Abstract
Background/Objectives: Late presentations of advanced hepatocellular carcinoma (HCC) indicate a lack of detection of underlying cirrhosis and a need to identify clinical and socioeconomic risk factors contributing to early-stage HCC recognition. This study tested associations between early diagnostics of HCC and demographic, socioeconomic, clinical, and healthcare-related indicators. Methods: A retrospective analysis of clinical data accumulated between February 2018 and February 2024 was completed at a quaternary care centre (South Australia). Results: We identified 388 cases of newly diagnosed HCC during a six-year period. There were 131 (33.7%) patients with early-stage (Barcelona clinic liver cancer (BCLC) stage 0–A) and 257 (66.3%) patients with late-stage (BCLC B–D) HCC. Late-stage HCC was found in 66.3% of patients, with half of the cohort not having a diagnosis of cirrhosis at the time of HCC detection. A retrospectively calculated Fibrosis Index (FIB-4) of >3.25 was present in nearly half of patients with newly diagnosed HCC with no prior diagnosis of cirrhosis. Engagement with healthcare (p < 0.05), a history of liver cirrhosis (p < 0.001), and gastroenterologist-led care with surveillance programmes (p < 0.001) was associated with early-stage presentation and curative treatment. Late-stage HCC was associated with male sex (p = 0.041), failing to attend appointments (p < 0.001), and liver function tests ordered by general physicians (p = 0.002) or non-gastroenterologist specialists (p = 0.023). Logistic regression analysis indicated that engaging in a surveillance programme, assessment by a gastroenterologist, and Model for End-Stage Liver Disease scores were important factors contributing to early detection of HCC; the area under the curve for this model on the ROC analysis was 0.892 (95% CI 0.855–0.929). Conclusions: Better cirrhosis detection is required, given that 60% of patients had a retrospectively calculated FIB-4 > 3.25. Routine use of non-invasive scores by all healthcare providers may increase engagement with surveillance and improve HCC screening.
1. Introduction
Hepatocellular carcinoma (HCC), the most common primary liver cancer, typically arises in patients with cirrhosis. It is the sixth most common cancer globally and the third leading cause of cancer-related mortality []. In Australia, HCC rose from the fifteenth to the sixth most common cause of cancer death between 2001 and 2023 []. Its financial burden is substantial, with an estimated cost of AUD 4.8 billion in 2019–2020 [].
Metabolic dysfunction-associated steatotic liver disease (MASLD) is a growing contributor to HCC []. MASLD is an increasing health burden in Australia, with rising cases of liver fibrosis, cirrhosis and HCC. Early diagnosis remains challenging []. Screening for advanced fibrosis has been improved with the use of non-invasive tools such as the Fibrosis-4 (FIB-4) score [,,]. Barcelona clinic liver cancer (BCLC) staging is an internationally accepted guideline which directs selection of HCC therapy and treatment modalities [,]. BCLC stages 0–A (early stage) are amenable to curative treatments such as surgical resection, microwave ablation (MWA), stereotactic ablative body radiotherapy (SABR), and liver transplantation []. Patients with BCLC stage B-C (intermediate-to-late stage) are treated with non-curative therapies such as transarterial chemoembolisation (TACE), selective internal radiation therapy (SIRT), and immunotherapy. BCLC D is managed supportively. HCC stage predicts survival, with early-stage HCC conferring 70% survival rates at 5 years [,].
Given the availability of curative treatment at early stages, HCC surveillance presents an opportunity for early diagnosis [,]. Guidelines recommend six monthly abdominal ultrasounds in patients with liver cirrhosis and subgroups of chronic hepatitis B virus (HBV) infection []. A meta-analysis involving 145,396 patients demonstrated surveillance was associated with early detection, curative treatment, and better survival []. Despite HCC surveillance being standard practice, an Australian cohort study in 2018 showed that survival of patients with HCC remains poor with an overall survival time of 20.8 months. Surveillance uptake was poor with only 40% of patients in the cohort participating in HCC surveillance at the time of diagnosis, with similar trends demonstrated in international studies. There is a lack of Australian data surrounding the efficacy of HCC screening, with only one previous study reporting rates of early HCC diagnosis. Despite an established surveillance programme, there remains a critical gap in knowledge around what rates of early HCC diagnosis constitute an effective surveillance programme [,,]. The aim of this study is to analyse real-world data of rates of early HCC detection and modifiable and non-modifiable factors, including aetiology, patient demographics, models of care, and effectiveness of non-invasive surveillance on HCC outcomes in an Australian setting. Our research objectives included the identification of the strongest predictors of early stage HCC and generation of a better surveillance model.
2. Materials and Methods
2.1. Study Cohort and Data Collection
Retrospective cohort data were collected from an Australian quaternary referral centre for patients diagnosed with HCC between February 2018 and February 2024. This centre receives referrals from three major urban hospitals, remote South Australia, the Northern Territory, and parts of regional Victoria and New South Wales. Treatment recommendations were made by a multidisciplinary team (MDT) comprising hepatologists, hepatobiliary surgeons, interventional radiologists, oncologists, and specialist nurses. The Central Adelaide Local Health Network (CALHN) Human Research Ethics Committee granted approval for the study (HREC/20/SAH/73). HCC cases were identified through MDT consensus. These patients were stratified into those with curative intent (BCLC A) and those receiving non curative therapy (BCLC B–D). Patients with recurrence of previous HCC at the same site were excluded as shown in Figure 1.
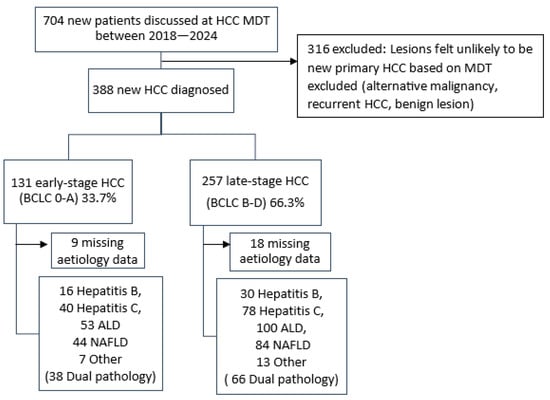
Figure 1.
Flowchart summarising new HCC cases discussed at MDT, with exclusions and distribution by BCLC staging and aetiology.
Demographic information (age, sex, Aboriginal and Torres Strait Islander status), distance from the referral centre, partner/spouse as next of kin, engagement with healthcare (i.e., attendance to pathology, imaging, clinic), attendance to liver clinics, pension health cardholder status, private insurance/DVA cardholder status, GP recorded, history of known cirrhosis, lost to follow-up or cancellations, screening/surveillance programme (defined as having a risk factor for HCC, requiring surveillance i.e., cirrhosis, and undergoing screening imaging within the 3 years preceding HCC diagnosis), interval between screening scans, aetiology contributing to cirrhosis, blood test results (in preceding 12 months) along with the specialty which had ordered them, were all obtained from EMR systems. Medians for Model for End-Stage Liver Disease (MELD) and fibrosis (FIB-4) scores were calculated [].
2.2. Statistical Analysis
Statistical analyses were performed using IBM © SPSS © Statistics version 29. Two sample t-tests or Chi-Square tests were used in univariate analysis with significance set at p value < 0.05. Binary logistic regression analysis was used to determine odds ratios, relative risks, and 95% confidence intervals. Factors that were significant in the univariant analysis were included in the logistic regression (binary) multivariate analysis. The model building was also based on a purposeful selection strategy for clinically important factors (such as aetiology) [,]. The diagnostic power of each model was assessed by the receiver operator characteristic (ROC) curve. The model robustness was checked using bootstrap, alternative model specification, internal validation, and calibration (Hosmer–Lemeshow plot) analyses.
3. Results
3.1. Analysis of Demographics and Treatment Modalities
In this study 388 new cases of HCC were diagnosed in a 5-year period (Figure 1 and Table 1). A total of 131 (33.7%) patients were classified as having early-stage (BCLC 0–A) and 257 (66.3%) as having late-stage (BCLC B–D) HCC. The median ages were 68 years (IQR: 62–75) and 65 years (IQR: 59–75), respectively. A total of 180 (46%) did not have a prior diagnosis of cirrhosis. Most patients were male (75%), with late-stage disease being more common in males (p = 0.041). Patients with a partner or spouse on record were also more likely to present with curable disease (p = 0.019). Surrogates for socioeconomic status and access to healthcare including private health insurance (p = 0.011) and proximity of the residence to the referral centre (p = 0.026) were also associated with early detection of HCC. No significant associations were found for Aboriginal and Torres Strait Islander status, pension cardholders, or liver disease aetiology (Table 1).

Table 1.
Demographics and clinical factors in the HCC patient cohort.
The average MELD score was significantly lower in early-stage HCC patients (9.6; IQR 6–11) compared to late-stage patients (12.1; IQR 8–15; p = 0.001, Table 2). FIB-4 scores were not significantly associated with stage (p = 0.065; Table 1). Grouped FIB-4 categories (Group 1: FIB-4 < 1.45; Group 2: FIB-4 > 1.45 through 3.25; Group 3: FIB-4 > 3.251 (6, 7, 8)) showed no significant association on Chi-square testing (χ2(2) = 4.01; p = 0.135). However, a linear-by-linear trend test indicated a borderline association (p = 0.050), suggesting that higher FIB-4 categories may be linked to reduced likelihood of early-stage HCC.

Table 2.
Treatment-related characteristics of HCC patients.
A total of 131 patients received curative intent treatment, 87 (66.4%) received MWA, 28 (21.3%) underwent surgical resection, 6 (4.5%) received liver transplantation, and 10 (7.7%) underwent definitive treatment with SABR. A total of 257 cases were ineligible to receive curative intent treatment: 99 (38.5%) received TACE, 66 (25.7%) received immunotherapy, 3 (1.2%) received SIRT sphere insertion, and 11 (4.3%) received combination therapies including TACE, SABR, MWA, and immunotherapy. Notably, 77 (30%) patients received best supportive care, and 2 patients (0.8%) were deceased prior to the MDT discussion (Table 2).
3.2. Testing Effects of the Recognition of Cirrhosis and Model of Care
Patient factors such as active engagement with any healthcare provider (p < 0.001), having a GP on record (p = 0.019), and a known history of liver cirrhosis (p < 0.001) were strongly associated with early-stage presentation and receipt of curative intent treatment (Table 3). Considering models of care, 100/131 (76.3%) of early-stage diagnoses were known to a specialist liver clinic, compared to 105/257 (31.9%) of late-stage cases (p < 0.001). Amongst patients known to a liver clinic, failing to attend appointments (p < 0.001) or being lost to follow-=up (p < 0.001) were strongly associated with late-stage presentations. Undergoing HCC surveillance was strongly associated with early detection, with 67.9% (89/131) of early-stage patients undergoing surveillance compared to 16.0% (41/257) of late-stage cases (p < 0.001).

Table 3.
Model of care.
Liver enzyme tests within the prior 12 months were analysed. Tests ordered by gastroenterologists were associated with early-stage HCC (p < 0.001), while those ordered by GPs (p = 0.002) and other specialists (p = 0.023) were linked to late-stage presentation. Tests from emergency departments trended toward late-stage disease but were not statistically significant; inpatient-ordered tests showed no association with curable HCC.
At diagnosis, 130/388 (62.8%) patients were undergoing HCC surveillance—122 for cirrhosis and 8 for other indications such as chronic HBV. Of these, 89/130 (68.5%) were diagnosed at early stage and 41/130 (31.5%) at late stage (Table 4). Among those with late-stage disease under surveillance, 18/41 (43.9%) had prolonged scan intervals (>7 months), 5/41 (12.2%) had inadequate imaging, 4/41 (9.8%) showed progression of a known nodule, 2/41 (4.9%) had early detection but were not curative candidates, 1/41 (2.4%) had a missed lesion, and 11/41 (26.8%) had unclear reasons, including remote or interstate residence.

Table 4.
Comparing HCC stages in “surveillance” vs. “non-surveillance” status patients.
3.3. The Impact of Non-Invasive Fibrosis Assessment and Other Surveillance Methods
Among the 180 HCC cases without prior cirrhosis (Table 5), only 11 (6.1%) lacked sufficient blood tests to calculate FIB-4. Of the remainder, 146/180 (81.1%) had FIB-4 > 1.45 and 86/180 (47.8%) exceeded > 3.25, indicating likely advanced fibrosis. An alternative non-invasive score, AST to Platelet Ratio Index (APRI) was also calculated. A total of 54/180 (30%) of patients met the threshold (APRI > 1.0) for advanced fibrosis/cirrhosis. Of the patients without a prior diagnosis of cirrhosis, at least 44.4% (80/180) had bloods tests performed > 6 months prior to their diagnosis of HCC. Nearly half (48.8%; 39/80) of these patients had a FIB-4 of > 3.25, suggestive of advanced fibrosis.

Table 5.
Non-invasive score distribution amongst patients with new HCC.
3.4. Multivariate Analysis: Logistic Regression Model for Early-Stage HCC Detection
We aimed to define the predictive role of healthcare-related modifiable factors using logistic regression. Several variables showed significant individual association with the early detection of HCC in univariate analysis (Table 1 and Table 2) and were strong candidates for inclusion in the multivariable model. Strong independent prognostic factors of early-stage HCC detection included engagement with healthcare services (p < 0.001, OR = 9, 95% CI 3.3–24.6), assessment by a gastroenterologist (p < 0.001, OR = 5.7, 95% CI 2.7–11.8), and having known cirrhosis (p < 0.001, OR = 5.8, 95% CI 2.7–12.2). Presence of dual pathology (two or more different liver diseases) was another independent predictor with a high OR (p = 0.042, OR = 3.7, 95% CI 1.1–12.7). A high MELD score (p = 0.012, OR = 0.92, 95% CI 0.87–0.98) or FIB-4 (p = 0.029, OR = 0.57, 95% CI 0.35–09.4) were independent negative predictors. Previous diagnosis with alcoholic liver disease (ALD) (p = 0.044; OR = 0.33, 95% CI 0.11–0.97) or fatty liver disease (MASLD) (p = 0.044; OR = 0.35, 95% CI 0.12–0.97) were also significant negative predictors. However, other factors, including viral hepatitis (or other liver diseases) and gastroenterologist-ordered blood tests were not independent predictors in this model (p > 0.05) (Supplementary Tables S1–S4). The ROC test was used to assess the quality of this model. The area under ROC curve (AUC) value was equal to 0.892 with CI: 0.855–0.929 (p < 0.001; Sensitivity = 92.1%, Specificity = 82%), indicating the selected model reliably and with high sensitivity distinguishes between HCC groups.
The constructed models were assessed for robustness using sensitivity analyses. Our analysis indicated that both logistic regression models are robust. Bootstrap confirmed that the results are not driven by random variation (robust) and are stable (bootstrap resampling is shown as Supplementary Data S5) and well-calibrated. The main predictors (defined using logistic regression approach) remain significant regardless of whether FIB-4 or MELD is used. However, MELD and FIB-4 both add prognostic value to the constructed models. This strengthens confidence that the associations (especially for the presence of cirrhosis, engagement behaviour, and specialist follow-up) are not artefacts of model choice and can potentially be implemented in practice, although further testing in larger cohort is recommended.
4. Discussion
This study highlights a major disparity in HCC diagnosis, with 66.3% of patients presenting at advanced stages despite surveillance guidelines. Key elements include the importance of healthcare engagement, limitations of current surveillance, and the potential of non-invasive scores for earlier detection. Factors most strongly associated with increased odds of early-stage HCC at diagnosis were engagement with healthcare (OR 5.133, 95% CI 2.551–10.332), being known to a liver clinic (OR 6.845, 95% CI 4.232–11.072), a low MELD score (p < 0.001, OR 0.94, 95% CI 0.895–0.982), and undergoing HCC surveillance (OR 11.164, 95% CI 6.798–18.334). Our data indicates that patients managed in a hepatology clinic were more likely to be diagnosed with early-stage HCC. However, this finding may be in part impacted by a selection effect. Patients with a known diagnosis of liver disease are more likely to be referred to hepatology clinic and undergo regular surveillance, increasing the likelihood of early detection. Whilst this referral bias could partly explain our results, it also highlights the importance of dedicated hepatology follow-up for at-risk populations. Expectedly, failing to attend liver clinic appointments (OR 0.245, 95% 0.119–0.503) or being lost to follow-up (OR 0.094, 95% CI 0.031–0.281) were strongly associated with late-stage presentations, emphasising the role of engagement. Logistic regression showed surveillance participation as the strongest predictor of early-stage HCC, followed by engagement with health services, liver clinic access, dual liver pathology, and a low MELD score. Grouped FIB-4, though not independently significant, improved prediction when combined with clinical variables. ALD and MASLD were independent negative predictors, highlighting the need to improve surveillance in these groups.
Several studies report socioeconomic disparities in HCC treatment and survival [,,]. Access to specialist clinics and liver nursing staff improves care and outcomes in liver cirrhosis []. Our data similarly show that surrogates for socioeconomic status predict curative treatment; private insurance (OR 1.84, 95% CI 1.15–2.96), proximity to the referral centre (<100 km; OR 1.78, inverse OR 0.56, 95% CI 0.34–0.94), and social support (partner/spouse; OR 1.62, 95% CI 1.09–2.55) were all associated with early HCC diagnosis. Social support (partner/spouse) was also associated with increased odds of early diagnosis of HCC (OR 1.622, 95% CI 1.085–2.546), consistent with findings that marital status is an independent prognostic factor in cancers including HCC [,]. These findings require cautious interpretation given the complexities of health inequity. Furthermore, other socioeconomic surrogates such as level of education, occupation and cultural/language barriers were not collected. Likely due to insufficient sample size (21/388; 5.4%), Aboriginal and Torres Strait Islander status trended toward reduced odds of curative HCC (OR 0.437, 95% CI 0.144–1.327) but did not reach statistical significance. Noting the applicability of subgroup analysis was limited by underrepresentation, no strong conclusions could be drawn. Prior studies report a reduced frequency of receiving curative therapy and reduced overall survival in First Nations Australians [,].
Our data analysis confirmed that a diagnosis of cirrhosis, irrespective of aetiology, is associated with early-stage HCC detection. Studies indicate that surveillance and monitoring for HBV leads to early diagnosis of HCC [,]. Chronic HBV patients are ideally identified and monitored to enable early HCC detection for the at-risk subgroups. In our data no association was found between HBV diagnosis and improved odds of curative HCC in this study. Key factors such as ethnicity and family history were unavailable, limiting this analysis. Thus, HBV screening quality in the community and subsequent surveillance of HCC warrants further assessment. In patients with obesity and MASLD, limitations were reported during utilisation of ultrasound-based HCC surveillance [,]. While the gold standard for diagnosis of cirrhosis is liver biopsy, assessment of liver stiffness with elastography was associated with a significantly reduced HCC mortality, suggesting a valuable use in diagnostics []. MELD and other scores also correlate with early HCC detection, likely reflecting an association with disease progression and decompensation with diagnosis of HCC [,].
Australian data is limited, with a previous Victorian cohort study demonstrating curative treatment in 32% of participants and a surveillance uptake (defined as percentage of new HCC diagnoses undergoing HCC surveillance) of 40% []. Our cohort had similar rates of delivery of curative treatment (33.7%) and a surveillance uptake of 34%. Given the effectiveness of treatment in early HCC, it is disappointing that only one third were diagnosed at BCLC 0–A stage. Early-stage HCC is detected at significantly lower rates than other cancers with screening programmes in Australia []. Low rates of early diagnosis are multifactorial, and engagement in HCC surveillance amongst patients with liver cirrhosis remains an area for improvement. Amongst 207 patients with known liver cirrhosis, only 122 (58.9%) had undergone surveillance scans within the past 36 months. Previously identified barriers to surveillance in this patient population included limited patient or clinician awareness, cultural and linguistic factors, scheduling difficulties, and socioeconomic constraints [,]. Despite undergoing surveillance, 41/130 (31.5%) of patients presented with late-stage disease. Prolonged surveillance intervals were the most common factor (44%), emphasising the need for improved engagement and follow-up.
Over 6 million Australians are affected by chronic liver disease, with an estimated 70,000 having cirrhosis []. As 80–90% of newly diagnosed HCC arise in liver cirrhosis, undiagnosed cirrhosis remains a critical barrier to early HCC detection []. Within our cohort, a known history of cirrhosis was associated with significantly increased odds of early HCC diagnosis (OR 5.058, 95% CI 3.124–8.189), with only 207/388 (53.4%) patients having a known history of cirrhosis prior to presentation with HCC. There was a discrepancy noted in our cohort between increased odds of presenting with early-stage HCC and blood tests ordered by gastroenterologists (OR 2.142, 95% CI 1.370–3.350), while decreased odds were detected when blood tests were ordered by a GP (OR 0.470, 95% CI 0.291–0.760) or other specialists (OR 0.499, 95% CI 0.272–0.918). This should be interpreted in context, given that primary care physicians and other specialists order blood tests for any indications, including symptoms immediately prior to the undiagnosed HCC, as opposed to gastroenterologists monitoring a patient’s known liver disease. These do allude to a significant burden of previously undiagnosed and unrecognised liver disease in the community.
The FIB-4 was validated for use in MASLD and viral hepatitis as a screening tool with a high negative predictive value at a score of <1.45 []. The score performed better than other non-invasive tools in classifying stages of fibrosis, with a positive predictive value of over 80% at a score of >3.25 [,]. Although we observed a trend toward significance for FIB-4 groups on univariate analysis, this did not reach statistical significance. Grouped FIB-4 became a significant predictor of early-stage HCC on multivariate analysis. Model robustness analysis indicated that both MELD and FIB-4 add prognostic value to the healthcare models. While the role of FIB-4 as a predictor of early-stage HCC remains to be confirmed in large studies, the application of non-invasive scoring may have a more delineated role in identifying at-risk patient populations that may benefit from early access to cirrhosis care and HCC screening. In our study, FIB-4 could have been calculated for 169/180 (94%) of patients without prior diagnoses of cirrhosis. Moreover, the FIB-4 score was >1.3 in 146 (81%) patients, indicating further investigation was required, and >3.25 in 86 (48%) patients, indicating high likelihood of severe fibrosis. APRI showed similar trends, while high MELD predicted poorer outcomes. Moreover, 44% of patients without a diagnosis of cirrhosis had blood tests > 6 months prior to HCC diagnosis. Almost half had a FIB-4 of >3.25, suggestive of advanced fibrosis. These patients represent a population with missed opportunities for an earlier screening and diagnosis of HCC. These cost and time efficient non-invasive indicators of liver fibrosis are suitable for most healthcare settings to identify at-risk patients.
This study’s limitations included the single referral centre which may limit the generalizability of findings to the broader population, a limited patient cohort, and the study’s retrospective nature. Certain variables were difficult to establish based on available medical records and reduced strength of analysis, such as date/time/frequency of visits for engagement with healthcare, blood test dates, and ethnicity (except indicated Aboriginal and Torres Strait Islanders). Potential confounders such as comorbidities (except aetiology of liver disease), medication use, and lifestyle factors were not accounted for in the analysis. GP involvement was recorded, but visit frequency was not available.
Improving diagnosis rates of cirrhosis requires awareness of disease-contributing risk factors, including a history of viral hepatitis, alcohol misuse, and metabolic risk factors. General practitioners play a key role in identifying patients with undiagnosed liver disease and diagnosis of HCC. Within our cohort, patients who were known to a GP had significantly increased odds of presenting with curable HCC (OR 2.828, 95% CI 1.146–6.981). A recent systematic review acknowledged lower rates of HCC surveillance in primary care settings compared to gastroenterology-led care, highlighting a lack of awareness of cirrhosis and HCC risk factors and surveillance recommendations, time constraints in primary care, and patient cost incursion as significant barriers []. Access to specialist liver clinics and streamlined referral pathways for high-risk individuals identified via FIB-4 or similar tools may enhance early diagnosis of cirrhosis and HCC.
5. Conclusions
This study highlights the ongoing challenge of late-stage HCC diagnosis, with 66.3% of patients presenting at an advanced stage despite established surveillance programmes. Nearly half of patients did not have a prior diagnosis of cirrhosis e in a surveillance programme, though non-invasive testing in this cohort would have supported a diagnosis or further testing. Engagement with healthcare, assessment by a liver clinic consultant, and active surveillance were strongly associated with early-stage detection and curative treatment eligibility. While the retrospective, single-centre nature of this study limits generalizability, our findings indicate the need for improved risk stratification using non-invasive scores (FIB-4) and enhanced engagement strategies within targeted surveillance protocols. Validation of these findings through multi-centre studies with larger sample sizes is warranted.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/livers5040057/s1, Table S1: Binary Logistic regression analysis with MELD (model 1, completed using SPSS statistical application); Table S2: ROC analysis for model 1. Area Under the ROC Curve; Table S3: Binary Logistic regression analysis with FIB-4 (model 2, completed using SPSS statistical application); Table S4: ROC analysis for model 2; Data S5: The assessment of model robustness (sensitivity analysis).
Author Contributions
Conceptualization, B.N., J.E.F. and E.T.; methodology, B.N., K.P., J.M. and O.S.; validation, J.M. and O.S.; formal analysis, B.N. and O.S.; investigation, B.N., A.L., D.N., K.P. and J.M.; resources, J.M., J.E.F. and E.T.; data curation, H.K., A.L., K.P., J.M., D.N. and O.S.; writing—original draft preparation, B.N., H.K., D.N. and O.S.; writing—review and editing, B.N., H.K., A.L., D.N., A.N., J.E.F. and E.T.; visualisation, A.L. and O.S.; supervision, E.T.; project administration, J.E.F. and E.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Central Adelaide Local Health Network Human Research Ethics Committee (HREC/20/SAH/73–12 November 2020).
Informed Consent Statement
Patient consent was waived because this study was a retrospective review of existing clinical data, involved no direct patient contact, used only de-identified information and was judged by the Central Adelaide Local Health Network Human Research Ethics Committee to be of low or negligible risk.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to the guidelines indicated by the CALHN Human Ethics Committee. For more information, please refer to: https://www.sahealth.sa.gov.au/wps/wcm/connect/public+content/sa+health+internet/resources/policies/research+ethics+and+governance+policy (accessed on 9 September 2025).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AST | Aspartate Aminotransferase |
| LFT | Liver function test |
| APRI | AST to Platelet Ratio Index |
| BCLC | Barcelona clinic liver cancer score |
| HCC | Hepatocellular carcinoma |
| IQR | Interquartile range |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| FIB-4 | Fibrosis Index-4 score |
| MELD | Model for End-Stage Liver Disease score |
| MWA | Microwave ablation |
| TACE | Transarterial chemoembolization |
| SIRT | Selective internal radiation therapy |
| HBV | Hepatitis B virus infection |
| HCV | Hepatitis C virus infection |
| MDT | Multidisciplinary team |
| OR | Odds Ratio |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Australian Institute of Health and Welfare. Cancer in Australia 2021; AIHW: Canberra, Australia, 2021. [Google Scholar] [CrossRef]
- Worthington, J.; He, E.; Caruana, M.; Wade, S.; de Graaff, B.; Nguyen, A.L.T.; George, J.; Canfell, K.; Feletto, E. A Health Economic Evaluation of Routine Hepatocellular Carcinoma Surveillance for People with Compensated Cirrhosis to Support Australian Clinical Guidelines. MDM Policy Pract. 2025, 10, 23814683251344962. [Google Scholar] [CrossRef] [PubMed]
- Phoolchund, A.G.S.; Khakoo, S.I. MASLD and the Development of HCC: Pathogenesis and Therapeutic Challenges. Cancers 2024, 16, 259. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Chen, T.I.; Yang, T.H.; Yin, S.C.; Lu, S.N.; Liu, X.R.; Gao, Y.Z.; Lin, C.J.; Huang, C.W.; Huang, J.F.; et al. Long-Term Risks of Cirrhosis and Hepatocellular Carcinoma Across Steatotic Liver Disease Subtypes. Am. J. Gastroenterol. 2024, 119, 2241–2250. [Google Scholar] [CrossRef]
- Tincopa, M.A.; Loomba, R. Noninvasive Tests to Assess Fibrosis and Disease Severity in Metabolic Dysfunction-Associated Steatotic Liver Disease. Semin. Liver Dis. 2024, 44, 287–299. [Google Scholar] [CrossRef]
- Taru, M.G.; Lupsor-Platon, M. Exploring Opportunities to Enhance the Screening and Surveillance of Hepatocellular Carcinoma in Non-Alcoholic Fatty Liver Disease (NAFLD) through Risk Stratification Algorithms Incorporating Ultrasound Elastography. Cancers 2023, 15, 4097. [Google Scholar] [CrossRef]
- Seif El Dahan, K.; Reczek, A.; Daher, D.; Rich, N.E.; Yang, J.D.; Hsiehchen, D.; Zhu, H.; Patel, M.S.; Molano, M.d.P.B.; Sanford, N.; et al. Multidisciplinary care for patients with HCC: A systematic review and meta-analysis. Hepatol. Commun. 2023, 7, e0143. [Google Scholar] [CrossRef]
- Kinsey, E.; Lee, H.M. Management of Hepatocellular Carcinoma in 2024: The Multidisciplinary Paradigm in an Evolving Treatment Landscape. Cancers 2024, 16, 666. [Google Scholar] [CrossRef]
- Trevisani, F.; Vitale, A.; Kudo, M.; Kulik, L.; Park, J.-W.; Pinato, D.J.; Cillo, U. Merits and boundaries of the BCLC staging and treatment algorithm: Learning from the past to improve the future with a novel proposal. J. Hepatol. 2024, 80, 661–669. [Google Scholar] [CrossRef]
- El-Serag, H.B. Hepatocellular carcinoma. N. Engl. J. Med. 2011, 365, 1118–1127. [Google Scholar] [CrossRef]
- Huang, D.Q.; Singal, A.G.; Kanwal, F.; Lampertico, P.; Buti, M.; Sirlin, C.B.; Nguyen, M.H.; Loomba, R. Hepatocellular carcinoma surveillance—Utilization, barriers and the impact of changing aetiology. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 797–809. [Google Scholar] [CrossRef]
- Singal, A.G.; Zhang, E.; Narasimman, M.; Rich, N.E.; Waljee, A.K.; Hoshida, Y.; Yang, J.D.; Reig, M.; Cabibbo, G.; Nahon, P.; et al. HCC surveillance improves early detection, curative treatment receipt, and survival in patients with cirrhosis: A meta-analysis. J. Hepatol. 2022, 77, 128–139. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, J.; Xiao, W.; Fan, X. A review of MASLD-related hepatocellular carcinoma: Progress in pathogenesis, early detection, and therapeutic interventions. Front. Med. 2024, 11, 1410668. [Google Scholar] [CrossRef]
- Hong, T.P.; Gow, P.J.; Fink, M.; Dev, A.; Roberts, S.K.; Nicoll, A.; Lubel, J.S.; Kronborg, I.; Arachchi, N.; Ryan, M.; et al. Surveillance improves survival of patients with hepatocellular carcinoma: A prospective population-based study. Med. J. Aust. 2018, 209, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Parikh, N.D.; Tayob, N.; Al-Jarrah, T.; Kramer, J.; Melcher, J.; Smith, D.; Marquardt, P.; Liu, P.-H.; Tang, R.; Kanwal, F.; et al. Barriers to Surveillance for Hepatocellular Carcinoma in a Multicenter Cohort. JAMA Netw. Open 2022, 5, e2223504. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Shim, S.G.; Sinn, D.H.; Song, J.E.; Kim, B.S.; Kim, H.G. Child-Pugh, MELD, MELD-Na, and ALBI scores: Which liver function models best predicts prognosis for HCC patient with ascites? Scand. J. Gastroenterol. 2020, 55, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Bursac, Z.; Gauss, C.H.; Williams, D.K.; Hosmer, D.W. Purposeful selection of variables in logistic regression. Source Code Biol. Med. 2008, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.Z.I.; Turin, T.C. Variable selection strategies and its importance in clinical prediction modelling. Fam. Med. Community Health 2020, 8, e000262. [Google Scholar] [CrossRef]
- Wagle, N.S.; Park, S.; Washburn, D.; Ohsfeldt, R.L.; Rich, N.E.; Singal, A.G.; Kum, H. Racial, Ethnic, and Socioeconomic Disparities in Curative Treatment Receipt and Survival in Hepatocellular Carcinoma. Hepatol. Commun. 2022, 6, 1186–1197. [Google Scholar] [CrossRef]
- Wu, C.; Chen, P.; Qian, J.-J.; Jin, S.-J.; Yao, J.; Wang, X.-D.; Bai, D.-S.; Jiang, G.-Q. Effect of marital status on the survival of patients with hepatocellular carcinoma treated with surgical resection: An analysis of 13,408 patients in the surveillance, epidemiology, and end results (SEER) database. Oncotarget 2016, 7, 79442–79452. [Google Scholar] [CrossRef]
- Wigg, A.J.; Narayana, S.K.; Hartel, G.; Medlin, L.; Pratt, G.; Powell, E.E.; Clark, P.; Davies, J.; Campbell, K.; Toombs, M.; et al. Hepatocellular carcinoma amongst Aboriginal and Torres Strait Islander peoples of Australia. eClinicalMedicine 2021, 36, 100919. [Google Scholar] [CrossRef]
- Parker, C.; Tong, S.Y.C.; Dempsey, K.; Condon, J.; Sharma, S.K.; Chen, J.W.C.; Sievert, W.; Davis, J.S. Hepatocellular carcinoma in Australia’s Northern Territory: High incidence and poor outcome. Med. J. Aust. 2014, 201, 470–474. [Google Scholar] [CrossRef]
- Rattanasupar, A.; Chartleeraha, S.; Akarapatima, K.; Chang, A. Factors that Affect the Surveillance and Late-Stage Detection of a Newly Diagnosed Hepatocellular Carcinoma. Asian Pac. J. Cancer Prev. 2021, 22, 3293–3298. [Google Scholar] [CrossRef]
- Tapper, E.B.; Hao, S.; Lin, M.; Mafi, J.N.; McCurdy, H.; Parikh, N.D.; Lok, A.S. The Quality and Outcomes of Care Provided to Patients with Cirrhosis by Advanced Practice Providers. Hepatology 2020, 71, 225–3423. [Google Scholar] [CrossRef] [PubMed]
- Esfeh, J.M.; Hajifathalian, K.; Ansari-Gilani, K. Sensitivity of ultrasound in detecting hepatocellular carcinoma in obese patients compared to explant pathology as the gold standard. Clin. Mol. Hepatol. 2020, 26, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Sukocheva, O.; Ow, T.-W.; Harding, D.; Le Mire, M.; Tse, E. Liver stiffness measurements in patients with metabolic dysfunction-associated steatotic liver disease: Updates on the method effectiveness and perspectives. World J. Hepatol. 2025, 17, 106675. [Google Scholar] [CrossRef] [PubMed]
- Chaiteerakij, R.; Chattieng, P.; Choi, J.; Pinchareon, N.; Thanapirom, K.; Geratikornsupuk, N. Surveillance for Hepatocellular Carcinoma Reduces Mortality: An Inverse Probability of Treatment Weighted Analysis. Ann. Hepatol. 2017, 16, 421–429. [Google Scholar] [CrossRef]
- Australian Institute of Health and Welfare. Cancer Data in Australia; AIHW: Canberra, Australia, 2024. Available online: https://www.aihw.gov.au/reports/cancer/cancer-data-in-australia/data (accessed on 16 March 2025).
- Singal, A.G.; Tiro, J.A.; Murphy, C.C.; Blackwell, J.-M.; Kramer, J.R.; Khan, A.; Liu, Y.; Zhang, S.; Phillips, J.L.; Hernaez, R. Patient-Reported Barriers Are Associated with Receipt of Hepatocellular Carcinoma Surveillance in a Multicenter Cohort of Patients with Cirrhosis. Clin. Gastroenterol. Hepatol. 2021, 19, 987–995. [Google Scholar] [CrossRef]
- Feng, G.; Yilmaz, Y.; Valenti, L.; Seto, W.; Pan, C.Q.; Méndez-Sánchez, N.; Ye, F.; Sookoian, S.; Targher, G.; Byrne, C.D.; et al. Global Burden of Major Chronic Liver Diseases in 2021. Liver. Int. 2025, 45, e70058. [Google Scholar] [CrossRef]
- Fattovich, G.; Stroffolini, T.; Zagni, I.; Donato, F. Hepatocellular carcinoma in cirrhosis: Incidence and risk factors. Gastroenterology 2004, 127, S35–S50. [Google Scholar] [CrossRef]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Sulkowski, M.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef]
- Xu, X.L.; Jiang, L.S.; Wu, C.S.; Pan, L.Y.; Lou, Z.Q.; Peng, C.T.; Dong, Y.; Ruan, B. The role of fibrosis index FIB-4 in predicting liver fibrosis stage and clinical prognosis: A diagnostic or screening tool? J. Formos. Med. Assoc. 2022, 121, 454–466. [Google Scholar] [CrossRef]
- Nguyen, A.L.T.; Milley, K.; Druce, P.; Qama, A.; McNamara, M.; Wong, J.; Karnchanachari, N.; de Graaff, B. Surveillance for liver cancer in primary care: A systematic review of the evidence. Aust. J. Gen. Pract. 2023, 52, 801–807. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).