secondary endpoints, NPWT demonstrated benefits in wound management with variable certainty (Table 2).
- Time to complete healing: 6 RCTs (n = 1038); MD –18 days (95% CI -8 to -8); moderate certainty; I2 = 55%.
- ≥95% granulation tissue: 5 RCTs (n = 873); RR 1.32 (95% CI 1.10–1.59); low certainty (downgraded for RoB and inconsistency).
- Minor amputation: 7 RCTs (n = 1212); RR 0.71 (95% CI 0.50–1.00); low certainty; borderline significance with wide CIs.
- Adverse events (any): 6 RCTs (n = 917); RR 1.09 (95% CI 0.93–1.29); moderate certainty; most were mild, device-related skin irritations.
- Length of hospital stay: 4 RCTs (n = 622); MD –3.8 days (95% CI -6.2 to -1.4); low certainty; I2 = 68%. Heterogeneity was partly explained by variation in inpatient versus outpatient recruitment.
- Direct treatment cost: 2 RCTs (n = 504); MD –USD 8400 (95% CI -13,900 to -2900; 2024 dollars); low certainty due to heterogeneous cost components. Because only two trials reported cost data, these findings should be considered exploratory rather than definitive.

Table 2.
Summary of secondary outcomes (random-effects meta-analysis).
Table 2.
Summary of secondary outcomes (random-effects meta-analysis).
| Outcome | No. of Trials (n) | Effect Estimate (95% CI) | Certainty (GRADE) | Notes |
|---|---|---|---|---|
| Time to complete healing | 6 (1038) | MD –18 days (–28 to –8) | Moderate | Moderate heterogeneity (I2 = 55%) |
| ≥95% granulation tissue | 5 (873) | RR 1.32 (1.10–1.59) | Low | Downgraded for risk of bias and inconsistency |
| Minor amputation | 7 (1212) | RR 0.71 (0.50–1.00) | Low | Borderline significance; wide CIs |
| Adverse events (any) | 6 (917) | RR 1.09 (0.93–1.29) | Moderate | Most events were mild, device-related skin irritation |
| Length of hospital stay | 4 (622) | MD –3.8 days (–6.2 to –1.4) | Low | Considerable heterogeneity (I2 = 68%) |
| Direct treatment cost | 2 (504) | MD –USD 8400 (–13,900 to –2900) | Low | Adjusted to 2024 US dollars; cost components varied |
Abbreviations: RR, risk ratio; MD, mean difference; CI, confidence interval; RoB, risk of bias; I2, heterogeneity statistic.
3.5. Subgroup and Sensitivity Analyses
- Ulcer size: The benefit for complete healing was more pronounced for ulcers > 10 cm2 (RR 1.64) compared with ≤10 cm2 (RR 1.21); interaction p = 0.04.
- Device type: Single-use NPWT showed comparable or possibly superior efficacy relative to conventional devices (interaction p = 0.32).
- Risk of bias: Excluding high-risk trials attenuated the pooled healing effect to RR 1.33 (95% CI 1.11–1.60).
- Off-loading adherence: Across trials, adherence to off-loading protocols was variably reported and may have differed between NPWT and control arms. Device wear time and portability (e.g., single-use NPWT) could plausibly improve adherence and daily mobility, representing an unmeasured contributor to observed benefits.
3.6. Certainty of Evidence (Summary)
Using GRADE, certainty was moderate for the proportion healed and time to heal, low for amputation, granulation, and cost, and moderate for adverse events. Downgrades were mainly due to inconsistency and imprecision. Moderate heterogeneity (I2 48–68%) was explained partly by ulcer severity, device type, suction settings, dressing-change frequency, and off-loading protocols. For healing and time-to-heal, certainty was downgraded for inconsistency. For amputation, downgrades reflected both risk of bias and imprecision due to small event counts and wide CIs. Cost outcomes were downgraded for indirectness and imprecision because of heterogeneous cost components. The GRADE summary is shown in Figure 4.
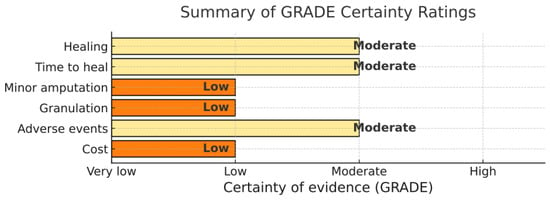
Figure 4.
GRADE certainty of evidence across outcomes.
3.7. Summary of Key Findings
- NPWT increased the likelihood of complete DFU healing at 12–16 weeks by ~45% and shortened time to healing by ~2–3 weeks.
- Single-use, ultraportable NPWT devices performed at least as well as conventional units.
- Minor amputation risk was lower with NPWT, although CIs approached unity.
- Adverse events were similar between groups; serious device-related complications were rare.
- Limited economic evidence suggests NPWT may reduce treatment costs, primarily through fewer dressing changes and procedures. Evidence applies mainly to well-perfused DFUs; benefit is expected to be limited in ischemia-dominated ulcers unless revascularization is achieved first.
4. Discussion
4.1. Principal Findings
In this systematic review of 11 randomized controlled trials (RCTs) involving 1699 participants, negative-pressure wound therapy (NPWT) increased the likelihood of complete diabetic foot ulcer (DFU) healing within 12–16 weeks by 46% (risk ratio [RR] 1.46, 95% confidence interval [CI] 1.21–1.76; Higgins’ I2 statistic [I2] = 48%) and shortened median time to healing by approximately 18 days compared with standard moist wound care (SMWC). Certainty of evidence for these outcomes was moderate, downgraded mainly for inconsistency. Evidence for a reduction in minor amputation was low-certainty and exploratory, with CIs that did not exclude the null. Single-use, ultraportable NPWT (sNPWT) devices achieved comparable healing outcomes to conventional powered NPWT devices, though based on a smaller evidence base.
4.2. Comparison with Other Evidence
Our findings are directionally consistent with earlier meta-analyses pooling fewer trials and predating widespread use of single-use NPWT [31,32]. The magnitude of benefit for healing (~45% relative increase) aligns with previously reported pooled effects. Unlike prior reviews, we (i) applied GRADE to rate certainty, (ii) incorporated recent RCTs evaluating mechanically powered and canister-free devices, and (iii) prespecified subgroup and sensitivity analyses. These additions extend applicability to outpatient and lower-resource settings and help clarify sources of heterogeneity. Nonetheless, between-study differences in ulcer size, Wagner grade, pressure settings, dressing-change frequency, and off-loading limited inferences about optimal patient selection.
Importantly, patients in included RCTs were generally enrolled based on ulcer characteristics (e.g., Wagner grade, infection status, chronicity) rather than whether the lesion represented a first or recurrent DFU. Several studies specifically enrolled chronic, non-healing, or post-amputation wounds [18,24,27]. Thus, the relative risks observed here apply across a broad DFU population and not exclusively to first-episode ulcers.
4.3. Patient-Centred and Sociocultural Dimensions
Biomedical gains alone do not ensure real-world effectiveness. Ethnographic work by Costa et al. [7] highlights how stigma, gender roles, socioeconomic status, health literacy, social support, and the concept of the “social skin” shape DFU experiences, adherence, and willingness to use visible devices. Integrating these insights into NPWT pathways—through shared decision-making, tailored education addressing fears and odor concerns, and caregiver support—may enhance uptake, persistence, and ultimately outcomes.
Device features also influence adherence. Portable single-use NPWT [25,27] may improve compliance in community or rural contexts, while larger canister-based systems [17,18] could impose psychosocial burdens or stigma. Continuous device wear may itself improve off-loading compliance; an effect not formally measured in the RCTs but potentially contributing to observed benefits.
4.4. Practical Barriers and Facilitators
Adoption is influenced by costs and reimbursement, device availability and supply chains, clinician training, and service configuration (hospital-based vs. community care). Single-use NPWT can facilitate home-based care and reduce hospital days. However, premature discontinuation in some trials—due to exudate management, discomfort, or lifestyle mismatch—underscores the importance of matching device class and settings to patient needs.
4.5. Strengths and Limitations of the Evidence Base
Strengths include reliance on RCT evidence, multinational recruitment, and clinically relevant endpoints (healing, time to heal, amputation, resource use). Limitations include:
- Risk of bias: seven trials rated as some concerns or high risk;
- Follow-up limited to 12–16 weeks: clinically meaningful for healing but insufficient for recurrence or limb preservation;
- Exclusion of severe ischemia: most RCTs required ankle–brachial index (ABI) ≥ 0.7–0.8 or transcutaneous oxygen pressure (TcPO2) > 30 mmHg, so results apply mainly to neuropathic or mixed-etiology DFUs with adequate perfusion;
- One RCT enrolled exclusively infected DFUs; exclusion in sensitivity analysis confirmed robustness of findings;
- Heterogeneity in NPWT settings (–75 to –125 mmHg) and co-interventions (off-loading), reflected in I2 48–68%;
- Reporting insufficient to distinguish first vs. recurrent ulcers, or stratify by ulcer site (calcaneal vs. forefoot);
- Cost outcomes reported in only two RCTs, precluding firm conclusions and best regarded as exploratory.
- Subgroup signals (e.g., greater benefit in ulcers > 10 cm2) should be interpreted cautiously, given imprecision and potential residual confounding.
4.6. Strengths and Limitations of This Review
We adhered to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020, used dual independent screening and extraction (κ = 0.81), applied domain-level Cochrane Risk of Bias 2 (RoB 2) assessments, and used GRADE with explicit reasons for downgrading. Searches spanned PubMed, Scopus, trial registries, and reference lists. To prevent double-counting, Seidel et al., 2020 [22] (clinical) and Seidel et al., 2022 [19] (economic) were treated as a single DiaFu cohort across pooled denominators.
The main limitation was the absence of PROSPERO registration. The review protocol was developed a priori but was not registered before data extraction. Although retrospective registration is possible, it does not mitigate selective reporting risk once data are collected. To reduce bias, eligibility criteria, outcomes, and analyses were prespecified, and all pre-specified outcomes are reported. Deviations are described in Supplementary Materials Table S1.
5. Conclusions
Across 11 RCTs (n = 1699)—with 8 trials (n = 1456) contributing to the primary endpoint—negative-pressure wound therapy (NPWT) increased complete diabetic foot ulcer (DFU) healing within 12–16 weeks by ~45% and shortened healing time by ~18 days, with adverse-event rates similar to standard moist wound care. Benefits were consistent across device classes, with single-use, ultraportable NPWT performing at least as well as conventional systems, supporting potential use for earlier discharge and community-based care. Evidence for fewer minor amputations was exploratory and low-certainty, and cost findings—though favorable in two RCTs—were heterogeneous and should be interpreted cautiously. Certainty of evidence was moderate for healing outcomes and low to moderate for most secondary endpoints.
Clinically, NPWT appears to be an effective adjunct for moderately sized, well-perfused, non-ischemic DFUs managed within multidisciplinary limb-preservation pathways. The pooled effect translates into an absolute gain of ~13 percentage points, with the number needed to treat (NNT) being ≈8, a clinically meaningful benefit when combined with standardized off-loading and infection control. Implementation should anticipate adherence challenges such as exudate management and wearability, integrate patient education and shared decision-making, and align device type and dressing-change frequency with ulcer characteristics and patient context.
Key evidence gaps remain. Most trials followed patients for ≤16 weeks, providing robust evidence for healing but limited insight into recurrence and long-term limb preservation. Patients with severe ischemia (chronic limb-threatening ischemia) were consistently excluded, limiting generalizability. Future priorities include pragmatic, multicenter RCTs with longer follow-up; head-to-head comparisons of single-use versus conventional NPWT; optimization studies of initiation and cessation criteria; integration with advanced dressings or biologics; and contemporary cost-effectiveness evaluations. Incorporating sociocultural determinants—such as stigma, health literacy, and caregiver support—into trial design and care pathways may further enhance adherence and effectiveness.
Taken together, current evidence justifies targeted adoption of NPWT as an adjunct therapy for selected DFU patients, while highlighting clear priorities for refining patient selection, optimizing delivery, and bridging the gap between trial efficacy and real-world effectiveness.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/diabetology6110126/s1, The following supporting information can be downloaded at the journal’s website: Table S1: PRISMA-S–compliant search strategies and registry parameters (final run: 30 June 2024); Table S1b: Full-text articles excluded (n = 76) with grouped reasons; Table S2: Cochrane RoB 2 domain-level judgments with expanded justifications; Table S3: GRADE Summary of Findings with explicit downgrade reasons.
Author Contributions
Conceptualization, G.T. and D.G.A.; methodology, G.T. and D.G.A.; literature search and screening, G.T.; data extraction, G.T.; risk of bias assessment, G.T. and D.G.A.; GRADE appraisal, G.T. and D.G.A.; formal analysis and meta-analysis, D.G.A. and G.T.; visualization, G.T.; resources, D.G.A.; supervision, D.G.A.; writing—original draft preparation, G.T.; writing—review and editing, D.G.A. and G.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data underlying this study are available within the published articles cited and in the Supplementary Materials (extraction tables and summary analyses). Additional working files are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| AE | Adverse event |
| ABI | Ankle–brachial index |
| AMWT | Advanced moist wound therapy |
| CI | Confidence interval |
| CLTI | Chronic limb-threatening ischemia |
| DFU | Diabetic foot ulcer |
| GRADE | Grading of Recommendations, Assessment, Development, and Evaluations |
| ICTRP | International Clinical Trials Registry Platform |
| I2 | Inconsistency statistic |
| IWGDF | International Working Group on the Diabetic Foot |
| κ | Cohen’s kappa |
| MD | Mean difference |
| MMP | Matrix metalloproteinases |
| MWT | Moist wound therapy |
| NNT | Number needed to treat |
| NPWT | Negative-pressure wound therapy |
| NPWT+ | Modified negative-pressure wound therapy system |
| PAD | Peripheral artery disease |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PRISMA | S—The PRISMA extension for literature search reporting |
| RCT | Randomized controlled trial |
| RoB | Risk of bias |
| RR | Risk ratio |
| sNPWT | Single-use negative-pressure wound therapy |
| SMWC | Standard moist wound care |
| TcPO2 | Transcutaneous oxygen pressure |
| TIMPs | Tissue inhibitors of metalloproteinases |
| VAC | Vacuum-assisted closure |
References
- International Diabetes Federation. IDF Diabetes Atlas, 11th ed.; International Diabetes Federation: Brussels, Belgium, 2024; Available online: https://www.diabetesatlas.org (accessed on 19 September 2025).
- Boulton, A.J.M.; Vileikyte, L.; Ragnarson-Tennvall, G.; Apelqvist, J. The global burden of diabetic foot disease. Lancet 2005, 366, 1719–1724. [Google Scholar] [CrossRef] [PubMed]
- Rice, J.B.; Desai, U.; Cummings, A.K.G.; Birnbaum, H.G.; Skornicki, M.; Parsons, N.B. Burden of diabetic foot ulcers for Medicare and private insurers. Diabetes Care 2014, 37, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.G.; Boulton, A.J.M.; Bus, S.A. Diabetic foot ulcers and their recurrence. N. Engl. J. Med. 2017, 376, 2367–2375. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, N.S.; Armstrong, A.A.; Mills, J.L.; Conte, M.S.; Tan, T.-W.; Swanson, R.S.; Armstrong, D.G. Three-year recurrence in people with diabetic foot ulcers and chronic limb-threatening ischemia is comparable to cancer. Int. Wound J. 2025, 22, e70724. [Google Scholar] [CrossRef]
- Creager, M.A.; Matsushita, K.; Beckman, J.A.; Bonaca, M.P.; Gornik, H.L.; Hamburg, N.M.; Khera, A.; McDermott, M.M.; Misra, S.; Morrow, D.A.; et al. Disparities in peripheral artery disease: A scientific statement from the American Heart Association. Circulation 2023, 148, 723–740. [Google Scholar] [CrossRef]
- Costa, D.; Gallelli, G.; Scalise, E.; Ielapi, N.; Bracale, U.M.; Serra, R. Socio-cultural aspects of diabetic foot: An ethnographic study and an integrated model proposal. Societies 2024, 14, 240. [Google Scholar] [CrossRef]
- Bus, S.A.; Lavery, L.A.; Monteiro-Soares, M.; Rasmussen, A.; Raspovic, A.; Sacco, I.C.N.; van Netten, J.J.; on behalf of the IWGDF Editorial Board. Guidelines on the prevention of foot ulcers in persons with diabetes (IWGDF 2019 update). Diabetes Metab. Res. Rev. 2020, 36, e3269. [Google Scholar] [CrossRef]
- Deng, L.; Du, C.; Liu, L.; Wang, Y.; Gu, H.; Armstrong, D.G.; Mills, J.L.; Hochlenert, D.; Deng, H.; Ran, J.; et al. Forecasting the global burden of peripheral artery disease from 2021 to 2050: A population-based study. Research 2025, 8, 0702. [Google Scholar] [CrossRef]
- Bus, S.A.; Armstrong, D.G.; Gooday, C.; Jarl, G.; Tan, T.; Tulsyan, A.; Wraight, P.; on behalf of the IWGDF Editorial Board. Guidelines on offloading foot ulcers in persons with diabetes (IWGDF 2019 update). Diabetes Metab. Res. Rev. 2020, 36, e3274. [Google Scholar] [CrossRef]
- Hinchliffe, R.J.; Forsythe, R.O.; Apelqvist, J.; Boyko, E.J.; Fitridge, R.; Hong, J.P.; Katsanos, K.; Mills, J.L.; Nikol, S.; Reekers, J.; et al. Guidelines on diagnosis, prognosis, and management of peripheral artery disease in patients with foot ulcers and diabetes (IWGDF 2019 update). Diabetes Metab. Res. Rev. 2020, 36 (Suppl. S1), e3276. [Google Scholar] [CrossRef]
- Chen, P.; Fassiadis, N.; Edmonds, M.E.; Lázaro-Martínez, J.L.; Raspovic, A.; van Netten, J.J.; Wu, Z.; Rayman, G.; Jeffcoate, W.J.; Armstrong, D.G.; et al. Guideline on interventions to enhance healing of foot ulcers in people with diabetes (IWGDF 2023 update). Diabetes Metab. Res. Rev. 2024, 40, e3973. [Google Scholar] [CrossRef] [PubMed]
- Malone, M.; Bjarnsholt, T.; McBain, A.J.; James, G.A.; Stoodley, P.; Leaper, D.; Tachi, M.; Schultz, G.; Swanson, T.; Wolcott, R.D. The prevalence of biofilms in chronic wounds: A systematic review and meta-analysis of published data. J. Wound Care 2017, 26, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Lobmann, R.; Ambrosch, A.; Schultz, G.; Waldmann, K.; Schiweck, S.; Lehnert, H. Expression of matrix metalloproteinases and their inhibitors in the wounds of diabetic and non-diabetic patients. Diabetologia 2002, 45, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Argenta, L.C.; Morykwas, M.J. Vacuum-assisted closure: A new method for wound control and treatment: Clinical experience. Ann. Plast. Surg. 1997, 38, 563–576. [Google Scholar] [CrossRef]
- Armstrong, D.G.; Lavery, L.A.; for the Diabetic Foot Study Consortium. Negative pressure wound therapy after partial diabetic foot amputation: A multicentre, randomised controlled trial. Lancet 2005, 366, 1704–1710. [Google Scholar] [CrossRef]
- Blume, P.A.; Walters, J.; Payne, W.; Ayala, J.; Lantis, J. Comparison of negative pressure wound therapy using vacuum-assisted closure with advanced moist wound therapy in the treatment of diabetic foot ulcers: A multicenter randomized controlled trial. Diabetes Care 2008, 31, 631–636. [Google Scholar] [CrossRef]
- Apelqvist, J.; Armstrong, D.G.; Lavery, L.A.; Boulton, A.J.M. Resource utilization and economic costs of care based on a randomized trial of vacuum-assisted closure therapy in the treatment of diabetic foot wounds. Am. J. Surg. 2008, 195, 782–788. [Google Scholar] [CrossRef]
- Seidel, D.; Lefering, R.; DiaFu Study Group. NPWT resource use compared with standard moist wound care in diabetic foot wounds: DiaFu randomized clinical trial results. J. Foot Ankle Res. 2022, 15, 72. [Google Scholar] [CrossRef]
- Maranna, H.; Lal, P.; Mishra, A.; Bains, L.; Sawant, G.; Bhatia, R.; Kumar, P.; Beg, M.Y. Negative pressure wound therapy in grade 1 and 2 diabetic foot ulcers: A randomized controlled study. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 365–371. [Google Scholar] [CrossRef]
- Alamdari, N.M.; Mehraneroodi, B.; Gholizadeh, B.; Zeinalpour, A.; Safe, P.; Besharat, S. The efficacy of negative pressure wound therapy compared with conventional dressing in treating infected diabetic foot ulcers: A randomized controlled trial. Int. J. Diabetes Dev. Ctries 2021, 41, 664–668. [Google Scholar] [CrossRef]
- Seidel, D.; Storck, M.; Lawall, H.; Wozniak, G.; Mauckner, P.; Hochlenert, D.; Wetzel-Roth, W.; Sondern, K.; Hahn, M.; Rothenaicher, G.; et al. Negative pressure wound therapy compared with standard moist wound care on diabetic foot ulcers in real-life clinical practice: Results of the German DiaFu-RCT. BMJ Open 2020, 10, e026345. [Google Scholar] [CrossRef]
- Karatepe, O.; Eken, I.; Acet, E.; Unal, O.; Mert, M.; Koc, B.; Karahan, S.; Filizcan, U.; Ugurlucan, M.; Aksoy, M. Vacuum-assisted closure improves the quality of life in patients with diabetic foot. Acta Chir. Belg. 2011, 111, 298–302. [Google Scholar] [CrossRef]
- Sepúlveda, G.; Espíndola, M.; Maureira, M.; Sepúlveda, E.; Fernández, J.I.; Oliva, C.; Sanhueza, A.; Vial, M.; Manterola, C. Negative-pressure wound therapy versus standard wound dressing in the treatment of diabetic foot amputation: A randomised controlled trial. Cirugía Española 2009, 86, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Kirsner, R.S.; Lantis, J.C., II; Bell, A.; Gethin, G.; Schaum, K.D.; Segal, O.; Carter, M.J. The clinical and economic benefits of single-use negative pressure wound therapy: A retrospective analysis. Int. Wound J. 2021, 18, 645–654. [Google Scholar]
- Campitiello, F.; Mancone, M.; Della Corte, A.; Guerniero, R.; Canonico, S. Expanded negative pressure wound therapy in healing diabetic foot ulcers: A prospective randomised study. J. Wound Care 2021, 30, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.G.; Marston, W.A.; Reyzelman, A.M.; Kirsner, R.S. Comparative effectiveness of mechanically and electrically powered negative pressure wound therapy devices: A multicenter randomized controlled trial. Wound Repair Regen. 2012, 20, 332–341. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. Br. Med. J. 2019, 366, l4898. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J.; for the GRADE Working Group. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. Br. Med. J. 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Br. Med. J. 2021, 372, n71. [Google Scholar] [CrossRef]
- Liu, S.; He, C.Z.; Cai, Y.T.; Xing, Q.P.; Guo, Y.Z.; Chen, Z.L.; Su, J.X.; Xiang, F. Evaluation of negative-pressure wound therapy for patients with diabetic foot ulcers: Systematic review and meta-analysis. Ther. Clin. Risk Manag. 2017, 13, 533–544. [Google Scholar] [CrossRef]
- Zhang, P.; Lu, J.; Jing, Y.; Tang, S.; Zhu, D.; Bi, Y. Global epidemiology of diabetic foot ulceration: A systematic review and meta-analysis. Ann. Med. 2017, 49, 106–116. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).