Abstract
Background/Objectives: Patients with isolated adult-onset growth hormone (GH) deficiency may present with hepatic steatosis and metabolic dysfunction. The effect of replacement therapy on metabolic phenotype has not been exhaustively studied yet. Methods: Patients with isolated adult-onset GH deficiency (GHD) were enrolled and prescribed GH-replacement therapy. DEXA scans for assessing body composition, anthropometric and biochemical parameters were evaluated at baseline and after 12 months of therapy. A fatty liver index, hepatic steatosis index and Fibrosis 4-test were calculated at baseline and after 12 months of therapy. Results and Conclusions: In our cohort, GH replacement therapy in adults with isolated adult-onset GHD is associated with weight loss and reduction of BMI (p < 0.001), amelioration in body composition with reduction in fat mass and trunk fat (respectively, p = 0.023 and p = 0.02), amelioration in lipid profile (significant reduction of total and LDL cholesterol and increase in HDL cholesterol) and reduction in fatty liver index (p = 0.021). Further long-term, randomized studies with bigger cohorts and advanced diagnostics are needed to confirm these results of our exploratory study.
1. Introduction
Growth hormone (GH) is a hormone with pleiotropic actions that influences many physiological aspects. Isolated GH deficiency (GHD) with onset in adult age is a rare condition associated with many pathological comorbidities, and it is not rarely associated with weight excess and an empty sella []. Adult GHD is a well-defined clinical entity characterized by decreased lean body mass and increased fat mass, dyslipidemia, cardiac dysfunction, decreased fibrinolysis and premature atherosclerosis, decreased muscle strength and exercise capacity, decreased bone mineral density (BMD), increased insulin resistance and impaired QoL []. The most common causes of adult GHD are isolated idiopathic GHD and hypothalamic-pituitary tumors and/or their treatment regimens. Idiopatic GHD results from decreased growth hormone (GH) secretion from the anterior pituitary gland that is more pronounced than the physiologic decline of the growth hormone–releasing hormone (GHRH)-GH-insulin-like growth factor-1 (IGF-1) axis associated with aging []. People with obesity often show a low GH status, with a blunted response to the GHRH + arginine test or ITT []. Nevertheless, GHD is often associated with excess weight []. GHD concurs with obesity in negatively affecting bone quality, immune response and cardiovascular risk, through metabolic derangements, altered body composition and cardiological morpho functional alterations [,,,]. GH replacement therapy is of fundamental importance in GHD patients to ameliorate quality of life, body composition, lipid profile and immunological defense [,,].
Hepatic steatosis represents the hepatic manifestation of metabolic syndrome. Its prevalence is continuously rising, in parallel with the increase in metabolic diseases. The relationship between liver steatosis and metabolic derangement is so strict that recently a new term was coined, MASLD (metabolic dysfunction-associated steatotic liver disease), in substitution for the old definition NAFLD (non-alcoholic fatty liver disease). The prevalence of hepatic steatosis in patients with obesity and type 2 diabetes is incredibly high, reaching almost 80%. Hepatic steatosis in patients with GHD is reported to be more common in some studies, but others do not observe differences between GHD patients and healthy controls [,,,]. Hepatic steatosis results from a complex interconnection between lipid in-flow and out-flow, dietary composition, sedentary life, microbiota, hormonal alterations and genetic factors [,,,].
A liver biopsy has historically been the gold standard for diagnosing hepatic disease and stratifying its severity. However, the cost and risk profile mandate pragmatic use of non-invasive tests, such as the fatty liver index (FLI), the hepatic steatosis index (HSI) and the Fibrosis-4 test (FIB-4) [].
A few studies have been conducted on the effects of GH replacement therapy on anthropometric and metabolic parameters and hepatic fat deposition in patients with hypopituitarism and GHD. However, studies on the effects of GH replacement therapy in patients with isolated adult-onset GH deficiency, which is a rare condition, are lacking. Our study is aimed at exploring the metabolic effects of GH replacement therapy in a small cohort of patients, in a real-life setting.
2. Materials and Methods
2.1. Study Design and Participants
The participants were patients with adult-onset isolated GHD, followed in our Endocrinology Clinic in Umberto I Polyclinic, Sapienza University of Rome. The patients had recently been diagnosed with GHD and were naïve to therapy. All of the patients were diagnosed with a GHRH plus Arginine stimulation test in our clinic. BMI-related cut-off values were used for diagnosis []. Inclusion criteria were age > 25 years, BMI > 25 kg/m2, diagnosis of adult onset GHD and absence of contraindication to GH replacement therapy. Medical history was recorded, and a complete clinical assessment was performed for all patients.
The GH replacement therapy dose was personalized for the individual patients, according to the current guidelines [], starting with a low dose (0.1 mg/day, for six days/week), and eventually increasing it, considering the individual clinical response, the serum IGF-1 levels and side effects.
2.2. Anthropometric Measurements
Anthropometric parameters were recorded in the morning, with subjects wearing light clothing, without shoes. Body weight was rounded to the nearest 0.1 kg and height to the nearest 0.1 cm. Waist circumference (WC) was measured with a non-stretchable tape over the unclothed abdomen just above the iliac crest at the end of a normal expiration, and hip circumference (HC) was measured around the pelvis at the widest point. The tape was parallel to the floor and did not compress the skin. Waist-to-Hip Ratio (WHR) was calculated using the parameters described above. BMI was calculated as weight (kg) divided by squared height (m2). Overweight was defined as a BMI ≥ 25 kg/m2 and <30 kg/m2, whereas obesity was defined as a BMI ≥ 30 kg/m2.
2.3. Biochemical Assessment
Blood samples were collected from fasting patients by using venipuncture between 8:00 and 9:00 a.m. The samples were then transferred to the local laboratory and handled according to the local standards of practice. A complete metabolic assessment including fasting glucose, insulin, HbA1c, total cholesterol, high-density lipoprotein (HDL) cholesterol and triglycerides (TG) was performed. Insulin resistance was assessed with the HOMAi (homeostatic model assessment of insulin resistance), calculated as fasting insulin (UI/L) × fasting glucose (mg/dL)/405, and patients were defined as insulin-resistant when HOMAi exceeded 2.5 [].
2.4. Evaluation of Hepatic Steatosis and Fibrosis
Hepatic steatosis and fibrosis were estimated with non-invasive methods, based on laboratory and anthropometric measurements. The formulas used for estimating steatosis were the fatty liver index (FLI) and the hepatic steatosis index (HSI).
FLI was calculated with the formula FLI = (e0.953 × loge (triglycerides) + 0.139 × BMI + 0.718 × loge (ggt) + 0.053 × waist circumference − 15.745)/(1 + e0.953 × loge (triglycerides) + 0.139 × BMI + 0.718 × loge (ggt) + 0.053 × waist circumference − 15.745) × 100. The cut-off value used to discriminate against hepatic steatosis was 60 [].
HSI was calculated with the formula HSI = 8 × ALT/AST + BMI (+2, in case of diabetic patients; +2, for females). The diagnosis of diabetes mellitus was based on a fasting glucose of ≥126 mg/dL, HbA1c ≥ 6.5% or therapy with anti-diabetic medication. The cut-off value used to discriminate hepatic steatosis was 36 [].
The Fibrosis-4 test (FIB-4) is an index used to estimate the risk of hepatic fibrosis, and was calculated with the formula: (age × AST)/(platelet count × √ALT). The cut-off value to rule-out advanced fibrosis was 1.3 [].
2.5. Dual Energy X-Ray Absorptiometry
All patients underwent DXA (Hologic-Discovery A, software version 12.5.3:2) to evaluate total body composition. DXA was performed with subjects wearing light clothing and no shoes. We considered the values of total body fat, trunk fat and lean mass.
2.6. Statistical Analysis
The statistical analysis was performed using the software Statistica, version 14 StatSoft Inc. (Tulsa, OK, USA), IBM SPSS, version 27 and Prism Graphpad version 10 (Graphpad Software Inc., San Diego, CA, USA).
Descriptive statistics were calculated for continuous variables. Data are expressed as the median (interquartile range). Frequencies and percentages were presented for categorical variables. Distribution of continuous variables was tested with the Shapiro–Wilk test. Comparison between groups was made with the paired Student’s t-test for normally distributed data, or the Mann–Whitney Test or Wilcoxon test if data were not normally distributed. The Pearson correlation was calculated for investigating the correlation between Delta FLI and weight loss.
3. Results
The characteristics of the patients at baseline are reported in Table 1. We evaluated 16 patients at baseline. The patients had been recently diagnosed with GHD and were naïve to therapy.

Table 1.
Characteristics of our cohort at baseline.
Among these, four were not adherent to the therapy or dropped out for personal reasons: two patients refused hormone therapy for fear of possible side effects; one patient refused therapy for fear of needles and was unwilling to undergo injection therapy; one patient was non-compliant due to reported family issues. A total of 12 patients were adherent to the therapy and attended the follow-up visit at 12 months. A total of 8 patients agreed to repeat the blood draw and 11 agreed to repeat the DEXA scan at the 12 months follow-up visit. No adverse effects of note were reported by any patient.
The GH replacement therapy dose was started and maintained at a low dose (0.1 mg/day, for six days/week) to avoid impairment of glucose metabolism and for the good clinical and biochemical response at a low dose achieved by all patients.
The anthropometric parameters at baseline are indicative of severe obesity, and FLI and HSI indicate the presence of hepatic steatosis, while fibrosis was not detected by the FIB-4 (Table 1).
In Table 2, blood pressure and biochemical metabolic parameters after 12 months of GH replacement therapy are reported. GH replacement therapy was associated with a reduction in weight (from 107.5 (13.33) Kg to 98.95 (13.61) Kg), BMI (from 35.74 (13.79 Kg/m2 to 32.96 (12.02) Kg/m2) and waist circumference (from 117 (27.1) cm to 109.5 (23.5) cm, as reported in Figure 1, panel 1. The waist-to-height ratio was significantly reduced (from 0.69 (0.22) to 0.64 (0.25), and the HOMAi index did not significantly change.

Table 2.
Blood pressure, IGF-1 values and biochemical metabolic parameters at baseline and after 12 months of GH replacement therapy.
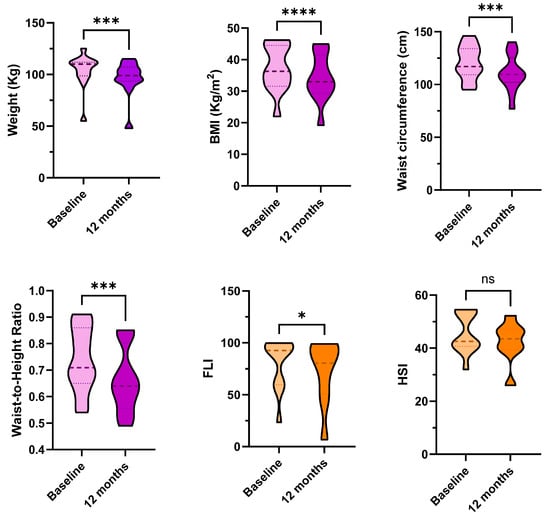
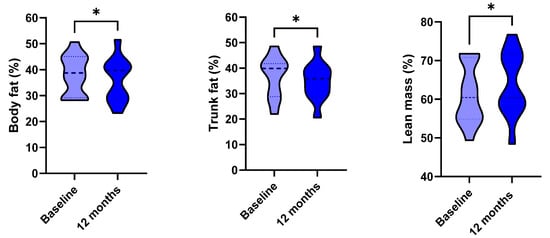
Figure 1.
Anthropometric parameters (in violet), dynamics of fatty liver index (FLI) and hepatic steatosis index (HSI) (in orange) and body composition parameters (in blue) at baseline and after 12 months of GH replacement therapy. * p < 0.05, *** p < 0.001, **** p < 0.0001. ns: non-significant. Weight and FLI were compared with non-parametric tests. Data are displayed in truncated violin plots, and the median and quartiles are shown, respectively, with dashed and dotted lines.
FLI significantly improved (from 92.68 (41.29) to 80.54 (46.88), p = 0.021, Figure 1, panel 2). The Pearson’s correlation between weight loss and Delta FLI was non-significant (p = 0.568). HSI and FIB-4 did not significantly change over time (respectively, p = 0.071 and p = 0.110). The DEXA-scans confirmed a slight amelioration of body composition, with a slight reduction of total body fat and trunk fat percentages (respectively, p = 0.023 and p= 0.02), and a slight increase in lean mass percentage (p = 0.023) (Figure 1, panel 3).
4. Discussion and Conclusions
GH and its main mediator IGF-1, play a crucial role in the regulation of hepatic steatosis and fibrosis []. GH exerts a lipolytic effect, that is, it promotes fat degradation by acting both in the adipose, promoting lipolysis, and in the liver, regulating fat uptake, synthesis and oxidation []. GH directly inhibits hepatic lipogenesis through suppression of factors such as SREBP-1c (Sterol Regulatory Element-Binding Protein 1c) and acetyl-CoA carboxylase (ACC) and fatty acid synthase (FAS) []. It also promotes mitochondrial fat oxidation through its influence on PPAR-α [].
In humans, low serum IGF-1 levels are associated with increased histologic severity of MASLD when rigorously controlled for age, BMI, the presence of diabetes and after the exclusion of subjects with cirrhosis [].
Also, chronic GH deficiency is associated with increased insulin resistance and increased risk of steatosis []. Insulin resistance is one of the main risk factors for the development of MAFLD steatosis. The resulting hyperinsulinemia has direct and indirect effects on hepatic lipid metabolism []. Firstly, hyperinsulinemia stimulates de novo fatty acid synthesis in the liver, while at the same time it inhibits adipose lipolysis, promoting the release of free fatty acids into the bloodstream, which are then accumulated in the liver []. In addition, insulin resistance alters the function of insulin receptors in the liver, reducing the organ’s ability to metabolize lipids properly and promoting the accumulation of intracellular fat []. The accumulation of triglycerides in liver cells is the main determinant of hepatic steatosis, which, if left unmanaged, can progress to more severe forms of liver disease, such as steatohepatitis and liver cirrhosis []. In addition, insulin resistance promotes a chronic inflammatory state that increases the risk of disease progression to forms of irreversible liver damage [].
Liver fibrosis is characterized by the excessive accumulation of the extracellular matrix, mainly due to the activation of hepatic stellate cells (HSCs). Under normal conditions, HSCs are quiescent, but in response to chronic liver damage they become activated, contributing to fibrogenesis []. Recent studies have shown that IGF-1 can induce senescence of HSCs in a p53 gene-dependent manner, thereby limiting the progression of fibrosis. In mouse models of nonalcoholic steatohepatitis (NASH) and cirrhosis, IGF-1 administration significantly improved steatosis, inflammation and liver fibrosis. This effect was associated with reduced oxidative stress and improved hepatic mitochondrial function [].
Moreover, IGF-1 demonstrated antifibrotic effects in animal models of acute carbon tetrachloride-induced liver damage (CCl4). IGF-1 administration modulated the expression of genes involved in fibrogenesis and extracellular matrix degradation, suggesting therapeutic potential in preventing progression to cirrhosis [].
Finally, GH and IGF-1 may decrease mitochondrial oxidative stress and improve hepatocellular function [,].
The purpose of our study was to evaluate the impact of GH replacement therapy for a period of 12 months on anthropometric and metabolic parameters and non-invasive markers of liver steatosis and fibrosis (FLI, HSI, FIB-4) in a group of adult patients with isolated adult-onset GHD. Noninvasive markers are useful tools for estimating the presence of hepatic steatosis, despite some limitations in terms of accuracy in diagnosing and monitoring more advanced stages of liver disease [,].
Results on the impact of GH replacement therapy on hepatic steatosis available in the literature are summarized in Table 3.
Our results show significant favorable effects on body-composition-related variables and a reduction in the index of hepatic steatosis. After 12 months of treatment, the patients showed a significant reduction in BMI, waist circumference, waist-to-height ratio, trunk fat and body fat percentage. The patients also demonstrated significant improvements in total HDL and LDL cholesterol values. These results confirm the known effect of GH in promoting lipolysis, reducing visceral fat and improving overall body composition []. Our data is consistent with what has been reported in previous studies of adult patients with GHD, in whom replacement therapy has been shown to improve anthropometric and metabolic parameters [,].

Table 3.
Evidence from the literature of the effects of GH replacement therapy on hepatic steatosis.
Table 3.
Evidence from the literature of the effects of GH replacement therapy on hepatic steatosis.
| Study | Patients | Duration | Evaluation of Steatosis | Results |
|---|---|---|---|---|
| Meienberg et al., 2016 [] | (a) 9 patients with hypopituitarism and GHD treated with GH replacement therapy (b) 9 patients with hypopituitarism and GHD not treated with GH replacement therapy | 6 months | Proton magnetic resonance spectroscopy | The change in intrahepatocellular lipid content did not differ between the groups |
| Matsumoto et al., 2014 [] | (a) 31 patients with hypopituitarism and GHD (b) Among these, 13 patients with NASH diagnosed with hepatic biopsy | 24 months | Liver enzymes and serum hyaluronic acid (marker of fibrosis), measured in all patients | Reduction in serum liver enzymes; serum hyaluronic acid was unchanged at 24 months |
| Gardner et al., 2012 [] | 12 GHD patients with or without other pituitary deficiencies | 6 months | Proton magnetic resonance spectroscopy | GH therapy did not reduce intrahepatocellular lipid content |
| Nishizawa et al., 2012 [] | (a) 19 patients with hypopituitarism and GHD (b) Among these, 5 patients underwent hepatic biopsy | 6 months | (a) Liver enzymes were measured in all patients (b) Liver biopsy was performed at baseline and after 6 months of therapy | (a) Reduction in serum liver enzymes (b) Improvement of steatosis and fibrosis in the histological analysis |
The effects of GH replacement therapy on indirect measurements of hepatic steatosis were encouraging. The fatty liver index (FLI) was significantly reduced after 12 months of substitutive treatment. Similarly, HSI showed a trend toward reduction, but without reaching statistical significance. However, even a slight reduction may be clinically relevant, considering that the HSI incorporates the ALT/AST ratio, BMI and the presence of diabetes. The discrepancy in results could be attributable to the different data that formulas consider for the estimation of the fat liver deposition. Conversely, no significant change was observed in FIB-4, an index used to estimate liver fibrosis. This finding is in line with available evidence indicating that FIB-4 is less sensitive to early changes in young patients or those without advanced fibrosis. Furthermore, as highlighted in the EASL guidelines (2021), FIB-4 has good accuracy in discriminating significant fibrosis, but it is not an optimal tool for monitoring subtle changes in the short term [].
Meienberg et al. [] evaluated liver fat in hypopituitary adults with GHD compared to matched controls, reporting no significant differences in the prevalence of steatosis between groups at baseline. After six months of GH replacement therapy, the authors found no significant change in liver fat content between GHD patients and controls. This lack of effect may be attributed to the relatively short duration of therapy, which was shorter than ours, as well as the potentially mild baseline steatosis in the cohort. Meienberg et al. [] relied on imaging modalities less sensitive than magnetic resonance spectroscopy, possibly underestimating subtle changes in hepatic fat content.
Gardner et al. [] similarly observed no difference in the prevalence of nonalcoholic fatty liver disease between GHD patients and controls. However, they noted a significantly higher proportion of visceral adipose tissue in GHD subjects. Importantly, six months of GH replacement resulted in a significant reduction of abdominal visceral fat, confirming our findings, and, differently from Meienberg [], improvement in hepatic fat content measured by magnetic resonance spectroscopy—but only in patients with elevated intrahepatocellular lipid at baseline. This suggests that GH therapy’s beneficial effect on liver fat may be contingent on the presence of clinically relevant hepatic steatosis prior to treatment. Patients without elevated liver fat at baseline might not demonstrate significant improvement, which could explain the null findings in other cohorts.
Similarly to us, other studies reported beneficial effects of GH replacement therapy. Nishizawa et al. The authors of [] reported a higher prevalence of NAFLD among hypopituitary patients with GHD compared to age-, sex-, and BMI-matched controls. Six months of GH replacement therapy significantly improved liver enzyme levels, particularly in patients with elevated baseline transaminases, and led to the amelioration of histological features and circulating fibrotic markers in those with biopsy-confirmed steatohepatitis. This study highlights the potential of GH therapy, not only for reducing biochemical markers, but also for inducing histopathological improvement in patients with more advanced liver disease, emphasizing the importance of baseline disease severity in treatment responsiveness as hypothesized from the study by Gardner [].
Similarly, Matsumoto et al. [] demonstrated that long-term GH replacement (24 months) improved liver enzymes in adult GHD patients with hypopituitarism, contingent on preventing weight gain during therapy. This underscores the crucial role of concomitant lifestyle or medical interventions targeting body weight, as weight gain may counteract the hepatic benefits of GH replacement. The longer duration of follow-up also suggests that sustained therapy may be necessary to observe significant hepatic improvements.
The discrepancies among these studies may thus be explained by several factors such as the baseline severity of hepatic steatosis, duration of GH therapy, diagnostic modalities, patients’ characteristics and other concomitant factors.
GH deficiency in adults is increasingly recognized as a condition associated with metabolic dysfunction and increased cardiovascular risk. Our study supports the efficacy of replacement therapy not only in improving body composition but also in reducing metabolic risk and potentially ameliorating metabolic liver disease.
Of note, since our patients lost weight during the treatment, improvement in steatosis indices may be related not only to GH replacement therapy, but also to weight loss per se, which, however, may be favored by the GH replacement therapy. Nevertheless, the correlation between weight loss and Delta FLI was not significant, and considering that the patients did not follow a structured nutritional intervention and considering the effects of GH on body composition, basal metabolism and lipid profile [,], it is likely that the observed benefits can be attributed to the restoration of the GH/IGF-1 axis, and weight loss may have had an additive effect.
From a clinical standpoint, these results underscore the importance of evaluating hepatic health and metabolic parameters in patients diagnosed with isolated adult-onset GHD, particularly those with excess weight or other risk factors for MASLD, for whom appropriate dietary strategies are fundamental for inducing and maintaining weight loss and ameliorating metabolic derangements and cardiovascular risk long-term []. GH replacement therapy should be considered not only for its well-established effects on body composition and quality of life but also for its potential to mitigate hepatic steatosis and associated metabolic derangements. Incorporating routine monitoring of non-invasive hepatic markers alongside body composition assessments such as DEXA scans can provide practical, cost-effective tools for clinicians to track therapy effectiveness and hepatic risk. This integrated approach facilitates early identification and management of liver involvement in GHD patients.
The management of adult-onset isolated GHD may benefit from assessing hepatic status at baseline and during follow-up, with considering GH replacement as part of a comprehensive metabolic management strategy. Multidisciplinary collaboration involving endocrinologists, hepatologists and nutritionists could optimize patient outcomes through tailored interventions addressing both hormonal deficiency and metabolic health.
Given the current evidence, GH therapy represents a promising adjunctive treatment for improving metabolic derangements and hepatic steatosis in adult patients with isolated GHD and metabolic risk factors. However, until data from larger, randomized controlled trials with advanced imaging and histological validation are available, clinicians should carefully weigh benefits against potential risks and individualize therapy decisions.
Our study has several limitations that warrant consideration. First, the small sample size limits the generalizability of our findings and restricts our ability to draw firm causal inferences. However, the rarity of isolated adult-onset GH deficiency limits the possibility of bigger cohorts in real-life settings. Moreover, the low adherence to therapy in the real-life setting further reduces the number of patients evaluable in longer follow-ups. Second, we did not include a quantitative assessment of hepatic steatosis via liver biopsy or imaging techniques such as the magnetic resonance imaging-proton density fat fraction (MRI-PDFF) or controlled attenuation parameter (CAP) by transient elastography. Liver biopsy remains the gold standard for evaluating liver fat content, inflammation and fibrosis; however, it is invasive, costly and carries potential procedural risks, making it less feasible in routine clinical or research settings, especially for rare conditions such as adult-onset isolated GHD. Imaging techniques, while they are less invasive, still pose logistical and economic challenges and may not be readily available in all clinical settings. In contrast, the use of non-invasive surrogate markers such as the FLI, HSI and FIB-4 offers practical, cost-effective and reproducible alternatives for estimating hepatic fat accumulation and fibrosis risk. Although these indices lack the precision of histological or imaging-based evaluation, their consistent reduction in our cohort—particularly the uniform improvement in FLI—supports the hypothesis of a GH-mediated reduction in hepatic fat content. Still, future studies incorporating imaging or histological validation are needed to confirm these findings and to more accurately characterize the impact of GH therapy on hepatic steatosis and fibrosis in this population. Another limitation is the impossibility of completely disentangling the metabolic effects of GH replacement therapy from the effects of weight loss itself. However, given the absence of a structured weight loss plan and the known positive effects of GH replacement therapy on metabolism, it is plausible that the therapy favored weight loss, which further contributed to the amelioration of body composition and hepatic fat deposition induced by the substitutive therapy.
As strength points, the follow-up at 12 months is longer than the majority of studies available in the literature, which lasted 6 months, and the patients were affected by a very rare condition for which they were treated, adult-onset isolated GH-deficiency, which specifically does not impair developmental age and does not involve other hormone deficiencies that require treatment. The majority of published studies include hypopituitarism patients and a miscellaneous of cases, in which multiple hormonal deficiencies and relative treatments overlap.
In conclusion, GH replacement therapy in adult patients affected by isolated GHD is associated with significant improvement in body composition and reduction in the fatty liver index. Our results are consistent with the existing literature on patients with hypopituitarism and GHD, suggesting that GH replacement therapy may ameliorate hepatic steatosis in patients with adult-onset isolated GHD and hepatic steatosis.
To build on these findings, future research should focus on prospective RCTs with larger sample sizes and longer follow-up periods to evaluate the sustained effects of GH replacement therapy on liver health, including advanced imaging such as magnetic resonance and histology confirmation with liver biopsy, when ethically and clinically feasible.
Key endpoints should include not only changes in hepatic fat content and the fibrosis stage but also metabolic parameters (e.g., insulin sensitivity, lipid profile), inflammatory markers and quality-of-life measures. Incorporating standardized and validated non-invasive biomarkers alongside imaging and histology would enable comprehensive assessment while balancing invasiveness and clinical applicability.
Moreover, mechanistic studies exploring the molecular pathways through which GH modulates lipid metabolism and inflammation in the liver could provide valuable insight to optimize therapeutic strategies. Such integrated approaches would strengthen the evidence base for GH replacement therapy in this patient population and help guide personalized treatment decisions.
Author Contributions
Conceptualization, E.G. and C.L.; data curation, E.G., R.R., G.S., M.C., O.G. and C.L.; formal analysis, E.G. and C.L.; investigation, E.G., R.R., G.S., M.C., O.G. and C.L.; methodology, C.L.; supervision, S.M. and C.L.; writing—original draft, E.G. and R.R.; writing—review and editing, S.M. and C.L. All authors have read and agreed to the published version of the manuscript.
Funding
Sapienza University of Rome, 000055_24_RS_LUBRANO_Ateneo_ProgMedi2023.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Ethics Committee of Polyclinic Umberto 1—Rome (protocol code CE5475, approved 24 October 2019, and amendment protocol n. 0513/2024, approved 6 June 2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the last author due to privacy reasons.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data, in the writing of the manuscript or in the decision to publish the results.
References
- Lubrano, C.; Tenuta, M.; Costantini, D.; Specchia, P.; Barbaro, G.; Basciani, S.; Mariani, S.; Pontecorvi, A.; Lenzi, A.; Gnessi, L. Severe growth hormone deficiency and empty sella in obesity: A cross-sectional study. Endocrine 2015, 49, 503–511. [Google Scholar] [CrossRef]
- de Boer, H.; Blok, G.-J.; van der Veen, E.A. Clinical Aspects of Growth Hormone Deficiency in Adults. Endocr. Rev. 1995, 16, 63–86. [Google Scholar] [CrossRef]
- Yuen, K.C.J.; Biller, B.M.K.; Radovick, S.; Carmichael, J.D.; Jasim, S.; Pantalone, K.M.; Hoffman, A.R. American Association of Clinical endocrinologists and American College of Endocrinology guidelines for management of growth hormone deficiency in adults and patients transitioning from pediatric to adult care. Endocr. Pract. 2019, 25, 1191–1232. [Google Scholar] [CrossRef]
- Di Somma, C.; Pivonello, R.; Pizza, G.; De Rosa, A.; Lombardi, G.; Colao, A.; Savastano, S. Prevalence of the metabolic syndrome in moderately-severely obese subjects with and without growth hormone deficiency. J. Endocrinol. Investig. 2010, 33, 171–177. [Google Scholar] [CrossRef]
- Savastano, S.; Di Somma, C.; Barrea, L.; Colao, A. The complex relationship between obesity and the somatropic axis: The long and winding road. Growth Horm. IGF Res. 2014, 24, 221–226. [Google Scholar] [CrossRef]
- Gangitano, E.; Curreli, M.I.; Gandini, O.; Masi, D.; Spoltore, M.E.; Gnessi, L.; Lubrano, C. Bone Quality Indices Correlate with Growth Hormone Secretory Capacity in Women Affected by Weight Excess: A Cross-Sectional Study. J. Clin. Med. 2024, 13, 5064. [Google Scholar] [CrossRef]
- Masi, D.; Gangitano, E.; Criniti, A.; Ballesio, L.; Anzuini, A.; Marino, L.; Gnessi, L.; Angeloni, A.; Gandini, O.; Lubrano, C. Obesity-Associated Hepatic Steatosis, Somatotropic Axis Impairment, and Ferritin Levels Are Strong Predictors of COVID-19 Severity. Viruses 2023, 15, 488. [Google Scholar] [CrossRef]
- Gangitano, E.; Barbaro, G.; Susi, M.; Rossetti, R.; Spoltore, M.E.; Masi, D.; Tozzi, R.; Mariani, S.; Gnessi, L.; Lubrano, C. Growth Hormone Secretory Capacity Is Associated with Cardiac Morphology and Function in Overweight and Obese Patients: A Controlled, Cross-Sectional Study. Cells 2022, 11, 2420. [Google Scholar] [CrossRef]
- Doga, M.; Bonadonna, S.; Gola, M.; Mazziotti, G.; Giustina, A. Growth hormone deficiency in the adult. Pituitary 2006, 9, 305–311. [Google Scholar] [CrossRef]
- Masi, D.; Spoltore, M.E.; Curreli, M.; Costa, D.; Gangitano, E.; Mariani, S.; Angeloni, A.; Gnessi, L.; Anastasi, E.; Lubrano, C. Growth hormone replacement therapy enhances humoral response to COVID-19 mRNA vaccination in patients with adult-onset growth hormone deficiency. J. Endocrinol. Investig. 2025, 48, 1283–1288. [Google Scholar] [CrossRef]
- Götherström, G.; Johannsson, G.; Svensson, J. Effects of 18 months of GH replacement on cardiovascular risk factors and quality of life in GH deficient adults; a randomized controlled trial using a fixed very low and a standard dose of GH. Growth Horm. IGF Res. 2022, 67, 101510. [Google Scholar] [CrossRef]
- Elbornsson, M.; Götherström, G.; Bosæus, I.; Bengtsson, B.; Johannsson, G.; Svensson, J. Fifteen years of GH replacement improves body composition and cardiovascular risk factors. Eur. J. Endocrinol. 2013, 168, 745–753. [Google Scholar] [CrossRef]
- Ichikawa, T.; Hamasaki, K.; Ishikawa, H.; Ejima, E.; Eguchi, K.; Nakao, K. Non-alcoholic steatohepatitis and hepatic steatosis in patients with adult onset growth hormone deficiency. Gut 2003, 52, 914. [Google Scholar] [CrossRef]
- Meienberg, F.; Yee, M.; Johnston, D.; Cox, J.; Robinson, S.; Bell, J.D.; Thomas, E.L.; Taylor-Robinson, S.D.; Godsland, I. Liver fat in adults with GH deficiency: Comparison to matched controls and the effect of GH replacement. Clin. Endocrinol. 2016, 85, 76–84. [Google Scholar] [CrossRef]
- Gardner, C.J.; Irwin, A.J.; Daousi, C.; McFarlane, I.A.; Joseph, F.; Bell, J.D.; Thomas, E.L.; Adams, V.L.; Kemp, G.J.; Cuthbertson, D.J. Hepatic steatosis, GH deficiency and the effects of GH replacement: A Liverpool magnetic resonance spectroscopy study. Eur. J. Endocrinol. 2012, 166, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, H.; Iguchi, G.; Murawaki, A.; Fukuoka, H.; Hayashi, Y.; Kaji, H.; Yamamoto, M.; Suda, K.; Takahashi, M.; Seo, Y.; et al. Nonalcoholic fatty liver disease in adult hypopituitary patients with GH deficiency and the impact of GH replacement therapy. Eur. J. Endocrinol. 2012, 167, 67–74. [Google Scholar] [CrossRef]
- Gangitano, E.; Scannapieco, F.; Lubrano, C.; Gnessi, L. Metabolic Syndrome, Hepatic Steatosis and Testosterone: A Matter of Sex. Livers 2024, 4, 534–549. [Google Scholar] [CrossRef]
- Gangitano, E.; Corradini, S.G.; Lubrano, C.; Gnessi, L. La Non-Alcoholic Fatty Liver Disease, una patologia epatica di interesse endocrinologico. L’Endocrinologo 2021, 22, 436–440. [Google Scholar] [CrossRef]
- Bellini, M.I.; Urciuoli, I.; Del Gaudio, G.; Polti, G.; Iannetti, G.; Gangitano, E.; Lori, E.; Lubrano, C.; Cantisani, V.; Sorrenti, S.; et al. Nonalcoholic fatty liver disease and diabetes. World J. Diabetes 2022, 13, 668–682. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef] [PubMed]
- Archer, A.J.; Belfield, K.J.; Orr, J.G.; Gordon, F.H.; Abeysekera, K.W. EASL clinical practice guidelines: Non-invasive liver tests for evaluation of liver disease severity and prognosis. Front. Gastroenterol. 2022, 13, 436–439. [Google Scholar] [CrossRef]
- Cook, D.M.; Yuen, K.C.; Biller, B.M.K.; Kemp, S.F.; Vance, M.L.; Camacho, P.M.; Duick, D.S.; Garber, A.J.; Garber, J.R.; Gharib, H.; et al. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for Growth Hormone Use in Growth Hormone-Deficient Adults and Transition Patients—2009 Update. Endocr. Pract. 2009, 15, 1–29. [Google Scholar] [CrossRef]
- Bonora, E.; Kiechl, S.; Willeit, J.; Oberhollenzer, F.; Egger, G.; Targher, G.; Alberiche, M.; Bonadonna, R.C.; Muggeo, M. Prevalence of insulin resistance in metabolic disorders: The Bruneck Study. Diabetes 1998, 47, 1643–1649. [Google Scholar] [CrossRef] [PubMed]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Kim, D.; Kim, H.J.; Lee, C.-H.; Yang, J.I.; Kim, W.; Kim, Y.J.; Yoon, J.-H.; Cho, S.-H.; Sung, M.-W.; et al. Hepatic steatosis index: A simple screening tool reflecting nonalcoholic fatty liver disease. Dig. Liver Dis. 2010, 42, 503–508. [Google Scholar] [CrossRef]
- Takahashi, Y. Nonalcoholic fatty liver disease and adult growth hormone deficiency: An under-recognized association? Best Pract. Res. Clin. Endocrinol. Metab. 2023, 37, 101816. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, A.; Novosyadlyy, R.; Wu, Y.; Yakar, S.; LeRoith, D. Biological effects of growth hormone on carbohydrate and lipid metabolism. Growth Horm. IGF Res. 2010, 20, 1–7. [Google Scholar] [CrossRef]
- Li, N.; Li, X.; Ding, Y.; Liu, X.; Diggle, K.; Kisseleva, T.; Brenner, D.A. SREBP Regulation of Lipid Metabolism in Liver Disease, and Therapeutic Strategies. Biomedicines 2023, 11, 3280. [Google Scholar] [CrossRef]
- Bougarne, N.; Weyers, B.; Desmet, S.J.; Deckers, J.; Ray, D.W.; Staels, B.; De Bosscher, K. Molecular actions of PPARα in Lipid Metabolism and Inflammation. Endocr. Rev. 2018, 39, 760–802. [Google Scholar] [CrossRef]
- Dichtel, L.E.; Corey, K.E.; Misdraji, J.; Bredella, M.A.; Schorr, M.; Osganian, S.A.; Young, B.J.; Sung, J.C.; Miller, K.K. The Association Between IGF-1 Levels and the Histologic Severity of Nonalcoholic Fatty Liver Disease. Clin. Transl. Gastroenterol. 2017, 8, e217. [Google Scholar] [CrossRef]
- Johansson, J.-O.; Fowelin, J.; Landin, K.; Lager, I.; Bengtsson, B.-Å. Growth hormone-deficient adults are insulin-resistant. Metabolism 1995, 44, 1126–1129. [Google Scholar] [CrossRef]
- Marušić, M.; Paić, M.; Knobloch, M.; Pršo, A.-M.L. NAFLD, Insulin Resistance, and Diabetes Mellitus Type 2. Can. J. Gastroenterol. Hepatol. 2021, 2021, 6613827. [Google Scholar] [CrossRef]
- Choi, S.S.; Diehl, A.M. Hepatic triglyceride synthesis and nonalcoholic fatty liver disease. Curr. Opin. Infect. Dis. 2008, 19, 295–300. [Google Scholar] [CrossRef]
- Santoro, A.; McGraw, T.E.; Kahn, B.B. Insulin action in adipocytes, adipose remodeling, and systemic effects. Cell Metab. 2021, 33, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Kawano, Y.; Cohen, D.E. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J. Gastroenterol. 2013, 48, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ballantyne, C.M. Skeletal muscle inflammation and insulin resistance in obesity. J. Clin. Investig. 2017, 127, 43–54. [Google Scholar] [CrossRef]
- Powell, E.E.; Wong, V.W.-S.; Rinella, M. Non-alcoholic fatty liver disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef]
- Nishizawa, H.; Iguchi, G.; Fukuoka, H.; Takahashi, M.; Suda, K.; Bando, H.; Matsumoto, R.; Yoshida, K.; Odake, Y.; Ogawa, W.; et al. IGF-I induces senescence of hepatic stellate cells and limits fibrosis in a p53-dependent manner. Sci. Rep. 2016, 6, 34605. [Google Scholar] [CrossRef]
- Morales-Garza, L.A.; Puche, J.E.; Aguirre, G.A.; Muñoz, Ú.; García-Magariño, M.; De la Garza, R.G.; Castilla-Cortazar, I. Experimental approach to IGF-1 therapy in CCl4-induced acute liver damage in healthy controls and mice with partial IGF-1 deficiency. J. Transl. Med. 2017, 15, 96. [Google Scholar] [CrossRef] [PubMed]
- García-Fernández, M.; Castilla-Cortázar, I.; Díaz-Sanchez, M.; Navarro, I.; Puche, J.E.; Castilla, A.; Casares, A.D.; Clavijo, E.; González-Barón, S. Antioxidant effects of insulin-like growth factor-I (IGF-I) in rats with advanced liver cirrhosis. BMC Gastroenterol. 2005, 5, 7. [Google Scholar] [CrossRef]
- Cristin, L.; Montini, A.; Martinino, A.; Pereira, J.P.S.; Giovinazzo, F.; Agnes, S. The Role of Growth Hormone and Insulin Growth Factor 1 in the Development of Non-Alcoholic Steato-Hepatitis: A Systematic Review. Cells 2023, 12, 517. [Google Scholar] [CrossRef] [PubMed]
- Kasper, P.; Demir, M.; Steffen, H.-M. Screening strategies for non-alcoholic fatty liver disease: A holistic approach is needed. Clin. Mol. Hepatol. 2023, 29, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Berzigotti, A.; Tsochatzis, E.; Boursier, J.; Castera, L.; Cazzagon, N.; Friedrich-Rust, M.; Petta, S.; Thiele, M. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis—2021 update. J. Hepatol. 2021, 75, 659–689. [Google Scholar] [CrossRef]
- Takahashi, Y. Essential roles of growth hormone (GH) and insulin-like growth factor-I (IGF-I) in the liver. Endocr. J. 2012, 59, 955–962. [Google Scholar] [CrossRef]
- Hoffman, A.R.; Kuntze, J.E.; Baptista, J.; Baum, H.B.A.; Baumann, G.P.; Biller, B.M.K.; Clark, R.V.; Cook, D.; Inzucchi, S.E.; Kleinberg, D.; et al. Growth Hormone (GH) Replacement Therapy in Adult-Onset GH Deficiency: Effects on Body Composition in Men and Women in a Double-Blind, Randomized, Placebo-Controlled Trial. J. Clin. Endocrinol. Metab. 2004, 89, 2048–2056. [Google Scholar] [CrossRef]
- Kargi, A.Y. Impact of long-acting growth hormone replacement therapy in adult growth hormone deficiency: Comparison between adolescent, adult, and elderly patients. Best Pract. Res. Clin. Endocrinol. Metab. 2023, 37, 101825. [Google Scholar] [CrossRef]
- Matsumoto, R.; Fukuoka, H.; Iguchi, G.; Nishizawa, H.; Bando, H.; Suda, K.; Takahashi, M.; Takahashi, Y. Long-term effects of growth hormone replacement therapy on liver function in adult patients with growth hormone deficiency. Growth Horm. IGF Res. 2014, 24, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Carroll, P.V.; Hormon, E.R.; Bengtsson, B.Å.; Carlsson, L.; Christiansen, J.S.; Clemmons, D.; Hintz, R.; Ho, K.; Laron, Z.; Sizonenko, P.; et al. Growth Hormone Deficiency in Adulthood and the Effects of Growth Hormone Replacement: A Review. J. Clin. Endocrinol. Metab. 1998, 83, 382–395. [Google Scholar] [CrossRef]
- Barrea, L.; Boschetti, M.; Gangitano, E.; Guglielmi, V.; Verde, L.; Muscogiuri, G. Long-Term Efficacy and Safety of Nutritional and Pharmacological Strategies for Obesity. Curr. Obes. Rep. 2025, 14, 1. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).