Abstract
Background/Objectives: In the present study, the Metabolic dysfunction-associated fatty liver disease (MAFLD) and Metabolic dysfunction-associated steatotic liver disease (MASLD) diagnostic criteria were applied to evaluate the relative performance in predicting short-term advanced fibrosis (AF) progression (AFpr) and hepatocellular carcinoma (HCC), as well as an ancillary outcome, i.e., the occurrence of acute cardiovascular events (ACEs) in steatotic liver disease (SLD) patients. Methods: We retrospectively analyzed the data stored in the University Hospital (UH)’s Official Health Documents Digitization Archive of 931 SLD patients, with a follow-up of 3 years. Based on the Body Mass Index (BMI), patients were subdivided into lean “L” (BMI < 25 kg/m2) (n = 134) and not-lean “NL” (n = 797), and, subsequently, into NL-MASLD (n = 206), NL-MASLD/MAFLD (n = 481), NL-MAFLD (n = 110), L-MASLD (n = 39), L-MASLD/MAFLD (n = 68), and L-MAFLD (n = 27). All study outcomes (AFpr, HCC, and ACE) were primarily evaluated in NL-SLD and by conducting a sub-analysis of L-SLD individuals. Results: MASLD and MAFLD criteria similarly estimated [p = 0.076] the overall 3-year risk of AF progression in NL-SLD. In the L-SLD sub-analysis, MAFLD criteria better estimated the overall 3-year risk of AF progression [p = 0.006]. Multivariate competing risk analysis (adjusted for sex, age, diabetes, steatosis, and fibrosis severity) revealed diabetes [adjusted Hazard Ratio (aHR) = 2.113, p = 0.001], high-sensitivity C-reactive protein (aHR = 1.441; p = 0.02), and Homeostatic Model Assessment for Insulin Resistance (aHR = 1.228; p = 0.03) as being associated with AF progression in L-MAFLD. Compared to MAFLD, MASLD diagnostic criteria similarly estimated the 3-year risk of HCC occurrence both in NL [HR = 1.104, C.I. 95%: 0.824–1.593, p = 0.741] and L [HR = 1.260, C.I. 95%: 0.768–2.104, p = 0.701] patients. Finally, no significant differences were reported between the MAFLD or MASLD criteria for ACE risk occurrence in all study groups. Conclusions: The MAFLD criteria better estimate the AF progression risk, limited to L-SLD patients.
1. Introduction
In the last decade, crucial modifications for the definition of hepatic steatosis have been progressively proposed, rapidly switching from non-alcoholic fatty liver disease (NAFLD) to Metabolic dysfunction-associated fatty liver disease (MAFLD), and, more recently, to Metabolic dysfunction-associated steatotic liver disease (MASLD) [1,2,3,4].
The intrinsic pathophysiological link with a systemic dysmetabolic status fueled by insulin resistance (IR), synergically with cumulating evidence presenting NAFLD as the hepatic manifestation of Metabolic Syndrome (MS), initially drove the abandonment of the term ‘non-alcoholic’ and the transition from “NAFLD” to “MAFLD” [2]. The NAFLD definition was based on the exclusion of other etiologies of chronic liver damage. In contrast, MAFLD diagnosis benefits from a confirmatory approach by emphasizing the association of steatosis with specifically identified metabolic risk abnormalities (MRAs) [at least two of seven to confirm Metabolic dysfunction (MD)], not excluding the coexistence of other etiologies [e.g., alcohol-related liver disease (ALD)] [2].
Recently, a multi-society Delphi consensus established paramount modifications by introducing the term “steatotic liver disease” (SLD), replacing MAFLD with MASLD, effectively retiring the term “NAFLD”, and proposing new MASLD diagnostic criteria [3]. SLD, by covering the entire etiological spectrum, currently represents an “umbrella entity” under which non-MD-SLD encloses cryptogenic SLD, specific etiology SLD [drug-induced liver injury (DILI), monogenic disease, and miscellaneous], and ALD [3]. On the other hand, MD-SLD includes MASLD (“Pure MASLD”) and MetALD (MASLD associated with harmful alcohol intake) [3].
The MASLD definition was mainly proposed to overcome some of the limitations imposed by its antecedents. Concerning the diagnosis, the new criteria, requiring only one of five identified cardiometabolic risk factors (CMRFs) for MD establishment, appear more inclusive, better embracing even a significant proportion of lean patients not considered in the MAFLD definition [5]. In MD-SLD scenarios, on one hand, the perpetuation of chronic liver damage, in the absence of adequate interventional management, inexorably promotes the progression to steatohepatitis (SH), advanced fibrosis (AF), cirrhosis, and the occurrence of hepatocellular carcinoma (HCC), whose onset has also been reported in non-AF disease stages [1,6,7]. On the other hand, the occurrence of acute cardiovascular events (ACEs) continues to represent the major prognostic factor burdening the prognosis in this setting [8,9,10]. For this purpose, evaluating whether and how the MASLD diagnostic criteria impact the clinical outcomes of the “old” MAFLD-defined patients in real life represents a crucial challenge.
Previous studies reported similarities in prevalence, risk factors, and clinical features, including AF frequency in a large United States cohort by comparing steatotic patients diagnosed with MAFLD and MASLD; conversely, differences in all-cause and cause-specific long-term mortality between MAFLD and MASLD individuals were revealed [11,12,13,14]. In particular, patients diagnosed with SLD under either definition exhibited comparable profiles with respect to age distribution, weight, glycemic status, and hepatic steatosis severity [11,12,13,14]. Furthermore, while the incidence of AF did not differ significantly between the two groups, when evaluating long-term outcomes, MAFLD-diagnosed individuals exhibited higher rates of all-cause and cause-specific mortality compared to those meeting the MASLD criteria.
However, the accuracy of MAFLD and MASLD criteria in predicting relatively short-term liver disease progression, HCC risk, and the occurrence of cardiovascular events has never been systematically investigated in a European cohort.
Based on this background, in this observational study, the MAFLD and MASLD diagnostic criteria were applied to a real-world scenario to evaluate the relative performance in predicting relatively short-term (3 years) liver disease worsening (AF progression) and HCC onset, as well as an ancillary outcome, i.e., the first ACE’s occurrence, in SLD patients.
2. Materials and Methods
2.1. Study Design and Participants
In this longitudinal observational retrospective cohort study, inpatients or outpatients admitted between Jan 2016 and May 2021 to the Hepatogastroenterology Division of the University of Campania “Luigi Vanvitelli” Hospital (UH) (Naples, Italy), receiving an ultrasound-based diagnosis of SLD, were consecutively identified, extracted, selected, and enrolled.
2.1.1. Identification Phase
SLD patients (24–78 years), giving their privacy consent upon admission, were consecutively identified by using the Health Documents Digitization Archive (HDDA) (http://10.30.10.16:8080/hero/ accessed on 2 September 2025). The HDDA is the official health cloud serving “Luigi Vanvitelli” UH, where demographic, biochemical, anthropometric, clinical, and imaging data of admitted patients, as well as clinical data of follow-up visits, are stored in personal electronic medical records (EMRs).
2.1.2. Extraction Phase
Of 2136 identified SLD patients, those regularly receiving EMR-supported and HDDA-available complete annual follow-up visits, according to the clinical practice guidelines [15], were exclusively extracted. Baseline data extraction and complete follow-up visits included the collection of anthropometrical, clinical, biochemical, liver transient elastography (LTE) data [Liver Stiffness Measurement (LSM) and Controlled Attenuation Parameter (CAP)] and the determination of noninvasive tools (NITs), as well as, the recording, through EMR-supported interviews (File S1), of the HCC occurrence, lifestyle habits [16], and drugs in the previous year.
2.1.3. Selection Process
The exclusion criteria during the identification and extraction phases were (1) discernible causes of hepatic steatosis, at baseline and on follow-up, defined by ongoing or excessive (>350 g/week) alcohol intake in the previous 12 months [3], drug-induced liver injury (DILI), monogenic diseases (lysosomal acid lipase deficiency, Wilson’s disease, and inborn errors of metabolism), viral hepatitis [Hepatitis C (HCV) and human immunodeficiency (HIV) virus], malnutrition, and celiac disease, as well as other discernible etiologies of chronic liver disease (CLD) [Hepatitis B virus (HBV), Autoimmune hepatitis, Primary Biliary Cholangitis, and Primary Sclerosing Cholangitis]; (2) the unavailability of LTE baseline data; (3) the evidence of advanced chronic liver disease (ACLD) at baseline [assumed when LSM > 15 kilopascal (kPa) [17]]; (4) previous HCC; (5) psychiatric problems (including severe depression, psychosis, bipolar disorder in manic or depressive phases, and major neurocognitive disorders) that can potentially invalidate the informed consent; and (6) the absence of EMR-supported documentation. Based on these, out of the initially 2136 identified individuals, we primarily excluded 184 patients whose complete follow-up visit data for the entire period of observation (3 years) were not HDDA-available, and 168 subjects revoked their consent to acquire EMR-supported HDDA data on follow-up visits. Moreover, 348 patients presented discernible causes of baseline steatosis, 74 showed another baseline discernible etiology of CLD, 101 had no baseline LTE estimation data available, 68 individuals presented baseline ACLD, 32 were not HCC-naïve subjects, and 101 patients did not show EMR-supported documentation during the interview, thus being excluded. One hundred and twenty-nine patients died because of extra-hepatic/cardiovascular-related events [solid extra-hepatic cancers complications (n = 58), infections and/or sepsis (n = 49), unknown causes (n = 18), or accidents (n = 4)] during the follow-up.
2.1.4. Identification of the Study Groups and Subgroups and Definition of Study Outcomes
At the end of the selection process, the HDDA-acquired data of 931 SLD participants were included in a specific ethically validated Case Report Form (CRF). According to the Body Mass Index (BMI), considering the baseline BMI and the relative cut-off of <25 kg/m2 as a shared MASLD and MAFLD diagnostic criteria element [2,3], patients were a posteriori subdivided into lean (“L”) (Body Mass Index < 25 kg/m2) (n = 134) and “NL” (n = 797) and, subsequently, by separately applying MAFLD and MASLD criteria, in NL-MASLD (n = 206), NL-MASLD/MAFLD (n = 481), NL-MAFLD (n = 110), L-MASLD (n = 39), L-MASLD/MAFLD (n = 68), and L-MAFLD (n = 27). In addition, by identifying individuals presenting a negative clinical history for previous ACEs [including Acute Myocardial Infarction (AMI), Acute Coronary Syndrome (ACS), Ictus cerebri (IC), and Transient ischemic attack (TIA)] [8], ACE-naïve subjects were independently further extracted from both NL and L patients to configure the study subgroups after applying the MAFLD and MASLD criteria, as follows: ACE-naïve NL-MASLD (n = 180), ACE-naïve NL-MASLD/MAFLD (n = 301), ACE-naïve NL-MAFLD (n = 98), ACE-naïve L-MASLD (n = 39), ACE-naïve L-MASLD/MAFLD (n = 66), and ACE-naïve L-MAFLD patients (n = 26). Figure 1 presents the recruitment flowchart and the constitution of the study groups and subgroups (Figure 1).
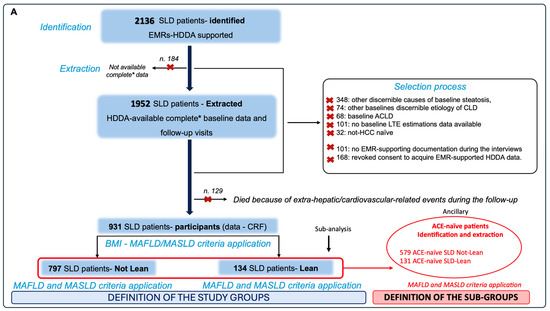
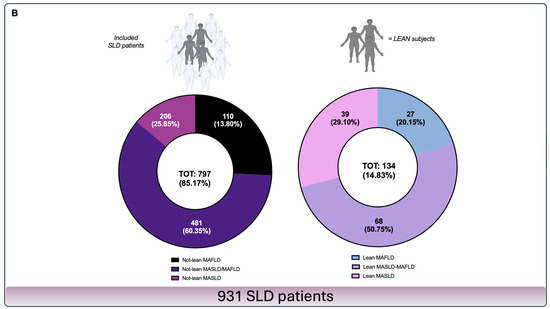
Figure 1.
Enrolment flowchart and frequency distribution of study population groups. Panel (A)—Enrolment processes. SLD patients who gave consent to privacy data treatment on admission were identified and extracted from HDDA by entering in the search field of the main diagnosis the keywords “bright liver”, “steatosis”, and “fatty liver”. Patients presenting discernible causes of hepatic steatosis and CLD, at baseline and on follow-up, including excessive alcohol intake history, defined as ongoing or excessive (>350 g/week) alcohol intake in the previous 12 months, were excluded. * Complete data included anthropometrical, clinical, biochemical, and noninvasive tools (NITs), as well as the recording of the HCC occurrence, lifestyle habits, and drugs in the previous year. ACE-naïve subjects were independently further extracted from both NL and L patients to configure the study subgroups after applying the MAFLD and MASLD criteria. Panel (B)—Frequency distribution of study population groups and subgroups. Based on the baseline BMI, the enrolled patients were further divided into lean “L” (BMI < 25 kg/m2) and “not-lean” and, subsequently, by separately applying MAFLD and MASLD criteria, into NL-MASLD (n = 206), NL-MASLD/MAFLD (n = 481), NL-MAFLD (n = 110), L-MASLD (n = 39), L-MASLD/MAFLD (n = 68), and L-MAFLD (n = 27). ACE-naïve patients that were used to configure the study subgroups were NL-MASLD (n = 180), ACE-naïve NL-MASLD/MAFLD (n = 301), ACE-naïve NL-MAFLD (n = 98), ACE-naïve L-MASLD (n = 39), ACE-naïve L-MASLD/MAFLD (n = 66), and ACE-naïve L-MAFLD patients (n = 26). SLD: Steatotic liver disease; HDDA: Health Documents Digitization Archive; MASLD: Metabolic dysfunction-associated steatotic liver disease; MAFLD: Metabolic dysfunction-associated fatty liver disease; ACE: Acute cardiovascular event; BMI: Body Mass Index; CLD: Chronic liver disease; CRF: Case Report Form; EMR: Electronic medical records; ACLD: Advanced chronic liver disease; HCC: Hepatocellular carcinoma; LTE: Liver transient elastography; TOT: Total.
This study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki (1975). The compilation of the CRFs received approval from the Ethics Committee of the University of Campania Luigi Vanvitelli, Naples (protocol no. 531/2016). Written informed consent was obtained from all adult participants.
In not-lean SLD patients, the comparison of MASLD vs. MAFLD diagnostic criteria in estimating the 3-year risk of AF progression [AF: LSM > 9.7 kPa [17]] (in baseline non-AF), as well as the comparison of HCC occurrence (including every fibrosis stage), represented the primary outcomes of this research. The comparison of MASLD vs. MAFLD criteria (sub-analysis) in estimating the same outcomes in lean SLD patients represented a secondary study objective. Finally, in the identified ACE-naïve SLD patients’ subgroups, the evaluation of MASLD and MAFLD diagnostic criteria in estimating the 3-year risk of the first ACE occurrence represented an ancillary outcome of the present study.
2.2. MAFLD and MASLD Diagnostic Criteria
MASLD diagnostic criteria were hepatic steatosis associated with overweight or obesity, defined as BMI > 25 kg/m2 in the case of NL patients, type 2 diabetes mellitus (T2DM), or the presence of at least one of the following: (A) waist circumference ≥ 94 cm (males) or ≥80 cm (females); (B) blood pressure ≥ 130/85 mmHg or a specific drug treatment; (C) plasma triglycerides (TG) ≥ 150 mg/dL or a specific drug treatment; (D) plasma high-density lipoprotein (HDL) cholesterol < 40 mg/dL (males) or <50 mg/dL (females) or a specific drug treatment; and (E) a fasting plasma glucose (FPG) level of 100–125 mg/dL, a 2 h post-load glucose level of 140–199 mg/dL, or glycated hemoglobin (HbA1c) > 5.7% [3].
MAFLD diagnostic criteria were hepatic steatosis associated with BMI > 25 kg/m2 in the case of NL patients, T2DM, or at least two of the following seven criteria: (A) waist circumference > 102 cm (males) or 88 cm (females); (B) systolic blood pressure (SBP) > 130 mmHg or a specific drug treatment; (C) plasma TG level > 150 mg/dL or a specific drug treatment; (D) plasma HDL cholesterol < 40 mg/dL and 50 mg/dL for men and women, respectively, or a specific drug treatment; (E) hyperglycemia, defined by an FPG level of 100–125 mg/dL; (F) Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) score > 2.5; and (G) a high-sensitivity C-reactive protein (hs-CRP) level > 2 mg/L [2].
Table 1 summarizes the head-to-head comparison of the MASLD and MAFLD diagnostic criteria (Table 1).

Table 1.
Diagnostic criteria comparison of MASLD and MAFLD definitions.
2.3. Data Collection
2.3.1. Abdominal Ultrasound-Based Diagnosis of SLD
Ultrasonography B-mode evaluation (ultrasound machinery version: Logiq E8 LogoR4, GE HealthCare Technologies Inc., Milan, Italy) performed by an expert physician was used to identify the presence of a bright liver, suggesting the diagnosis of hepatic steatosis. For this purpose, hepatic parenchymal brightness was assessed by evaluating the liver-to-kidney echogenicity ratio, in conjunction with the visual characteristics of hepatic veins and diaphragmatic contours [18].
2.3.2. Clinical and Biochemical Parameters
Clinical data, including comorbidities, especially cardiometabolic ones, lifestyle habits (see File S1), and the complete medical history, were recorded and included in the CRF.
Anthropometrical parameter collection included the determination of the BMI by dividing the weight by the square of height (kg/m2), the waist-to-hip ratio (WHR), SBP, and diastolic blood pressure (DBP). The biochemical parameters collected were platelet count (PLT), hs-CRP, aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transferase (GGT), alkaline phosphatase (ALP), total cholesterol (TC), HDL cholesterol, low-density lipoprotein (LDL), TG, insulin, HbA1c, and FPG.
All biochemical parameters were analyzed employing standardized, fully automated laboratory methodologies, such as AST, ALT, GGT, and ALP; TC, HDL, LDL, and TG were quantified using enzymatic colorimetric techniques on the Roche Cobas® 8000 modular analyzer (Roche Diagnostics, Mannheim, Germany). PLT count was measured via an automated hematology system (Sysmex XN-Series, Sysmex Corporation, Kobe, Japan). Hs-CRP concentrations were determined through a high-sensitivity immunoturbidimetric assay conducted on the same Roche platform. FPG and insulin levels were assessed using enzymatic and electrochemiluminescence immunoassay (ECLIA) methods, respectively. HbA1c was measured by high-performance liquid chromatography (HPLC) utilizing the Bio-Rad D-10 system (Bio-Rad Laboratories, Hercules, CA, USA). All analytical procedures adhered strictly to the manufacturers’ specifications and internal quality assurance protocols, with results interpreted according to reference intervals validated by the institutional clinical laboratory. Finally, HOMA-IR was calculated by using the following formula: fasting insulin (μU/mL) × FPG (mmol/L)/22.5 [19].
2.3.3. Transient Elastography: Definition of Cut-Offs for Fibrosis and Steatosis Severity
The instrumental assessment of liver fibrosis was obtained at the baseline and during the follow-up by using a FibroScan®-based LSM [version 502 (Echosens, Paris, France)] equipped with M and XL probes (18). When the BMI was >30 or when the ultrasound-measured distance between the skin and liver capsule was >2.5 cm, an XL probe was used. Ten acceptable measurements defined a successful LSM. The criteria used to determine the quality of measurements were the ones proposed by Boursier et al. based on the interquartile range (IQR): “very reliable” (IQR/M ≤ 0.1), “reliable” (0.1 < IQR/M ≤ 0.3 or IQR/M > 0.3 with a liver stiffness median < 7.1 kPa), or “poorly reliable” (IQR/M > 0.3 with a LS median ≥ 7.1 kPa [20]. The following LSM cut-offs were used to identify the different liver fibrosis stages according to the Metavir score: (a) F0–F2 ≤ 9.6 kPa; (b) F3: 9.7–13.5 kPa; and (c) F4 ≥ 13.6 kPa [21]. AF (F3–F4) was defined by LSM values > 9.7 kPa. F0, F1, and F2, respectively, indicated “no fibrosis”, “mild”, and “moderate” fibrosis. The transition from F0–F2 to F3–F4 defined AF progression [17].
Hepatic steatosis was quantitatively assessed using the CAP, which evaluates ultrasonic signal attenuation at a frequency of 3.5 MHz. Measurements were obtained via FibroScan® M and XL probes, in accordance with established physical principles detailed in the prior literature [22,23].
CAP values were considered only when derived from validated measurements, applying the same quality control criteria established for LSM [20,22,23].
Specifically, the following CAP thresholds were applied to classify hepatic steatosis grades based on the attenuation of ultrasonic signals measured via liver transient elastography (LTE): S0, no steatosis (0–10% hepatic fat content; CAP 0–237 dB/m); S1, mild steatosis (11–33% fat; CAP 238–259 dB/m); S2, moderate steatosis (34–66% fat; CAP 260–292 dB/m); and S3, severe steatosis (>67% fat; CAP ≥ 293 dB/m) [20,22,23]. The transition from S1/S2 to S3 defined severe steatosis progression.
2.3.4. Determination of Noninvasive Tools (NITs): FIB-4, NFS, and BARD Scores
The validated NITs that were used to estimate the liver disease progression status were the Fibrosis-4 score (FIB-4), NAFLD fibrosis score (NFS), and “BMI, AST/ALT ratio, diabetes” (BARD) scores. The FIB-4 score was calculated by using the originally described formula: [Age × AST (U/L)]/[PLT count (109/L) × ALT1/2]. The results from this formula were used to derive the three FIB-4 categories: low risk for advanced fibrosis (<1.45); indeterminate risk for AF (1.45–3.25); and high risk for AF (>3.25) [24]. The NFS score was calculated by using the following equation: NFS = −1.675 + 0.037 − age + 0.094 − body mass index + 1.13 × impaired fasting glucose/diabetes mellitus + 0.99 × aspartate aminotransferase/alanine aminotransferase ratio − 0.013 × PLT count − 0.66 × serum albumin [25]. The BARD score was composed of 3 variables: an AST/ALT ratio ≥ 0.8 (2 points); a BMI ≥ 28 (1 point); and the presence of diabetes (1 point). A BARD score of 0 to 1 was referred to as a high (96%) negative predictive value for AF [26].
2.3.5. Recording of HCC and ACEs
During the entire follow-up, at each annual visit, the onset of HCC and ACEs in the previous year, supported by EMR documentation, was recorded for each patient. HCC was diagnosed following the European Association for the Study of the Liver (EASL) clinical practice guidelines [27]. Moreover, in the case of progression to AF/ACLD, patients received a semiannual screening ultrasound [27], with results being directly included in his/her EMR-available documentation. The considered ACEs were Transient ischemic attack (TIA), Ictus cerebri (IC), Acute Myocardial Infarction (AMI), and Acute Coronary Syndrome (ACS) [8].
2.4. Statistical Analysis
Continuous variables were summarized using mean values and standard deviations (mean ± SD), whereas categorical data were reported as absolute frequencies and percentages (n [%]). To assess the distributional characteristics of continuous variables, the Kolmogorov–Smirnov test was employed to determine the appropriateness of parametric versus non-parametric statistical approaches. Depending on the outcome of the normality assessment, comparisons between independent groups were conducted using either the t-test or Mann–Whitney U test. For multiple group comparisons, the Kruskal–Wallis test was applied in the presence of non-normal distributions, while one-way ANOVA, followed by Tukey’s post hoc analysis, was used for normally distributed data.
The χ2 test or Fisher’s exact test and the extended McNemar test were used to assess the MAFLD and MASLD diagnostic criteria in predicting disease progression risk by comparing the frequency data (at baseline: the difference in the frequency of AF and S3; after 3 years: the difference in the frequency of AF progression and HCC occurrence in patients) and estimating the relative odds ratios (ORs) of the identified study groups. The log-rank test analysis with a Kaplan–Meier curve comparison, including the time-to-event (TTE) analysis, was adopted to determine the risk (Hazard Ratio) (HR) and compare the cumulative incidence (incidence proportion) (IP) and incidence rate ratio (IRR) of HCC occurrence in the study groups.
Univariate and multivariate competing risk regression analyses were conducted to evaluate event occurrence during follow-up, specifically focusing on 3-year AF progression and hepatocellular carcinoma (HCC) development within predefined study subgroups. The multivariate models were adjusted for key covariates, including sex, age, BMI, presence of T2DM, degree of hepatic steatosis, and baseline fibrosis severity. This approach enabled the identification of independent predictors, expressed as adjusted sub-distribution Hazard Ratios (aHRs), associated with the respective outcomes under competing risk conditions.
Statistical significance was p < 0.05 in a two-tailed test with a 95% confidence interval (C.I.). GraphPad Prism® version 10.2.1 (GraphPad Software, San Diego, CA, USA) was used for the analysis.
The sample size estimation, performed by using a chi-square test comparing two independent proportions with a prediction of a 20% difference in terms of AF progression frequency in NL-SLD patients meeting MASLD criteria (A) exclusively, compared to NL-SLD patients meeting MAFLD criteria (B) exclusively, resulted in n = 110 for (A) and n = 110 for (B) (significance: p ≤ 0.05, type II error: 0.1; power: 0.9) (STATA14 for MacOS) (StataCorp LP, College Station, TX, USA).
3. Results
3.1. Baseline Characteristics of the Study Population
A total of 931 SLD patients (797 NL and 134 L) were ultimately enrolled and subdivided into 206 NL-MASLD, 481 NL-MASLD/MAFLD, 110 NL-MAFLD, 39 L-MASLD, 68 L-MASLD/MAFLD, and 27 L-MAFLD. The baseline characteristics (demographic, anthropometric, clinical, biochemical, and NITs) of the study population groups are reported in Supplementary Table S1 (Table S1).
In general, L-SLD patients were younger than NL-SLD (46.21 ± 5.74 vs. 60.1 ± 3.48, p < 0.0001), whereas no significant differences in terms of gender distribution were reported among the study groups. Moreover, in L-SLD patients, a higher prevalence of individuals presenting compliance with the Mediterranean Diet regimen (43.28% vs. 14.3% p < 0.0001) and active physical exercise (65.67% vs. 52.44%, p = 0.005), as well as a lower percentage of smoker individuals (38.81% vs. 54.71%, p = 0.0007), was revealed in comparison to NL-SLD. Concerning MD-related comorbidities, a higher prevalence of T2DM (p = 0.005) [decompensated T2DM, NL-SLD 38.21% (n = 180), L-SLD 28.57% (n = 14)] and dyslipidemia (p < 0.0001) was evidenced in NL-SLD compared to L-SLD, as well as in NL-MAFLD compared to NL-MASLD (both p < 0.0001). On the contrary, arterial hypertension was globally more represented in L-SLD in comparison to NL-SLD, as well as in NL-MAFLD compared to NL-MASLD (both p < 0.0001); however, no significant differences in SBP and DBP values emerged (Table S1).
Along the same lines, NL and L patients presented with substantially similar biochemical features. In addition, higher HOMA-IR and hs-CRP levels were reported in L-SLD (vs NL-SLD, HOMA-IR, p = 0.04; hs-CRP, p = 0.03) and L-MAFLD (vs L-MASLD, HOMA-IR, hs-CRP, both p = 0.002) (Table S1).
Regarding the baseline liver disease progression status, steatosis severity and fibrosis severity prevalence were not significantly different across the groups in NL (NL-MASLD, S1–S2: 154 (75%); NL-MASLD/MAFLD, S1–S2: 351 (73%); NL-MAFLD, S1–S2: 70 (64%); p = 0.103; NL-MASLD, F0–F2: 171 (83%); NL-MASLD/MAFLD, F0–F2: 390 (81%); NL-MAFLD, F0–F2: 82 (75%); p = 0.277). Similar results were reported in the sub-analysis of L patients (L-MASLD, S1–S2: 28 (72%); L-MASLD/MAFLD, S1–S2: 50 (74%); L-MAFLD, S1–S2: 18 (67%); p = 0.163; L-MASLD, F0–F2: 32 (82%); L-MASLD/MAFLD, F0–F2: 54 (79%); L-MAFLD, F0–F2: 20 (73%); p = 0.718) (Figure 2).
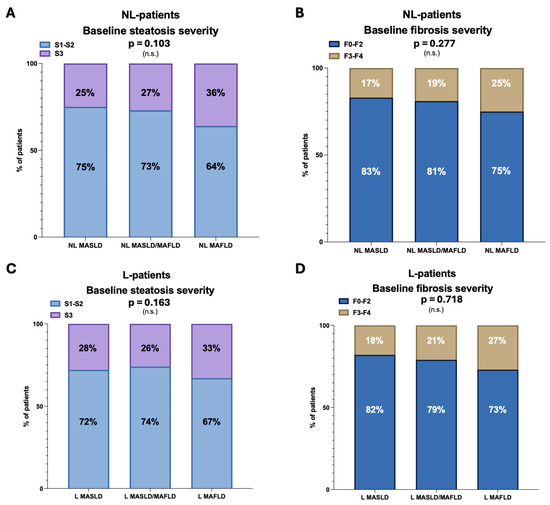
Figure 2.
Baseline liver disease progression status across the groups. Panel (A)—Frequency distribution (%) of baseline mild–moderate (S1–S2) and severe steatosis (S3) in NL patients. Panel (B)—Frequency distribution (%) of baseline no fibrosis, mild fibrosis, moderate fibrosis (F0–F2), and advanced fibrosis (F3–F4) in NL patients. Panel (C)—Frequency distribution (%) of baseline mild–moderate (S1–S2) and severe steatosis (S3) in L patients. Panel (D)—Frequency distribution (%) of baseline no fibrosis, mild fibrosis, moderate fibrosis (F0–F2), and advanced fibrosis (F3–F4) in L patients. Chi-square test analysis. NL: Not-lean; L: Lean; MAFLD: Metabolic dysfunction-associated fatty liver disease; MASLD: Metabolic dysfunction-associated steatotic liver disease; and n.s.: Not statistically significant.
3.2. MASLD and MAFLD Diagnostic Criteria in Estimating Liver Disease Progression Risk
3.2.1. Estimating the 3-Year Risk of AF Progression
Of the NL-MASLD individuals not presenting baseline AF (total, n = 171), 108 (63.15%) progressed to AF after 3 years, whereas of 35 patients with baseline AF, 7 (20%) regressed to F0–F2 fibrosis (OR: 2.334, C.I. 95%: 1.878–3.028, p < 0.0001).
Of 82 NL-MAFLD patients not showing AF at the baseline, 49 (59.75%) progressed to AF after 3 years, whereas in 6 (21.43%) of 28 individuals presenting baseline AF, a regression to F0–F2 fibrosis was observed (OR: 2.469, C.I. 95%: 1.892–3.668, p < 0.0001). MASLD and MAFLD criteria similarly estimated the 3-year risk of AF progression in NL-SLD patients [(MASLD, sensitivity: 0.205, C.I. 95% 0.146–0.281, specificity: 0.901, C.I. 95%: 0.807–0.951, positive predictive value (PPV): 0.800, C.I. 95%: 0.641–0.899); (MAFLD: sensitivity: 0.309, C.I. 95% 0.214–0.424, specificity: 0.846, C.I. 95%: 0.702–0.927, positive predictive value (PPV): 0.785, C.I. 95%: 0.604–0.897)] [p = 0.076].
These results were preserved even after stratifying for gender [(Male MASLD, sensitivity: 0.189, C.I. 95% 0.058–0.207, specificity: 0.809, C.I. 95%: 0.774–0.902, PVV: 0. 767, C.I. 95%: 0.522–0.835); (Female MASLD, sensitivity: 0.195, C.I. 95% 0.062–0.203, specificity: 0.804, C.I. 95%: 0.769–0.891, PVV: 0. 751, C.I. 95%: 0.519–0.825); (Male MAFLD: sensitivity: 0.212, C.I. 95% 0.159–0.298, specificity: 0.780, C.I. 95%: 0.420–0.985, PPV: 0.760, C.I. 95%: 0.560–0.804); (Female MAFLD: sensitivity: 0.223, C.I. 95% 0.174–0.275, specificity: 0.762, C.I. 95%: 0.412–0.931, PPV: 0.741, C.I. 95%: 0.545–0.815); (Male MASLD vs. Male MAFLD, p = 0.741; Female MASLD vs. Female MAFLD, p = 0.451; Male MASLD vs. Female MASLD, p = 0.423; Male MAFLD vs. Female MAFLD, p = 0.311)].
The sub-analysis of lean SLD patients showed that 19 (59.37%) of 32 L-MASLD not presenting baseline AF progressed to AF after 3 years, whereas 7 (36.84%) of 19 patients showing baseline AF regressed to F0–F2 fibrosis (OR: 0.912, C.I. 95%: 0.815–2.102, p < 0.0001).
On the contrary, in L-MAFLD, after 3 years, AF progression was observed in 14 (70%) of 20 patients without baseline AF, and 2 (28.57%) of 7 patients presenting baseline AF regressed to F0–F2 fibrosis (OR: 1.071, C.I. 95%: 0.911–1.157, p < 0.0001). Overall, MAFLD criteria better estimated the 3-year risk of AF progression in L-SLD patients [(MASLD, sensitivity: 0.173, C.I. 95% 0.069–0.214, specificity: 0.812, C.I. 95%: 0.769–0.934, PPV: 0.571, C.I. 95%: 0.405–0.841); (MAFLD: sensitivity: 0.263, C.I. 95% 0.178–0.287, specificity: 0.750, C.I. 95%: 0.409–0.955, PPV: 0.714, C.I. 95%: 0.558–0.794)] [p = 0.006].
The variations (Δ: baseline (T0) vs. last visit (T1)) in LTE (both in LSM and CAP: Δ LSM and Δ CAP) are reported in Supplementary Figure S1 (Figure S1).
In lean MAFLD, physical exercise (HR: 0.349; p = 0.03) and Mediterranean Diet compliance (HR: 0.491; p = 0.02) represented expected AF progression predictive variables, whereas dyslipidemia (HR: 1.683; p = 0.002), T2DM (HR: 2.572; p < 0.0001), HOMA-IR (HR: 1.721; p < 0.0001), and hs-CRP (HR: 1.543: p < 0.0001) were evidenced as variables significantly associated with 3-year AF progression (Table 2). The multivariate analysis revealed T2DM (p = 0.001), HOMA-IR (p = 0.02), and hs-CRP (p = 0.03) as the exclusive variables significantly impacting the outcome in these individuals, independently of sex, age, BMI, diabetes, steatosis, and baseline fibrosis severity (Table 2).

Table 2.
Variables associated with 3-year advanced fibrosis (AF) progression in patients meeting MAFLD criteria.
3.2.2. Estimating 3-Year Risk of Hepatocellular Carcinoma Occurrence
Compared to MAFLD, MASLD diagnostic criteria similarly estimated the 3-year risk of HCC occurrence in NL-SLD patients [NL-MASLD vs. NL-MAFLD, HR: 1.104, C.I 95%: 0.824–1.593, median time (in months): 21 vs. 16, p = 0.741] (Figure 3A). Consistently, during a median follow-up of 35.8 (6–36) months, the cumulative incidence of HCC at 12, 24, and 36 months was, respectively, 3.88%, 10.67%, and 16.51% in NL-MASLD and 1.81%, 11.81%, and 15.45% in NL-MAFLD (NL-MASLD vs. NL-MAFLD: p = 0.735).
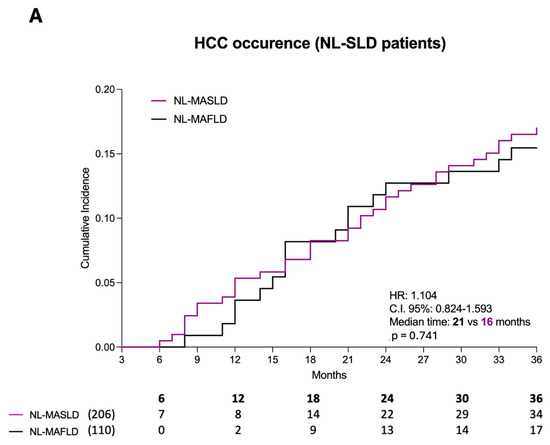
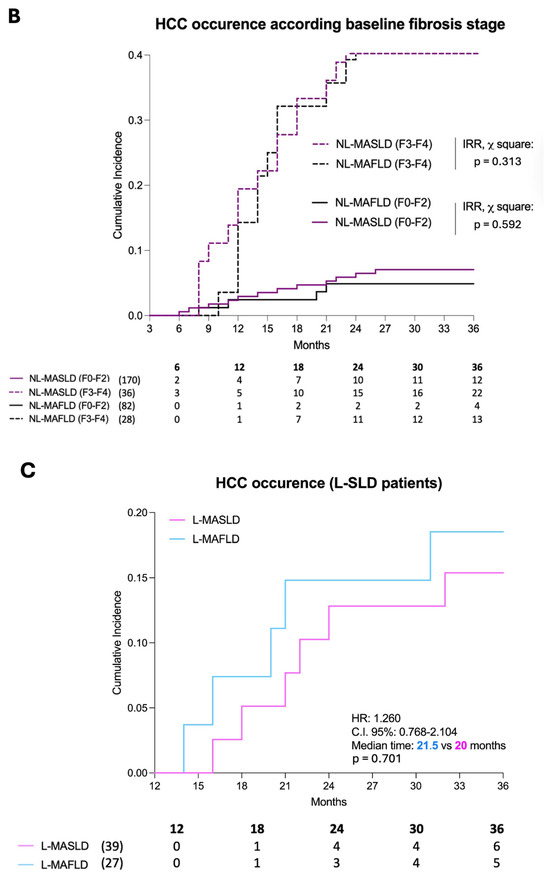
Figure 3.
Hepatocellular carcinoma occurrence. Panel (A)—Hepatocellular carcinoma occurrence in not-lean SLD patients. The 3-year risk of HCC occurrence with the relative cumulative incidence in NL-MASLD (purple) and NL-MAFLD (black). Panel (B)—The incidence rate of the event (HCC occurrence) in NL-MASLD and NL-MAFLD according to the baseline fibrosis severity. HCC: Hepatocellular carcinoma; SLD: Steatotic liver disease; NL: Not-lean. Log-rank test analysis with a Kaplan–Meier curve comparison, including the time-to-event (TTE) analysis. Panel (C)—Hepatocellular carcinoma occurrence in lean SLD patients. The 3-year risk of HCC occurrence with the relative cumulative incidence in L-MASLD (pink) and L-MAFLD (blue). HCC: Hepatocellular carcinoma; SLD: Steatotic liver disease; NL: Not-lean. Log-rank test analysis with a Kaplan–Meier curve comparison, including the time-to-event (TTE) analysis. MAFLD: Metabolic dysfunction-associated fatty liver disease; MASLD: Metabolic dysfunction-associated steatotic liver disease.
In this setting, the results were preserved after stratifying for gender [Male NL-MASLD vs. Female NL-MASLD, HR: 1.091, C.I. 95%: 0.811–1.349, p = 0.561; Male NL-MAFLD vs. Female NL-MAFLD, HR: 1.113, C.I. 95%: 0.772–1.318, p = 0.451; Male NL-MASLD vs. Male NL-MAFLD, HR: 1.011, C.I. 95%: 0.834–1.219, p = 0.566; Female NL-MASLD vs. Female NL-MAFLD, HR: 1.026, C.I. 95%: 0.765–1.282, p = 0.072].
Even after stratifying for the baseline fibrosis stage (sub-analysis), no significant differences were reported both in F0–F2 (NL-MASLD vs. NL-MAFLD, IRR, p = 0.313) and in AF patients (NL-MASLD vs. NL-MAFLD, IRR, p = 0.592) (Figure 3B). The cumulative HCC incidence at 12, 24, and 36 months in the groups was NL-MASLD F0–F2 (2.35%, 5.88%, and 7.05%), NL-MAFLD F0–F2 (1.21%, 2.43%, and 4.87%), NL-MASLD F3–F4 (8.33%, 41.66%, and 61.11%), and NL-MAFLD F3–F4 (3.57%, 39.28%, and 46.42%) (NL-MASLD F0–F2 vs. NL-MAFLD F0–F2, p: 0.321; NL-MASLD F3–F4 vs. NL-MAFLD F3–F4, p = 0.288).
In line with NL-SLD patients, even the sub-analysis of lean individuals showed that MASLD criteria, compared to MAFLD, similarly estimated the 3-year risk of HCC occurrence [L-MAFLD vs. L-MASLD, HR: 1.260, C.I. 95%: 0.768–2.104, median time (in months): 21.5 vs. 20, p = 0.701] (Figure 3C). In this setting of patients, the cumulative incidence of HCC at 18, 24, and 36 months was, respectively, 2.56%, 10.25%, and 15.38% in L-MASLD and 3.70%, 11.11%, and 18.51% in L-MAFLD (L-MASLD vs. L-MAFLD: p = 0.664).
3.3. MASLD and MAFLD Diagnostic Criteria in Estimating 3-Year Risk of ACEs’ Occurrence
Compared to MAFLD, MASLD diagnostic criteria similarly estimated the 3-year risk of ACEs’ occurrence in NL-SLD patients [NL-MASLD vs. NL-MAFLD, HR: 1.188, C.I. 95%: 0.626–2.252, median time (in months): 27 vs. 28, p = 0.606] (Figure 4A).
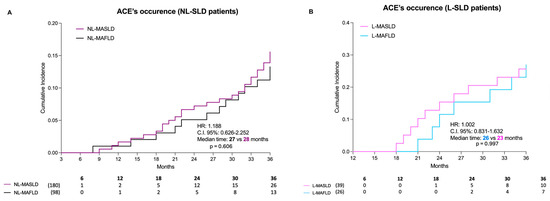
Figure 4.
Occurrence of acute cardiovascular events. Panel (A)—Occurrence of acute cardiovascular events in not-lean SLD patients. The 3-year risk of ACEs’ occurrence with the relative cumulative incidence in NL-MASLD (purple) and NL-MAFLD (black). ACE: Acute cardiovascular event; SLD: Steatotic liver disease; NL: Not-lean. Log-rank test analysis with a Kaplan–Meier curve comparison, including the time-to-event (TTE) analysis. Panel (B)—Occurrence of acute cardiovascular events in lean SLD patients. The 3-year risk of ACEs’ occurrence with the relative cumulative incidence in L-MASLD (pink) and L-MAFLD (blue). ACE: Acute cardiovascular event; SLD: Steatotic liver disease; NL: Not-lean. Log-rank test analysis with a Kaplan–Meier curve comparison, including the time-to-event (TTE) analysis. MAFLD: Metabolic dysfunction-associated fatty liver disease; MASLD: Metabolic dysfunction-associated steatotic liver disease.
In this setting, the results were preserved after stratifying for gender [Male NL-MASLD vs. Female NL-MASLD, HR: 1.089, C.I. 95%: 0.842–1.215, p = 0.556; Male NL-MAFLD vs. Female NL-MAFLD, HR: 1.125, C.I. 95%: 0.791–1.239, p = 0.435; Male NL-MASLD vs. Male NL-MAFLD, HR: 1.023, C.I. 95%: 0.854–1.221, p = 0.421; Female NL-MASLD vs. Female NL-MAFLD, HR: 1.018, C.I. 95%: 0.792–1.193, p = 0.062].
Consistently, the cumulative incidence of ACEs at 12, 24, and 36 months was, respectively, 1.11%, 6.67%, and 14.44% in NL-MASLD and 1.02%, 5.10%, and 13.26% in NL-MAFLD (NL-MASLD vs. NL-MAFLD: p = 0.265).
Even the sub-analysis of lean individuals showed that the MASLD criteria, compared to MAFLD, similarly estimated the 3-year risk of ACEs’ occurrence [L-MAFLD vs. L-MASLD, HR: 1.002, C.I. 95%: 0.831–1.632, median time (in months): 26 vs. 23, p = 0.997] (Figure 4B). In this setting, the cumulative incidence of ACEs at 24 and 36 months was, respectively, 12.82% and 25.64% in L-MASLD and 15.38% and 26.92% in L-MAFLD (L-MASLD vs. L-MAFLD: p = 0.917). The frequency distribution of ACEs, both in NL and L patients, is reported in Supplementary Table S2 (Table S2).
4. Discussion
In the last decades of the modern hepatologic era, the emerging “Hepatocentric theory,” considering liver disease as the centrum surrounded by several extra-hepatic MS-related comorbidities, has been progressively and rapidly affirmed in the SLD field [8,28].
According to this novel view, the concomitance of hepatic steatosis and CMRFs configures “cardiometabolic steatotic” patients whose prognosis is simultaneously burdened by the natural clinical history of liver disease and, even more relevant, elevated MD-related cardiovascular risk mortality [8,28].
Altogether, these reasons have initially driven the transition from the “exclusive” diagnostic criteria of “NAFLD” to the more inclusive criteria of “MAFLD” [2,29], whose advantages were subsequently supported in real-life experience by various studies. First, Yamamura et al. compared MAFLD and NAFLD definitions, evidencing that MAFLD criteria better identify SLD patients presenting significant fibrosis (AF) evaluated by noninvasive tests [30]. Subsequently, MAFLD and NAFLD were also compared by Lin et al., reporting that the MAFLD definition is more practical for identifying patients with fatty liver disease at high risk of disease progression [31].
However, when it seemed that stability had been reached, a multi-society Delphi consensus replaced “MAFLD” with “MASLD”, proposing new MASLD diagnostic criteria, furtherly revolutionizing SLD scenarios [3]. In response to this, a consistent part of the science community expressed perplexity and concern that this new definition update may confuse physicians and patients due to frequent changes (from “NAFLD” to “MAFLD” and “MASLD”) that occurred in less than five years [4,32,33]. For this reason, evaluating whether and how the new definition of MASLD would impact the “old” MAFLD is a critically concrete and relevant research goal. Moreover, the new proposed MASLD criteria, despite the removal of HOMA-IR and hs-CRP, as MD currently requires only one of five established CMRFs, have been presented as being more inclusive, also better embracing a significant proportion of lean patients who would have been excluded by the previous MAFLD definition [3,34]. Therefore, considering this complex background, evaluating the potential benefits of adopting MASLD rather than MAFLD criteria in defining disease progression risk represented the primum movens driving our research.
Song et al., by dynamically exploring the implications of the newer definition, consistent with other studies [11,12,13], suggested the superiority of MASLD compared to MAFLD in detecting more individuals showing a long-term poor prognosis, globally defined as an increased all-cause mortality [35]. However, despite evidence of all-cause mortality differences in comparing MASLD vs. MAFLD in American individuals [13,35], in a European cohort, the MAFLD and MASLD diagnostic criteria have never been compared to evaluate the accuracy in predicting relatively short-term (3 years) AF progression, HCC onset, and the first ACE’s occurrence in SLD. Therefore, to the best of our knowledge, the present research represents the first study proposing a head-to-head comparison of MAFLD and MASLD diagnostic criteria, revealing no significant differences in predicting these outcomes, except for the non-negligible superiority of MAFLD in predicting the 3-year risk of AF progression in the sub-setting of non-obese individuals.
In particular, concerning AF progression, no statistically significant differences (p = 0.076) between adopting MASLD and MAFLD criteria in estimating the 3-year risk were reported in NL patients. On the contrary, the sub-analysis of L patients showed that the MAFLD criteria better estimated the 3-year risk of AF progression overall (p = 0.006).
This difference (NL vs. L) might be explained by the presence of active visceral fat in NL patients, which may masquerade and influence this outcome. In contrast, in L patients, the analysis may reveal the “pure” independent factors associated with the fibrosis progression. In our findings, T2DM (p = 0.001), HOMA-IR (p = 0.02), and hs-CRP (p = 0.03) were consistently identified by properly adjusted multivariable analysis as being significantly associated with a higher risk of AF progression over three years in L subjects [5,36].
From a more pathogenetic point of view, on the one hand, the well-recognized crucial role of IR [37,38] in fueling liver disease progression to AF can explain the results regarding HOMA-IR and T2DM [39,40]; on the other hand, the equally well-supported relevance of systemic (extra-hepatic) inflammation in promoting MASLD/Metabolic dysfunction-associated steatohepatitis (MASH) complications can justify the association of hs-CRP with this outcome [9,41,42]. Specifically, MAFLD incorporates HOMA-IR and hs-CRP—markers that reflect systemic IR and inflammation, respectively—both of which are mechanistically implicated in hepatic fibrogenesis [43,44]. IR promotes hepatic lipotoxicity, oxidative stress, and stellate cell activation, thereby accelerating fibrotic remodeling [37,43,44].
Concurrently, elevated hs-CRP levels denote extra-hepatic inflammatory burden, which may amplify hepatic injury through cytokine-mediated pathways, simultaneously correlating with liver disease progression and body composition status [45]. These mechanisms emerge as particularly relevant in the lean setting, where overt cardiometabolic features (e.g., hypertension and dyslipidemia) may be absent or less pronounced, limiting the sensitivity of MASLD’s simplified criteria [36,38]. In line with this, also considering that increased levels of hs-CRP and IR have been revealed to be strongly correlated with the risk of developing MASH in normal-weight individuals [41], these results suggest these variables (hs-CRP and HOMA-IR)—particularly in lean individuals—may enhance the discriminatory power of diagnostic frameworks, as well as proposing the potential reintroduction of hs-CRP and HOMA-IR into MD-defining criteria as a subject for further prospective investigation, ideally within larger and more diverse cohorts [3,32].
Moreover, in estimating both the 3-year risk of HCC and the first ACE occurrence, there were no statistically significant differences between NL-MAFLD and NL-MASLD, and the results for the HCC onset were preserved even after stratifying for baseline fibrosis severity. In the L patients’ sub-analysis, MAFLD better estimated the 3-year risk of HCC occurrence compared to MASLD without reaching statistical significance (p = 0.664). These findings suggest the need for further basic science/clinical research to identify discriminant HCC-promoting factors in SLD. Notably, a significant proportion (36.3%) of lean patients presented a baseline decompensated T2DM. This report contributes to explaining the relatively elevated IRR observed in this setting, considering the well-documented role of this MD-contributing CMRF as an independent HCC risk factor [46].
Concerning the follow-up period, a brief observation interval (three years) was voluntarily considered with the aim of simulating the short-term repercussions in terms of disease progression (both in terms of liver-related and cardiovascular-risk-related complications) estimation using “MASLD” or “MAFLD” criteria in everyday clinical practice. On the other hand, equally consistent with routine reproducibility, a practical reason guided the identification of this relatively short interval, considering it is unlikely for MASLD patients to transition to MAFLD (and vice versa) within such a restricted period in real-life scenarios. In line with this, in our study, no patient transitioned from MASLD to MAFLD and vice versa within 36 months.
Regarding HCC, in addition to preventing this possible transition, evidence-based findings sustained the choice of such a limited interval of observations. As widely described, in the dysmetabolic context (NAFLD/MAFLD/MASLD), a higher risk of HCC onset is not limited to ACLD/liver cirrhosis scenarios, with studies reporting a relevant occurrence even in the AF stage (≥F3) [47,48]. In line with this, patients were further stratified according to fibrosis severity, consistently reporting a higher incidence rate of HCC in AF patients. On the other hand, T2DM, representing a crucial CMRF configuring “MD”, is also a well-recognized independent risk factor for both HCC and ACEs [10,46,49], thus contributing to enhancing the risk of this neoplasm and ischemic events, independently (both chronologically and physio-pathologically) of the liver disease progression status. Relevantly, a significant part of the study population (NL-SLD 38.21% and L-SLD 28.57%) presented decompensated T2DM at the baseline.
Finally, focusing more deeply on the duration of follow-up for HCC, a multicentric study revealed the impact of acute lifestyle changes on SLD evolution: a large cohort of patients was followed two years pre-lockdown and two years during the lockdown, with social restrictions (including stay-at-home orders, the closure of public venues, limits on gatherings, travel bans, and mandatory distancing measures to reduce viral transmission) in three Italian medical centers [50]. Simultaneously, with lifestyle modifications (decreased physical exercise and increased food intake) associated with changes in body composition, the overall HCC and Milan-out criteria for HCC occurrence during the lockdown (i.e., 2-year interval) revealed a significantly higher HCC risk [Hazard Ratio (HR): 2.398, p = 0.02, and HR: 5.931, p = 0.008, respectively], regardless of the hepatic fibrosis severity and availability of screening strategies during the pandemic period [50]. Consistent with this, considering that the screened (and then potentially enrolled) patients in our study were admitted between January 2016 and May 2021, a significant portion of the cohort experienced the lockdown period. In particular, of 931 participants, 410 (44.03%) experienced the two years of social restrictions, with a relative worsening of lifestyle habits (decreased physical exercise and increased food intake), and although none experienced severe SARS-CoV-2-related complications or hepatic involvement during the infection, the forced limitations would have impacted the observed outcomes via body composition modification. Altogether, these findings highlight how, even in a short period (“acute”), lifestyle changes may worsen SLD, negatively influencing HCC occurrence. Nonetheless, the experimental design of the present research must be considered to be pioneering, providing preliminary findings and opening pathways to future perspectives that may lead to future research with a follow-up of the studied cohort.
Interestingly, all the observed results concerning the research outcomes (AF progression, HCC, and ACE occurrence) were preserved even after stratifying for gender. Although preliminary trends indicated potential sex-specific differences in risk profiles [51] —particularly with respect to cardiovascular outcomes—these did not reach statistical significance within our not-lean (NL) cohort. Nonetheless, it is biologically plausible that gender-related metabolic and hormonal factors may modulate disease progression and influence outcome prediction [51]. For instance, sex hormones, adipose tissue distribution, and differential inflammatory responses may contribute to distinct pathophysiological trajectories [51,52,53]. In light of these considerations, future investigations employing larger, sex-stratified cohorts are required to more robustly evaluate gender-specific risk modulation in NL individuals.
In this sense, the present study presents various limitations. First, the retrospective nature of the recruitment implies potential biases. Nevertheless, by utilizing the UH’s official HDDA system, which is an entirely electronic recording system, all information and recorded events, including HCC occurrence, were constantly supported by genuine clinical documentation, and the robustness of the findings was consolidated by properly excluding—in the relative selection process—patients presenting with ACLD, subjects that were not HCC- or ACE-naïve, and individuals who were lost to follow-up. On the contrary, patients presenting with baseline AF (LSM: ≥F3) and severe steatosis (CAP: S3) were not excluded to both propose a faithful portrait of real-life scenarios and evaluate the impact of baseline disease burden, as well as the impact of the variations (Δ: baseline (T0) vs. last visit (T1)) in LTE (both in LSM and CAP: Δ LSM and Δ CAP) on the assessed outcomes. Moreover, despite the inclusion of lifestyle-related variables, contrary to gender, no information regarding genetic background was available. This may have critically influenced the observed results since, in the multifactorial SLD pathogenesis, simultaneously with the role of T2DM [46], various single-nucleotide polymorphisms have been revealed to contribute to disease progression [54], particularly in L subjects [55,56]. Finally, as previously discussed, this is a monocentric study considering a relatively small (despite not being insignificant for a single-center study and properly estimated through adequate sample size determination) number of patients, particularly L individuals.
However, as this research represented a real-life evaluation, the number of L patients included in this study reflects the true percentage of L patients within the total cohort in the real-world population. In this sense, the limited number of considered lean individuals represents a faithful portrait of the rarity of this condition in this setting of subjects, as clinicians meet in their routine clinical practice guidelines, simultaneously representing the consequence of a “rigid” identification–extraction–selection process, which was performed to “clean” the data and observe the true “effects” of MAFLD and MASLD application criteria on the study population. The findings for the L group were obtained by performing a sub-analysis, and consequently, all the observations must be considered as secondary outcomes (in contrast to what was performed and observed for the NL-SLD group) and should be interpreted with caution, while the emerging results can lead to further larger investigations and validations.
Conclusively, this pioneering study practically evidenced how belonging to the MASLD or MAFLD group does not confer an advantage in terms of baseline steatosis and fibrosis severity and does not imply a different risk of HCC and occurrence of the first ACE over three years. However, when it focused on predicting the occurrence of AF, L-MAFLD patients appeared to be at the highest risk. Therefore, the ultimate noble and translational aim of the present research is to underscore the need for tailored monitoring and management of L individuals to prevent the progression to further severe conditions, potentially determining dramatic health and socioeconomic repercussions worldwide, even considering their herein-evidenced younger age.
5. Conclusions
In our experience, the adoption of the recently proposed MASLD rather than MAFLD criteria does not imply concrete benefits in estimating liver disease progression risk in NL-SLD patients. On the contrary, focusing on L-SLD individuals, our research revealed the superiority of MAFLD vs. MASLD criteria in estimating 3-year AF progression risk, thus identifying L-MAFLD patients as subjects requiring close monitoring to prevent cirrhosis and related complications promptly. In the era of Precision Medicine, it appears imperative to ensure personalized heightened vigilance and tailored proactive management strategies for these individuals, as well as to implement preventive measures in clinical practice for patients with L-MASLD to avoid their progression to L-MAFLD.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/livers5040058/s1, Figure S1: Dynamicity of Liver Transient Elastography data along the follow-up (Lean patients); Table S1: Baseline characteristics of study groups; Table S2: Distribution of the first acute cardiovascular event according to the study groups and subgroups; File S1: INTERVIEW-COLLECTING DATA CARD.
Author Contributions
M.D. and M.R.: guarantor of the article, conceptualization, methodology, investigation, and writing of the original draft; F.D.N., P.V., C.N. and C.B.: conceptualization, methodology, formal analysis, investigation, and writing of the original draft. M.N., G.S., A.S. and A.C.: investigation, resources, data curation, and visualization; A.F.: conceptualization, data curation, and supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the University of Campania “Luigi Vanvitelli” in Naples (prot. n. 531/2016), approval date: 20 January 2016.
Informed Consent Statement
All study participants, or their legal guardians, provided informed written consent before enrolment in this study.
Data Availability Statement
The original contributions presented in this study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ACEs | Acute Cardiovascular Events |
| ACLD | Advanced Chronic Liver Disease |
| AF | Advanced Fibrosis |
| ALD | Alcohol-Related Liver Disease |
| ALP | Alkaline Phosphatase |
| ALT | Alanine Aminotransferase |
| AST | Aspartate Aminotransferase |
| BARD | BMI, AST/ALT Ratio, Diabetes |
| BMI | Body Mass Index |
| CAP | Controlled Attenuation Parameter |
| CLD | Chronic Liver Disease |
| CMRF | Cardiometabolic Risk Factor |
| CRF | Case Report Form |
| DBP | Diastolic Blood Pressure |
| EMRs | Electronic Medical Records |
| FPG | Fasting Plasma Glucose |
| GGT | Gamma-Glutamyl Transferase |
| HBV | Hepatitis B Virus |
| HCC | Hepatocellular Carcinoma |
| HCV | Hepatitis C Virus |
| HDDA | Health Documents Digitization Archive |
| HDL | High-Density Lipoprotein |
| HOMA-IR | Homeostatic Model Assessment for Insulin Resistance |
| Hs-CRP | High-Sensitivity C-Reactive Protein |
| IR | Insulin Resistance |
| L-MAFLD | Lean-Metabolic Dysfunction-Associated Fatty Liver Disease |
| L-MASLD | Lean-Metabolic Dysfunction-Associated Steatotic Liver Disease |
| LDL | Low-Density Lipoprotein |
| LSM | Liver Stiffness Measurement |
| LTE | Liver Transient Elastography |
| MAFLD | Metabolic Dysfunction-Associated Fatty Liver Disease |
| MASLD | Metabolic Dysfunction-Associated Steatotic Liver Disease |
| MD | Metabolic Dysfunction |
| NAFLD | Non-Alcoholic Fatty Liver Disease |
| NFS | NAFLD Fibrosis Score |
| NITs | Noninvasive Tools |
| NL | Not-Lean |
| SH | Steatohepatitis |
| SLD | Steatotic Liver Disease |
| T2DM | Type 2 Diabetes Mellitus |
| Whr | Waist-to-Hip Ratio |
References
- Chan, W.-K.; Chuah, K.-H.; Rajaram, R.B.; Lim, L.-L.; Ratnasingam, J.; Vethakkan, S.R. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A State-of-the-Art Review. J. Obes. Metab. Syndr. 2023, 32, 197–213. [Google Scholar] [CrossRef]
- Eslam, M.; Sanyal, A.J.; George, J. International Consensus Panel MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014.e1. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A Multisociety Delphi Consensus Statement on New Fatty Liver Disease Nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef]
- Emanuele, E.; Minoretti, P. Letter to the Editor: NAFLD, MAFLD or MASLD? Cut the Gordian Knot with “Ludwig Disease”. Hepatology 2024, 79, E4. [Google Scholar] [CrossRef]
- De, A.; Bhagat, N.; Mehta, M.; Taneja, S.; Duseja, A. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) Definition Is Better than MAFLD Criteria for Lean Patients with NAFLD. J. Hepatol. 2024, 80, e61–e62. [Google Scholar] [CrossRef]
- Pennisi, G.; Enea, M.; Romero-Gomez, M.; Viganò, M.; Bugianesi, E.; Wong, V.W.-S.; Fracanzani, A.L.; Sebastiani, G.; Boursier, J.; Berzigotti, A.; et al. Liver-Related and Extrahepatic Events in Patients with Non-Alcoholic Fatty Liver Disease: A Retrospective Competing Risks Analysis. Aliment. Pharmacol. Ther. 2022, 55, 604–615. [Google Scholar] [CrossRef]
- Foerster, F.; Gairing, S.J.; Müller, L.; Galle, P.R. NAFLD-Driven HCC: Safety and Efficacy of Current and Emerging Treatment Options. J. Hepatol. 2022, 76, 446–457. [Google Scholar] [CrossRef]
- Ren, Z.; Wesselius, A.; Stehouwer, C.D.A.; Brouwers, M.C.G.J. Cardiovascular Implications of Metabolic Dysfunction-Associated Fatty Liver Disease. Endocrinol. Metab. Clin. N. Am. 2023, 52, 459–468. [Google Scholar] [CrossRef]
- Targher, G.; Byrne, C.D.; Tilg, H. MASLD: A Systemic Metabolic Disorder with Cardiovascular and Malignant Complications. Gut 2024, 73, 691–702. [Google Scholar] [CrossRef]
- Dallio, M.; Romeo, M.; Di Nardo, F.; Vaia, P.; Napolitano, C.; Ventriglia, L.; Coppola, A.; Silvestrin, A.; Olivieri, S.; Federico, A. FLAME: Training and Validating a Newly Conceived Model Incorporating Alpha-Glutathione-S-Transferase Serum Levels for Predicting Advanced Hepatic Fibrosis and Acute Cardiovascular Events in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Int. J. Mol. Sci. 2025, 26, 761. [Google Scholar] [CrossRef]
- Perazzo, H.; Pacheco, A.G.; Griep, R.H. Collaborators Changing from NAFLD through MAFLD to MASLD: Similar Prevalence and Risk Factors in a Large Brazilian Cohort. J. Hepatol. 2024, 80, e72–e74. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xie, W. Are There All-Cause Mortality Differences between Metabolic Dysfunction-Associated Steatotic Liver Disease Subtypes? J. Hepatol. 2024, 80, e53–e54. [Google Scholar] [CrossRef]
- Zhao, Q.; Deng, Y. Comparison of Mortality Outcomes in Individuals with MASLD and/or MAFLD. J. Hepatol. 2024, 80, e62–e64. [Google Scholar] [CrossRef]
- Ciardullo, S.; Carbone, M.; Invernizzi, P.; Perseghin, G. Exploring the Landscape of Steatotic Liver Disease in the General US Population. Liver Int. 2023, 43, 2425–2433. [Google Scholar] [CrossRef] [PubMed]
- Boursier, J.; Guillaume, M.; Bouzbib, C.; Lannes, A.; Pais, R.; Smatti, S.; Cariou, B.; Bureau, C.; Ganne-Carrié, N.; Bourlière, M.; et al. Non-Invasive Diagnosis and Follow-up of Non-Alcoholic Fatty Liver Disease. Clin. Res. Hepatol. Gastroenterol. 2022, 46, 101769. [Google Scholar] [CrossRef]
- Panagiotakos, D.B.; Pitsavos, C.; Stefanadis, C. Dietary patterns: A Mediterranean diet score and its relation to clinical and biological markers of cardiovascular disease risk. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 559–568. [Google Scholar] [CrossRef] [PubMed]
- de Franchis, R.; Bosch, J.; Garcia-Tsao, G.; Reiberger, T.; Ripoll, C. Baveno VII Faculty Baveno VII—Renewing Consensus in Portal Hypertension. J. Hepatol. 2022, 76, 959–974. [Google Scholar] [CrossRef]
- Mishra, P.; Younossi, Z.M. Abdominal Ultrasound for Diagnosis of Nonalcoholic Fatty Liver Disease (NAFLD). Am. J. Gastroenterol. 2007, 102, 2716–2717. [Google Scholar] [CrossRef]
- Salgado, A.L.F.D.A.; Carvalho, L.D.; Oliveira, A.C.; Santos, V.N.D.; Vieira, J.G.; Parise, E.R. Insulin Resistance Index (HOMA-IR) in the Differentiation of Patients with Non-Alcoholic Fatty Liver Disease and Healthy Individuals. Arq. Gastroenterol. 2010, 47, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Boursier, J.; Zarski, J.-P.; de Ledinghen, V.; Rousselet, M.-C.; Sturm, N.; Lebail, B.; Fouchard-Hubert, I.; Gallois, Y.; Oberti, F.; Bertrais, S.; et al. Determination of Reliability Criteria for Liver Stiffness Evaluation by Transient Elastography. Hepatology 2013, 57, 1182–1191. [Google Scholar] [CrossRef] [PubMed]
- Eddowes Peter, J.; Sasso, M.; Allison, M.; Tsochatzis, E.; Anstee, Q.M.; Sheridan, D.; Guha, I.N.; Cobbold, J.F.; Deeks, J.J.; Paradis, V.; et al. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1717–1730. [Google Scholar] [CrossRef]
- Sasso, M.; Beaugrand, M.; de Ledinghen, V.; Douvin, C.; Marcellin, P.; Poupon, R.; Sandrin, L.; Miette, V. Controlled Attenuation Parameter (CAP): A Novel VCTETM Guided Ultrasonic Attenuation Measurement for the Evaluation of Hepatic Steatosis: Preliminary Study and Validation in a Cohort of Patients with Chronic Liver Disease from Various Causes. Ultrasound Med. Biol. 2010, 36, 1825–1835. [Google Scholar] [CrossRef]
- Sasso, M.; Miette, V.; Sandrin, L.; Beaugrand, M. The Controlled Attenuation Parameter (CAP): A Novel Tool for the Non-Invasive Evaluation of Steatosis Using Fibroscan. Clin. Res. Hepatol. Gastroenterol. 2012, 36, 13–20. [Google Scholar] [CrossRef]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.S.; Sulkowski, M.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a Simple Noninvasive Index to Predict Significant Fibrosis in Patients with HIV/HCV Coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef]
- Angulo, P.; Hui, J.M.; Marchesini, G.; Bugianesi, E.; George, J.; Farrell, G.C.; Enders, F.; Saksena, S.; Burt, A.D.; Bida, J.P.; et al. The NAFLD Fibrosis Score: A Noninvasive System That Identifies Liver Fibrosis in Patients with NAFLD. Hepatology 2007, 45, 846–854. [Google Scholar] [CrossRef]
- Harrison, S.A.; Oliver, D.; Arnold, H.L.; Gogia, S.; Neuschwander-Tetri, B.A. Development and Validation of a Simple NAFLD Clinical Scoring System for Identifying Patients without Advanced Disease. Gut 2008, 57, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of Hepatocellular Carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Pennisi, G.; Enea, M.; Romero-Gomez, M.; Bugianesi, E.; Wai-Sun Wong, V.; Fracanzani, A.L.; de Ledinghen, V.; George, J.; Berzigotti, A.; Viganò, M.; et al. Risk of Liver-Related Events in Metabolic Dysfunction-Associated Steatohepatitis (MASH) Patients with Fibrosis: A Comparative Analysis of Various Risk Stratification Criteria. Hepatology 2024, 79, 912–925. [Google Scholar] [CrossRef]
- Zeng, J.; Fan, J.-G. From NAFLD to MAFLD: Not Just a Change in the Name. Hepatobiliary Pancreat. Dis. Int. 2022, 21, 511–513. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, S.; Eslam, M.; Kawaguchi, T.; Tsutsumi, T.; Nakano, D.; Yoshinaga, S.; Takahashi, H.; Anzai, K.; George, J.; Torimura, T. MAFLD Identifies Patients with Significant Hepatic Fibrosis Better than NAFLD. Liver Int. 2020, 40, 3018–3030. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Huang, J.; Wang, M.; Kumar, R.; Liu, Y.; Liu, S.; Wu, Y.; Wang, X.; Zhu, Y. Comparison of MAFLD and NAFLD Diagnostic Criteria in Real World. Liver Int. 2020, 40, 2082–2089. [Google Scholar] [CrossRef]
- Rinella, M.E.; Sookoian, S. From NAFLD to MASLD: Updated Naming and Diagnosis Criteria for Fatty Liver Disease. J. Lipid Res. 2024, 65, 100485. [Google Scholar] [CrossRef] [PubMed]
- Sanal, M.G. Is the Change from NAFLD to MASLD Driven by Political Correctness? J. Hepatol. 2024, 80, e74–e76. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Sarin, S.K.; Wong, V.W.-S.; Fan, J.-G.; Kawaguchi, T.; Ahn, S.H.; Zheng, M.-H.; Shiha, G.; Yilmaz, Y.; Gani, R.; et al. The Asian Pacific Association for the Study of the Liver Clinical Practice Guidelines for the Diagnosis and Management of Metabolic Associated Fatty Liver Disease. Hepatol. Int. 2020, 14, 889–919. [Google Scholar] [CrossRef]
- Song, R.; Li, Z.; Zhang, Y.; Tan, J.; Chen, Z. Comparison of NAFLD, MAFLD and MASLD Characteristics and Mortality Outcomes in United States Adults. Liver Int. 2024, 44, 1051–1060. [Google Scholar] [CrossRef] [PubMed]
- Vesković, M.; Pejović, M.; Šutulović, N.; Hrnčić, D.; Rašić-Marković, A.; Stanojlović, O.; Mladenović, D. Exploring Fibrosis Pathophysiology in Lean and Obese Metabolic-Associated Fatty Liver Disease: An In-Depth Comparison. Int. J. Mol. Sci. 2024, 25, 7405. [Google Scholar] [CrossRef]
- Palma, R.; Pronio, A.; Romeo, M.; Scognamiglio, F.; Ventriglia, L.; Ormando, V.M.; Lamazza, A.; Pontone, S.; Federico, A.; Dallio, M. The Role of Insulin Resistance in Fueling NAFLD Pathogenesis: From Molecular Mechanisms to Clinical Implications. J. Clin. Med. 2022, 11, 3649. [Google Scholar] [CrossRef]
- Crocetto, F.; Barone, B.; Manfredi, C.; Trama, F.; Romano, L.; Romeo, M.; Russo, G.; Sicignano, E.; Persico, F.; Aveta, A.; et al. Are Insulin Resistance and Non-Alcoholic Fatty Liver Disease Associated with Peyronie’s Disease? A Pilot Study. J. Physiol. Pharmacol. 2022, 73, 53–62. [Google Scholar] [CrossRef]
- Tuleta, I.; Frangogiannis, N.G. Diabetic Fibrosis. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166044. [Google Scholar] [CrossRef]
- Singh, A.; Garg, R.; Lopez, R.; Alkhouri, N. Diabetes Liver Fibrosis Score to Detect Advanced Fibrosis in Diabetics with Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2022, 20, e624–e626. [Google Scholar] [CrossRef]
- Muriel, P.; Cardoso-Lezama, I.; Vargas-Pozada, E.E.; Ramos-Tovar, E. Mechanisms of Non-Alcoholic Fatty Liver Disease Development in Normal-Weight Individuals. Eur. J. Gastroenterol. Hepatol. 2023, 35, 521–529. [Google Scholar] [CrossRef]
- Dogru, T.; Genc, H.; Bagci, S. C Reactive Protein Levels in Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2012, 56, 507–508, author reply 508–510. [Google Scholar] [CrossRef][Green Version]
- Romeo, M.; Silvestrin, A.; Senese, G.; Di Nardo, F.; Napolitano, C.; Vaia, P.; Coppola, A.; Federico, P.; Dallio, M.; Federico, A. From “Traditional” to “Trained” Immunity: Exploring the Novel Frontiers of Immunopathogenesis in the Progression of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Biomedicines 2025, 13, 2004. [Google Scholar] [CrossRef]
- Dallio, M.; Sangineto, M.; Romeo, M.; Villani, R.; Romano, A.D.; Loguercio, C.; Serviddio, G.; Federico, A. Immunity as Cornerstone of Non-Alcoholic Fatty Liver Disease: The Contribution of Oxidative Stress in the Disease Progression. Int. J. Mol. Sci. 2021, 22, 436. [Google Scholar] [CrossRef]
- Dallio, M.; Romeo, M.; Coppola, A.; Martinelli, G.; Basile, C.; Di Nardo, F.; Napolitano, C.; Vaia, P.; De Gregorio, A.; Silvestrin, A.; et al. Phase Angle/C-Reactive Protein-Index as a Novel Combined Tool for Predicting Liver-Related Hospitalizations in MASLD-Decompensated Cirrhosis. Arch. Med. Res. 2025, 56, 103306. [Google Scholar] [CrossRef]
- Meroni, M.; Longo, M.; Dongiovanni, P. Cardiometabolic Risk Factors in MASLD Patients with HCC: The Other Side of the Coin. Front. Endocrinol. 2024, 15, 1411706. [Google Scholar] [CrossRef]
- Provera, A.; Vecchio, C.; Sheferaw, A.N.; Stoppa, I.; Pantham, D.; Dianzani, U.; Sutti, S. From MASLD to HCC: What’s in the Middle? Heliyon 2024, 10, e35338. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Dong, B. Molecular Mechanisms in MASLD/MASH-Related HCC. Hepatology 2025, 82, 1303–1324. [Google Scholar] [CrossRef]
- Cusi, K.; Abdelmalek, M.F.; Apovian, C.M.; Balapattabi, K.; Bannuru, R.R.; Barb, D.; Bardsley, J.K.; Beverly, E.A.; Corbin, K.D.; ElSayed, N.A.; et al. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) in People With Diabetes: The Need for Screening and Early Intervention. A Consensus Report of the American Diabetes Association. Diabetes Care 2025, 48, 1057–1082. [Google Scholar] [CrossRef]
- Dallio, M.; Sangineto, M.; Romeo, M.; Cipullo, M.; Coppola, A.; Mammone, S.; Di Gioia, G.; Masarone, M.; Persico, M.; Serviddio, G.; et al. The Influence of Acute Lifestyle Changes on NAFLD Evolution in a Multicentre Cohort: A Matter of Body Composition. Nutr. Diabetes 2024, 14, 33. [Google Scholar] [CrossRef]
- Penmetsa, R.; Kapil, S.; VanWagner, L.B. Sex and Gender Differences in Metabolic Dysfunction-Associated Liver Disease. Indian J. Gastroenterol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Alpízar Salazar, M.; Olguín Reyes, S.E.; Medina Estévez, A.; Saturno Lobos, J.A.; De Aldecoa Castillo, J.M.; Carrera Aguas, J.C.; Alaniz Monreal, S.; Navarro Rodríguez, J.A.; Alpízar Sánchez, D.M.F. Natural History of Metabolic Dysfunction-Associated Steatotic Liver Disease: From Metabolic Syndrome to Hepatocellular Carcinoma. Medicina 2025, 61, 88. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.-T.; Liao, Q.-Q.; Tian, H.-Y.; Yu, D.-J.; Xie, T.; Sun, X.-L.; Zhou, X.-M.; Han, Y.-X.; Zhao, Y.-J.; El-Kassas, M.; et al. Estrogen: The Forgotten Player in Metaflammation. Front. Pharmacol. 2024, 15, 1478819. [Google Scholar] [CrossRef] [PubMed]
- Dallio, M.; Masarone, M.; Romeo, M.; Tuccillo, C.; Morisco, F.; Persico, M.; Loguercio, C.; Federico, A. PNPLA3, TM6SF2, and MBOAT7 Influence on Nutraceutical Therapy Response for Non-Alcoholic Fatty Liver Disease: A Randomized Controlled Trial. Front. Med. 2021, 8, 734847. [Google Scholar] [CrossRef]
- Lin, H.; Wong, G.L.-H.; Whatling, C.; Chan, A.W.-H.; Leung, H.H.-W.; Tse, C.-H.; Shu, S.S.-T.; Chim, A.M.-L.; Lai, J.C.-T.; Yip, T.C.-F.; et al. Association of Genetic Variations with NAFLD in Lean Individuals. Liver Int. 2022, 42, 149–160. [Google Scholar] [CrossRef]
- Sookoian, S.; Pirola, C.J. Genetic Predisposition in Nonalcoholic Fatty Liver Disease. Clin. Mol. Hepatol. 2017, 23, 1–12. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).