1. Introduction
Glucagon-like peptide-1 receptor agonist (GLP-1RA) is an incretin-based therapy used as a treatment for type 2 diabetes. It lowers blood glucose through glucose-dependent stimulation of insulin secretion, suppression of glucagon release, and delayed gastric emptying, while also promoting weight reduction and favorable cardiovascular effects [
1]. Semaglutide, formulated as a peptide, was initially administered only via subcutaneous injection, as peptide medications have low oral bioavailability due to rapid enzymatic degradation. An oral formulation of GLP-1RA semaglutide has been developed, using the absorption enhancer sodium N-(8-[2-hydroxybenzoyl]amino) caprylate to improve its uptake [
2,
3]. Semaglutide is well-suited for once-daily oral administration because of its long half-life, which remains consistent regardless of the method of administration. This extended half-life reduces the effects of day-to-day variability in absorption associated with oral dosing [
2]. Oral semaglutide offers a unique option for administering GLP-1Ras orally, making it easier to use during routine outpatient visits [
4]. Pharmacokinetically, oral semaglutide has a half-life of about one week, similar to the subcutaneous formulation, though daily administration is required to achieve stable plasma concentrations. Steady state is generally reached in 4 to 5 weeks. While exposure shows more day-to-day variability compared to the injectable form, clinical outcomes are broadly comparable at approved doses. In the PIONEER randomized clinical trial program, oral semaglutide proved efficacy and safety in treating people with type 2 diabetes in different disease stages, and in combinations with other oral antidiabetic drugs (OAD) and insulin, in addition to reducing weight and cardiovascular biomarkers, leading to non-inferiority compared to placebo in cardiovascular safety [
1,
2,
3].
Renal impairment, often associated with type 2 diabetes, limits the selection of OAD that can be administered in these patients. Much like with subcutaneous semaglutide and liraglutide, the drug metabolism and kinetics of oral semaglutide is not affected by renal impairment. Moreover, the PIONEER 5 study demonstrated a decrease in the urine albumin–creatinine ratio (uACR), a risk marker for cardiovascular disease and kidney damage [
5]. Oral semaglutide reduced both mean systolic and diastolic blood pressure compared to placebo, as demonstrated in both the SUSTAIN 6 and PIONEER 6 studies. This may be advantageous for patients with longstanding diabetes and chronic kidney disease, conditions often accompanied by hypertension [
5,
6].
Oral semaglutide has shown superior efficacy in reducing HbA1c and body weight compared to empagliflozin, sitagliptin, and placebo, and is comparable to subcutaneous liraglutide [
1,
2,
7,
8,
9]. In the PIONEER 8 trial, 54.2% of insulin-treated patients achieved HbA1c < 7.0% at 52 weeks with oral semaglutide [
9]. Real-world studies from multiple countries confirmed significant HbA1c and weight reductions, both as add-on therapy and after switching from other agents [
4,
10,
11]. A pooled analysis across seven countries reported average reductions of 1% in HbA1c and 5% in BMI, with similar outcomes in primary and specialist care and increasing treatment satisfaction over time [
12]. Since GLP-1RAs are usually injectable and limited by GI side effects, the oral formulation may improve adherence and represents an important advance in diabetes management, though more real-world data are needed [
3,
13,
14].
Current guidelines recommend metformin as the first-line medication for treating type 2 diabetes mellitus, primarily because of its efficacy, safety, and affordability [
1,
8]. If patients do not reach their glycemic targets, a second-line therapy such as sulfonylureas, SGLT-2 inhibitors, DPP-4 inhibitors, or basal insulin should be introduced based on the patient’s profile and any existing health conditions [
1]. GLP-1 receptor agonists such as semaglutide are generally considered a third-line treatment, particularly for patients who fail to achieve glycemic control on dual therapy or in those with obesity, where weight reduction is also an important therapeutic target [
1]. There are still knowledge gaps regarding the effectiveness and tolerability of oral semaglutide in routine clinical practice, especially among populations in Central and Eastern Europe. Real-world studies are essential to complement clinical trial findings since they reflect actual prescribing habits, patient adherence, and comorbid conditions that may not always be included in randomized controlled trials. Furthermore, the impact of oral semaglutide on liver biomarkers, such as aspartate aminotransferase (AST) and alanine aminotransferase (ALT), has been studied less extensively in real-world cohorts.
In this non-sponsored, real-world, multicentric Croatian study, we evaluated the effectiveness and safety of oral semaglutide as a third-line treatment for patients with T2DM. Our focus was on dosing, effectiveness, side effects, and patient usage for a minimum of six months. The primary endpoint was the change in HbA1c levels, while secondary endpoints included changes in weight/BMI and biochemical markers (fasting and prandial blood glucose, lipids, liver enzymes) from the start of treatment to the last follow-up. We also recorded adverse events, along with the reasons for discontinuing the medication.
2. Materials and Methods
This was an observational retrospective study that collected electronic data available for T2DM patients treated in outpatient tertiary clinical center settings in Croatia between October 2022 and December 2024. Adult patients (aged over 18 years) with T2DM were included if they were taking oral semaglutide as an add-on to two antidiabetic agents (metformin, SGLT2 inhibitors, sulphonyureas, pioglitazone, or insulin), or if they switched from DPP-4i-based therapy. Exclusion criteria were applied in cases where follow-up data were not available, patients had type 1 diabetes mellitus or gestational diabetes, or in cases of treatment duration shorter than 6 months. Collected data included patients’ demographic characteristics (age, sex), disease duration, medication (withdrawn and/or associated, if any), oral antidiabetic therapy if any, insulin therapy if any, chronic diabetes-related complications and other documented medical diagnoses, biochemical parameters (including fasting plasma glucose (FPG), postprandial blood glucose (PPG), glycated hemoglobin (HbA1c), urea, creatinine, estimated glomerular filtration rate (eGFR), cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglycerides, aspartate aminotransferase (AST), and alanine aminotransferase (ALT)), as well as body weight, and BMI at last follow-up, frequency and cause of drug discontinuation, and frequency and type of adverse events.
All recorded clinical diagnoses were categorized into seven groups: overweight/obesity, cardiovascular diseases, psychiatric disorders, nephrological diseases, other endocrinological disorders, gastrointestinal diseases, and urological conditions. The most common comorbidities within each group were as follows:
Cardiovascular diseases: arterial hypertension, stable angina pectoris, acute coronary syndrome, post-myocardial infarction status, cardiomyopathy, atherosclerosis, intermittent claudication, valvular defects (mitral stenosis, mitral regurgitation, aortic stenosis, aortic regurgitation), and atrial fibrillation.
Psychiatric disorders: bipolar affective disorder, post-traumatic stress disorder (PTSD), depression, emotional instability, and adjustment disorder.
Nephrological diseases: chronic kidney disease, renal cysts, hyperuricemia, nephrolithiasis, adrenal tumor, and neurogenic bladder.
Other endocrinological disorders: dyslipidemia, nodular goiter, hypothyroidism, hyperthyroidism, autoimmune thyroiditis, Addison’s disease, and osteoporosis.
Gastrointestinal diseases: gastroesophageal reflux disease (GERD), chronic gastritis, dyspepsia, colitis, duodenitis, post-appendectomy status, post-abdominal hernia repair, rectal adenocarcinoma, hemorrhoids, hepatic steatosis, hepatic lesions, and cholelithiasis.
Urological conditions: benign prostatic hyperplasia, chronic prostatitis, and chronic urinary tract infections.
Due to the retrospective design of this study, informed consent was not necessary. Data were obtained solely from existing electronic medical records, without any direct contact with patients or interventions. All information was anonymized prior to analysis to ensure confidentiality and prevent the identification of individual participants. In accordance with national regulations, the Declaration of Helsinki, and the approval granted by the Institutional Ethics Committee (2158-61-46-24-45), retrospective studies based on de-identified clinical data are exempt from the requirement for individual informed consent.
Data was analyzed using SPSS software (IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY, USA: IBM Corp.). Descriptive statistics were used to describe the basic features of the study sample (proportions for categorical data, and mean +/− standard deviation for normally distributed continuous variables, or median and interquartile range for variables deviating from normal distribution). Data normality was tested using the Shapiro–Wilk test. Binary logistic regression was used to examine which factors influence goal attainment/response to therapy. To evaluate treatment effects, both paired ttests and repeated-measures ANCOVA were performed. The paired t-test compared pre- and post-treatment values within the same participants. Repeated-measures ANCOVA was used to adjust for potential confounding factors (age, sex, and diabetes duration) and to account for both between- and within-subject variability, thus allowing for a reduction in error variance and lowering the denominator of the F-ratio. Consequently, the statistical power of the analysis was enhanced, enabling the detection of smaller effect sizes within the same sample size. Two-way repeated measures ANCOVA was used when age effects were calculated. The results were considered significant when p < 0.05.
3. Results
3.1. Patient Demographics
The study involved 163 patients who started therapy with oral semaglutide (Rybelsus). Among these patients, 55.8% were male, and 44.2% were female (
Table 1). The largest age group was between 61 and 70 years, accounting for 40.5% of the participants (
Table 1). In terms of disease duration, 35.3% had been diagnosed with diabetes for 6 to 10 years. A significant majority, 90.1%, were classified as obese (with a BMI of 30 or higher). At the beginning of the study, 81.3% were undergoing treatment with metformin, while 24.3% were receiving insulin therapy. Nearly half of the patients were on DPP-4 inhibitors, and only 4.2% had switched from injectable GLP-1 receptor agonists to the oral formulation (
Table 2).
3.2. Comorbidities
The presence of comorbidities was high in this specific population. Cardiovascular disease was reported in 84% of patients (arterial hypertension present in all patients), while 96.5% had obesity. Additionally, 67.4% reported other endocrine disorders, and 9.0% had nephrological conditions (
Table 3).
3.3. Adverse Events
Gastrointestinal side effects were reported in 21.9% of patients. Nausea occurred in 17.3% and heartburn in 2.0%, while abdominal pain was reported by 9.8% of patients. The drug was discontinued in 22.1% of cases, as shown in
Table 4.
3.4. Changes in Clinical Parameters
An ANCOVA was conducted to evaluate changes in clinical parameters following the introduction of oral semaglutide, while controlling for age, sex, and duration of diagnosis (see
Table 5). Statistically significant improvements were observed in HbA1c, BMI, fasting and postprandial glucose levels, as well as HDL, AST, and ALT, with all results showing
p-values less than 0.05. Notably, HDL cholesterol displayed a significant increase compared to baseline levels, with a
p-value of 0.007.
A paired sample t-test was used to compare baseline and follow-up measurements. A p-value of <0.05 was considered statistically significant. Significant improvements were observed in HbA1c, BMI, fasting blood glucose, postprandial blood glucose, total cholesterol, HDL cholesterol, triglycerides, and AST levels. No statistically significant changes were found in urea, creatinine, eGFR, LDL cholesterol, or ALT levels.
3.5. Composite Outcomes
Two composite treatment outcomes were evaluated (
Table 6). The first combined HbA1c reduction below 7% with a minimum 5% weight loss, while the second required a ≥0.5% reduction in HbA1c and ≥5% weight loss. The first outcome was achieved in 27.9% of patients, while 30.3% met the second criterion.
3.6. Predictors of Composite Outcome
Binary logistic regression was employed to identify predictors for achieving the second composite outcome (HbA1c reduction of ≥0.5% and weight reduction of ≥5%). A shorter duration of diabetes showed a tendency for better odds of success (
p = 0.064) (
Table 7). Patients who had previously been treated with insulin had a nearly 85% lower chance of reaching the composite outcome (OR = 0.152, CI: 0.029–0.794,
p = 0.026) (
Table 8).
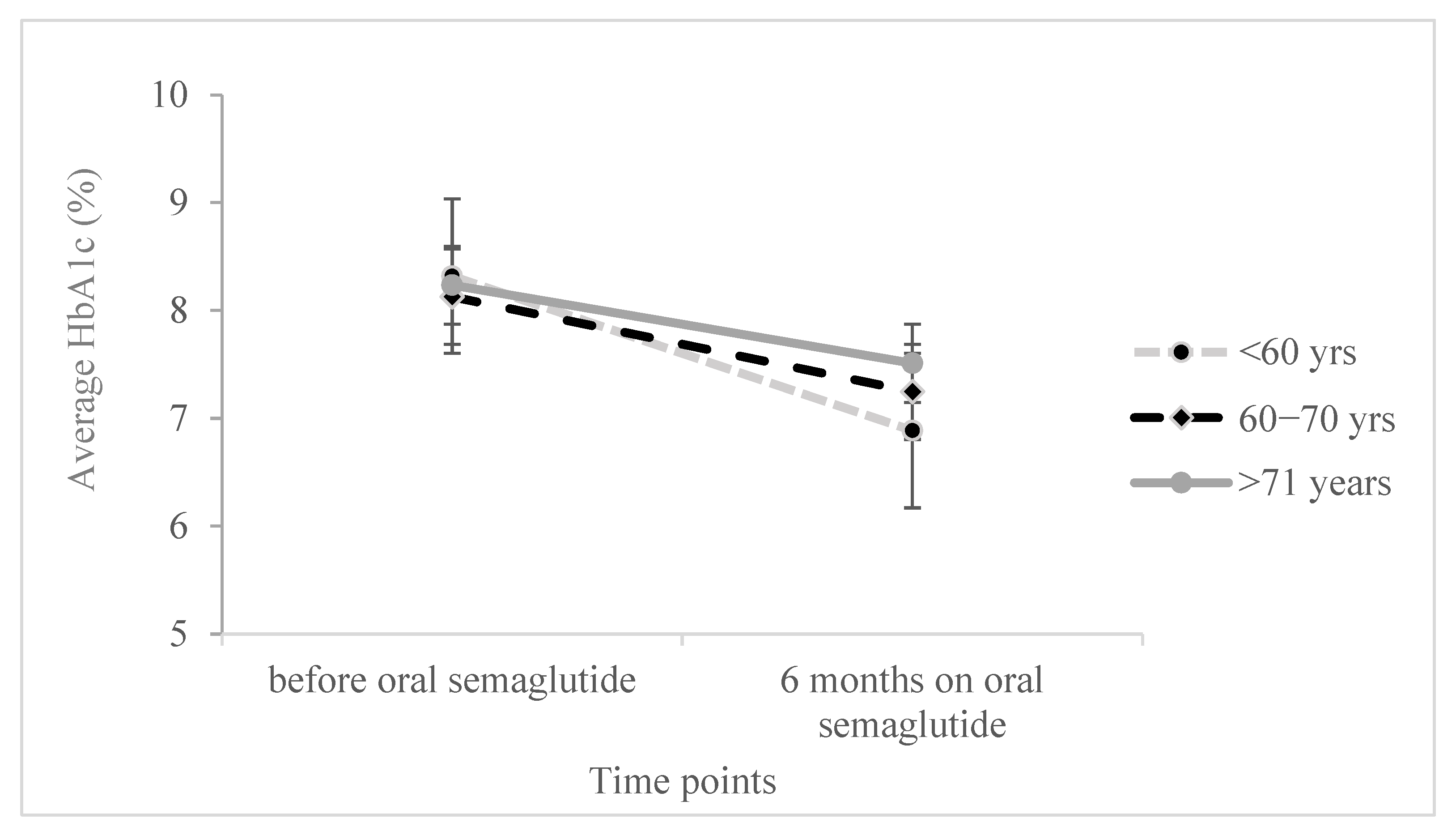
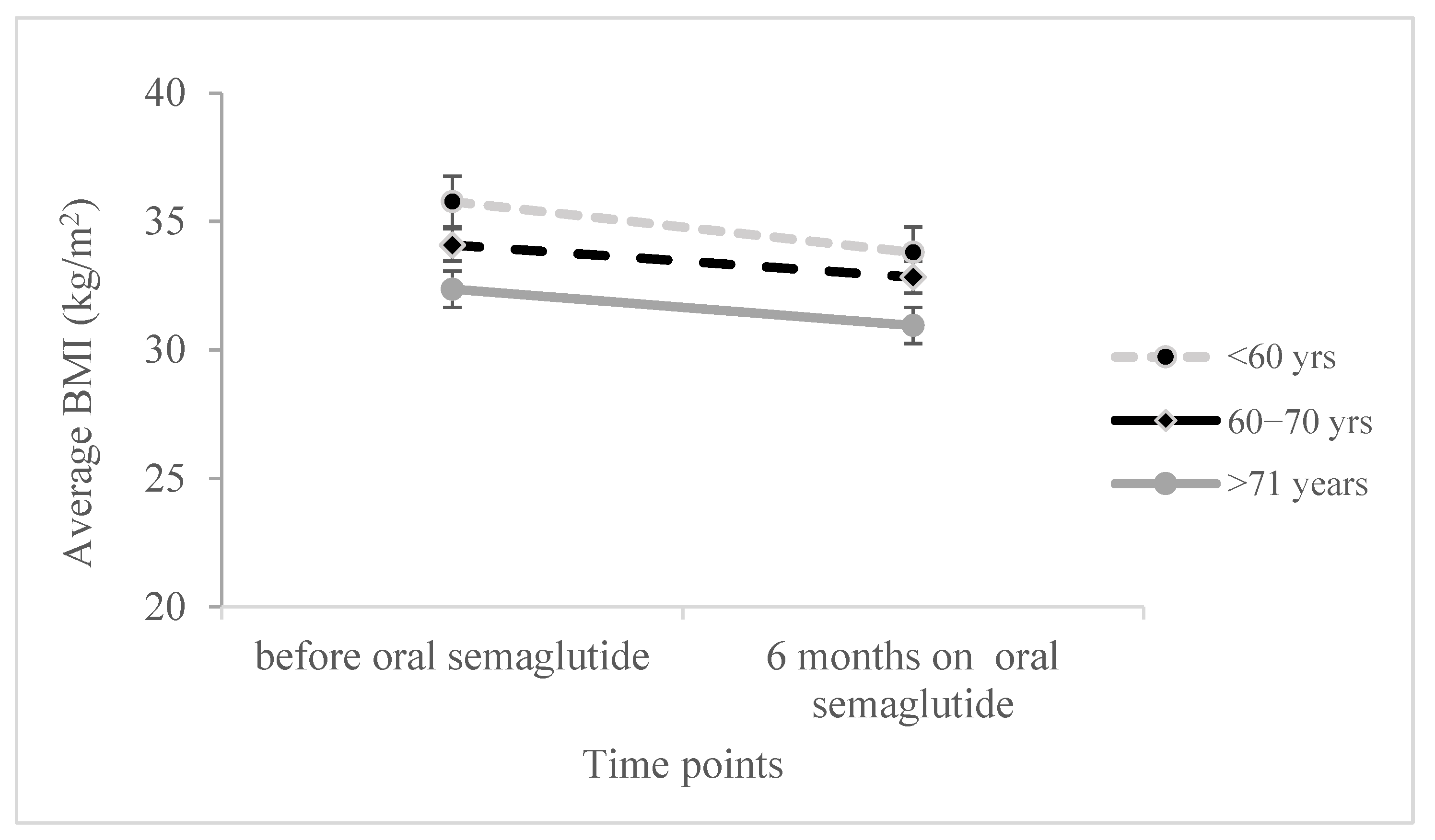
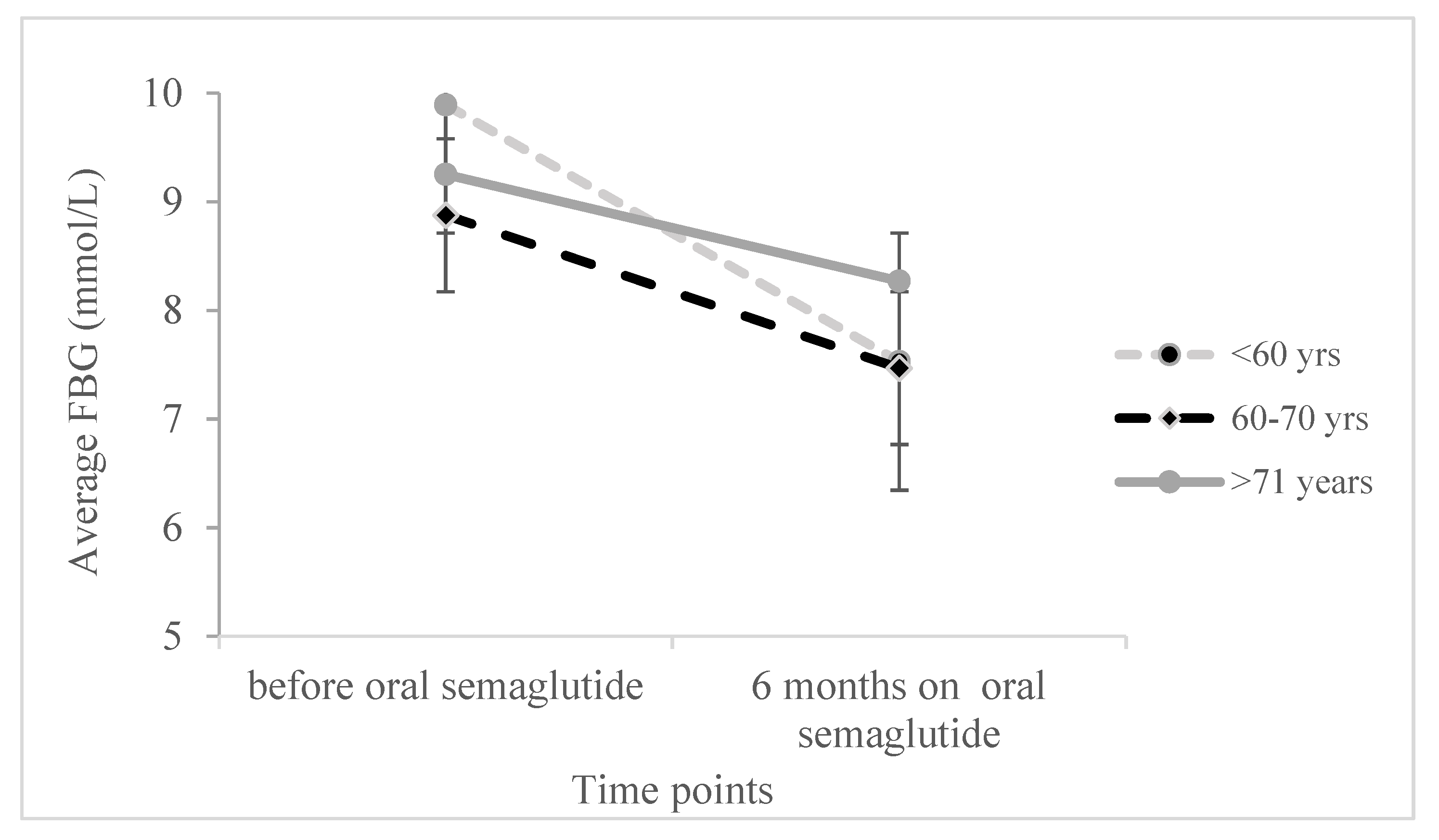
Moreover, we conducted a two-sided ANOVA with repeated measures to examine the effect of age and its interaction with changes in various parameters during therapy, while controlling for covariates, sex, and the duration of diabetes. Significant results were observed for HbA1c, BMI, and FBG (as shown in
Figure 1,
Figure 2 and
Figure 3), while nonsignificant in other reported parameters.
There was a significant effect on HbA1c decrease during the oral semaglutide treatment (F
(1,116) = 3.925,
p = 0.050), as well as a significant interaction of HbA1c with age (F
(2,116) = 2.986,
p = 0.054), suggesting a greater HbA1c reduction in the age group below 60 and the lowest reduction in the age group above 71 years. The age itself was not statistically significant when adjusted for diabetes duration and sex (F
(1,116) = 0.014,
p = 0.986). Similarly, when two-sided ANOVA was performed for BMI changes over treatment period (
Figure 2) there was no significant effect (F
(1,107) = 1.845,
p = 0.177), although the interaction of BMI with age was significant (F
(2,107) = 3.077,
p = 0.050) and suggested the steepest BMI reduction in the age group up to 60 years, together with the significant effect of age itself (F
(1,107) = 3.836,
p = 0.025), with Scheffe post hoc test results showing the average BMI being significantly lower in the group up to 60 years compared to the group older than 71 years (
p = 0.020).
Moreover, the reduction in fasting blood glucose levels was most significant in the patients up to 60 years of age (F
(2,90) = 3.196,
p = 0.046), as shown in
Figure 3.
4. Discussion
This multicentric, real-world study conducted in Croatia demonstrates that oral semaglutide is effective and well-tolerated as a third-line treatment in routine clinical practice. It leads to clinically significant improvements in glycemic control and body weight among patients with type 2 diabetes
mellitus. These findings are particularly relevant given the stringent criteria set by the Croatian Health Insurance Fund, which limits reimbursement for GLP-1 receptor agonists (GLP-1 RAs) to patients with a BMI greater than 30 kg/m
2 and HbA1c levels above 7% after failing two oral agents, or to those with cardiovascular disease and a BMI greater than 28 kg/m
2 under similar conditions. The clinical profile of our cohort aligns with these reimbursement criteria. A significant majority of patients (90.1%) had a BMI of 30 kg/m
2 or higher, and 83.3% had cardiovascular disease. Moreover, over 80% had previously used both metformin and a second oral agent before starting semaglutide. Therefore, our sample represents a highly comorbid and treatment-experienced population that aligns with the Croatian health insurance reimbursement policy requirements and guidelines for initiating GLP-1 RAs. In our study, the use of oral semaglutide resulted in a mean HbA1c reduction of 0.95%, along with significant improvements in fasting and postprandial glucose levels, BMI, liver enzymes, and HDL cholesterol. These results are consistent with those from the pivotal PIONEER 1 to 8 trials, which showed HbA1c reductions ranging from 0.9% to 1.5% and weight reductions between 2.5 kg and 5.0 kg, depending on the dose and background therapy [
8]. In PIONEER 2, for example, semaglutide 14 mg daily led to a 1.3% reduction in HbA1c and approximately 4.3 kg weight loss compared to empagliflozin over 52 weeks [
1]. Similarly, PIONEER 4 found semaglutide to be superior to liraglutide and placebo in reducing both HbA1c and weight [
7].
Our real-world findings align with those from the IGNITE study, which assessed the early use of oral semaglutide in routine clinical settings. This study reported an HbA1c reduction of 1.0% and a mean weight loss of 4.4 kg [
5]. Similarly, the PIONEER REAL Canada and PIONEER REAL Europe/Netherlands/Switzerland observational cohorts confirmed average HbA1c improvements of 1.0–1.2% and weight loss of 4–5%, aligning closely with our findings [
6]. These studies consistently highlight the therapeutic potential of oral semaglutide in real-life patients, despite heterogeneity in background therapy and baseline risk.
We achieved an overall response rate of about 30% for composite outcomes, which included patients with an HbA1c level of less than 7% and a weight reduction of 5% or more. Although these figures are modest, they hold clinical significance. However, they are somewhat lower than the success rates of approximately 33–35% reported in other real-world studies [
15,
16,
17]. This discrepancy may be due to our cohort having a higher baseline burden of comorbidities and longer duration of diabetes. In fact, logistic regression analysis in our study identified a longer disease duration (6 to 10 years) and previous insulin use as significant negative predictors of response. These findings align with the results from the IGNITE and PIONEER REAL studies, which suggest that introducing GLP-1 receptor agonists earlier—especially in insulin-naïve patients—leads to more favorable outcomes [
12,
14].
In our cohort, which primarily consisted of older adults (67.5% over the age of 60) with a high burden of comorbidities—including obesity (90.1%) and cardiovascular disease (84%)—oral semaglutide showed promising safety and efficacy. The incidence of gastrointestinal side effects was relatively low at 21.9%, and there were no severe adverse events reported, highlighting its tolerability in this population. These findings are consistent with the data from PIONEER 5, which specifically examined semaglutide in patients with moderate renal impairment—a common comorbidity among older adults. The study found that the therapy was both safe and effective, with an adverse event profile similar to that of a placebo [
5]. Moreover, the pooled PIONEER REAL analysis, incorporating data from over 2500 real-world patients across seven countries, confirmed consistent efficacy across age groups, with older individuals experiencing comparable HbA1c and weight reductions to younger counterparts, and without increased adverse event rates [
6]. The Canadian and Dutch PIONEER REAL cohorts also included older, comorbid patients and observed meaningful clinical improvements with good tolerability [
4,
11]. However, age-based subgroup analyses using two-way repeated measures ANOVA revealed important interaction effects. Patients under 60 years exhibited significantly greater reductions in HbA1c, BMI, and fasting glucose levels, indicating a more robust response in younger individuals. While the main effect of age was not statistically significant when controlling for diabetes duration and sex, the age-by-time interaction was significant for key parameters. This suggests that while older patients can still benefit from oral semaglutide, earlier initiation may yield more pronounced improvements, a finding confirmed in prior studies such as PIONEER 7 and IGNITE [
12,
18].
Importantly, predictors of composite outcome success underscore the clinical value of early GLP-1 RA intervention. Logistic regression revealed that prior insulin use significantly decreased the odds of achieving both glycemic and weight loss goals (OR = 0.152, p = 0.026). Additionally, patients with a diabetes duration of 6–10 years had reduced odds of composite goal attainment compared to those with a duration of ≤5 years. These findings support a therapeutic strategy that prioritizes the timely initiation of GLP-1 RAs before irreversible β-cell failure limits responsiveness.
Regarding safety, our study identified gastrointestinal adverse events in 21,9% of patients—primarily nausea and heartburn—which is consistent with previously reported data from the PIONEER program and real-world trials [
4,
8,
9,
11]. The overall discontinuation rate was mostly due to gastrointestinal side effects and occurred in the dose titration period, highlighting the need for careful dose titration and patient education.
This study has several strengths. As one of the first multicentric real-world evaluations of oral semaglutide in Croatia, it offers important insight into how this therapy performs outside of randomized controlled trial settings. The patient population is representative of individuals typically treated in Croatian endocrinology centers, providing data from a Central/Eastern European healthcare context where prescription patterns, socioeconomic factors, and adherence may differ from those in randomized clinical trial (RCT) populations. The study includes an analysis of hepatic biomarkers (AST and ALT), which have been underexplored in real-world studies of oral semaglutide. Additionally, it offers evidence of treatment effectiveness in routine clinical practice, complementing the controlled environment of randomized clinical trials. Overall, these findings may help guide regional clinical practices and inform health policy decisions regarding the use of GLP-1 receptor agonists, thereby enhancing the relevance of the results to everyday clinical scenarios.
However, certain limitations should be acknowledged. The retrospective observational design introduces the risk of various biases, including selection bias and incomplete data reporting, and inherently limits the ability to draw causal conclusions. Since the cohort included only individuals who qualified under the current Croatian reimbursement criteria, the results may not be generalizable to patients with lower BMI, less advanced disease, or without cardiovascular comorbidity.
5. Conclusions
Our findings encourage earlier use of this therapeutic class, particularly in eligible patients before insulin is required. While our retrospective design and lack of a comparator group are limitations, the multicentric, representative nature of our sample, and alignment with results from other published data support this conclusion. Furthermore, our results add to the growing body of evidence supporting oral semaglutide’s role in managing complex T2DM cases in real-life settings, including older and multimorbid individuals, a group often underrepresented in RCTs yet highly prevalent in everyday clinical practice.
In summary, oral semaglutide provides effective glycemic and metabolic control in a real-world Croatian population, particularly if initiated early and in insulin-naïve patients. These results complement global evidence from both randomized controlled and observational studies, and support the integration of oral GLP-1 RAs into national diabetes treatment strategies.