Abstract
This review examines the combustion characteristics of hydrogen-enriched natural gas with a specific focus on residential appliances, where safety, efficiency, and emission performance are critical. Drawing on experimental studies, numerical simulations, and regulatory considerations, the paper synthesizes current knowledge on how hydrogen addition influences flame stability, flashback phenomenon, thermal efficiency, pollutant formation, and flame geometry. Results across cooktop burners, boilers, and other domestic systems show that moderate hydrogen blending not only can reduce CO and CO2 emissions and enhance combustion efficiency but also can increase burning velocity, diffusivity, and flame temperature, thereby elevating flashback and NOx risks. The review highlights the blending limits, design adaptations, and operational strategies required to ensure safe and effective integration of hydrogen into residential gas infrastructures, supporting its role as a transitional low-carbon fuel.
1. Introduction
The Paris Agreement [1] emphasizes the development of low- or zero-carbon solutions, with the ultimate goal of achieving carbon neutrality. As a result of this commitment, nations worldwide are striving to reduce their dependence on carbon-based fuels, thereby contributing to a significant reduction in environmental pollution [2,3].
Following a period of accelerated global coal consumption, the situation has stabilized in recent years, as natural gas has gradually taken over as the primary energy source [4]. In terms of consumption, the residential sector is a major user (often second only to the industrial sector), where natural gas remains the dominant fuel for heating and cooking [5,6,7,8,9]. However, pollution-related challenges persist: conventional cooking systems fueled by natural gas emit carbon dioxide (CO2) as their primary pollutant [10].
To meet climate commitments aimed at increasing the share of non-fossil energy, states must transition toward zero-carbon fuels. Hydrogen is therefore considered to hold major potential as a primary energy source, including in residential applications. A transitional solution increasingly explored today is the blending of hydrogen with natural gas, which represents an effective means of reducing harmful carbon dioxide (CO2) [11,12] emissions while improving the thermal performance of gas-fired appliances [13]. A study conducted by Field and Derwent shows that, for instance, in the United Kingdom, replacing natural gas with hydrogen in residential use could reduce annual CO2 emissions by 76 million tons [14].
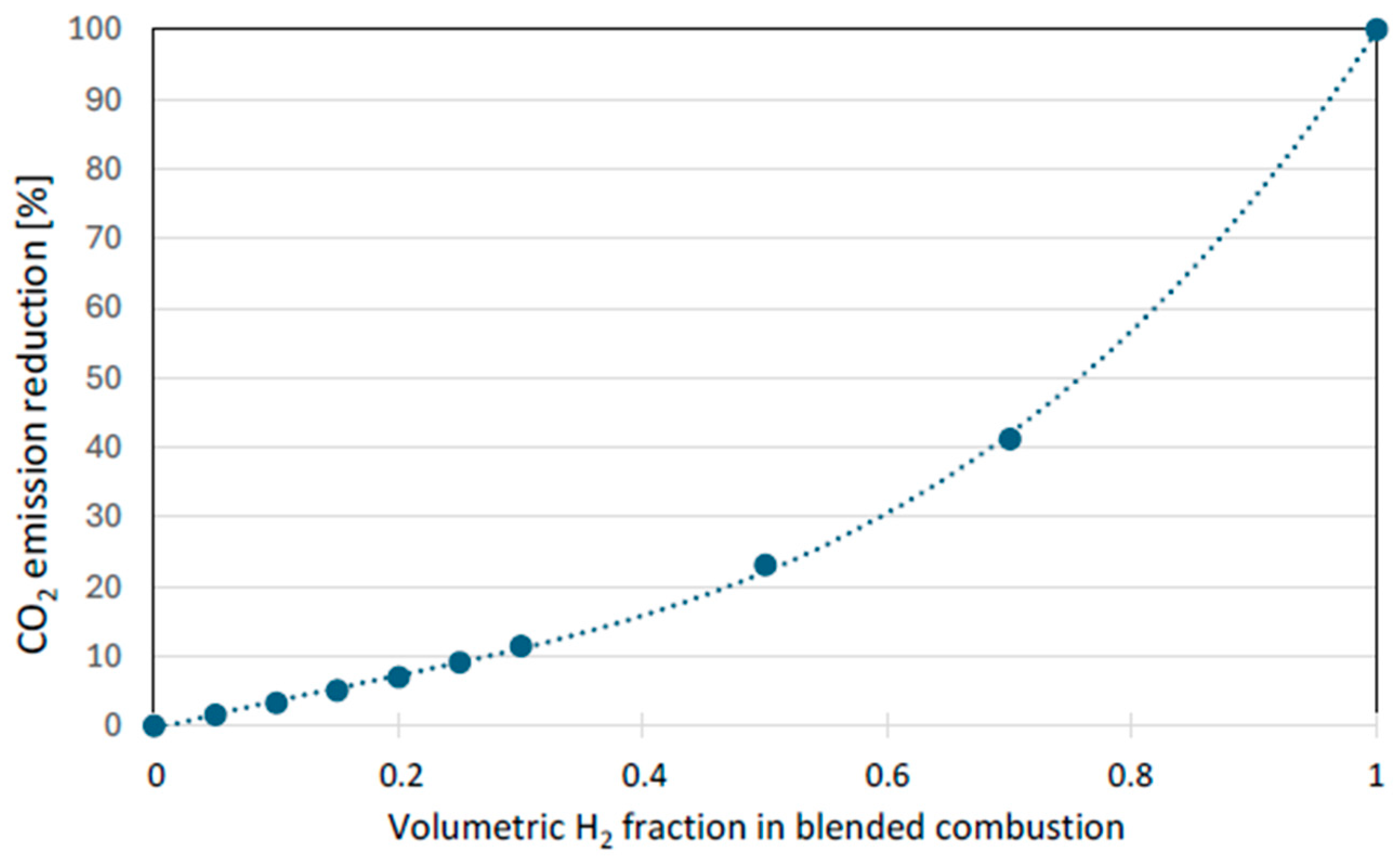
The use of hydrogen as an additive to natural gas in household applications, while offering benefits through reduced CO2 emissions (Figure 1) and enhanced thermal efficiency, is governed by critical safety concerns. Hydrogen is recognized as an extremely flammable and explosive gas, with the highest rating of 4 on the NFPA 704 scale [10]. Attention must therefore be paid to concentration limits. The most significant risk identified is the phenomenon of flame flashback, which can occur at relatively low concentrations in the gas network.
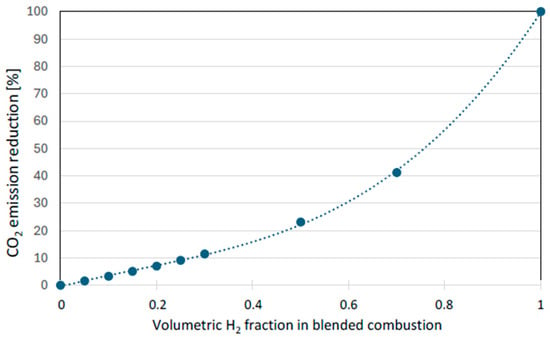
Figure 1.
Potential emission reduction as a function of hydrogen volumetric fraction in the fuel. (Reprinted with permission from [3]).
Di Lullo et al. [15] analyze the impact of blending blue hydrogen with natural gas in the existing infrastructure for transport and direct consumption, through a comprehensive evaluation of well-to-combustion (WTC) emissions. Their study shows that a mixture of 15% H2 and 85% NG reduces combustion emissions by approximately 4–5%, but increases pipeline transport emissions by about 8% and decreases the energy capacity of the network by 11%. In the absence of CO2 capture, total WTC emissions rise slightly (+2%), whereas scenarios with partial or full capture can maintain or even reduce emissions (up to −3.6% in the optimal scenario).
A previous review dedicated to the enhancement of domestic gas stoves [16] also touched on the potential role of hydrogen addition; however, as the author explicitly noted, the analysis could not fully address hydrogen-enriched natural gas due to the limited scientific documentation available at that time. The rapid expansion of experimental and numerical studies in recent years now allows for a more comprehensive assessment of HENG behavior in residential appliances, filling the gap left by earlier reviews.
The purpose of this review is to synthesize current knowledge on the combustion characteristics of hydrogen-enriched natural gas, with particular emphasis on residential appliances. By integrating findings from experimental studies, computational analyses, and regulatory perspectives, the paper aims to clarify how hydrogen blending influences flame stability, pollutant emissions, thermal efficiency, and safety concerns in domestic-scale applications. To provide a clear and systematic overview, the selected literature has been organized into four categories: (i) studies addressing the phenomenon of flashback; (ii) investigations focused on efficiency, emissions, and temperature behavior; (iii) research examining flame geometry; and (iv) experimental works that do not fall neatly into the previous categories but contribute valuable insights. This structure highlights both the potential of hydrogen as a transitional fuel toward carbon neutrality and the technical challenges that must be addressed to enable its safe and efficient adoption in household energy systems.
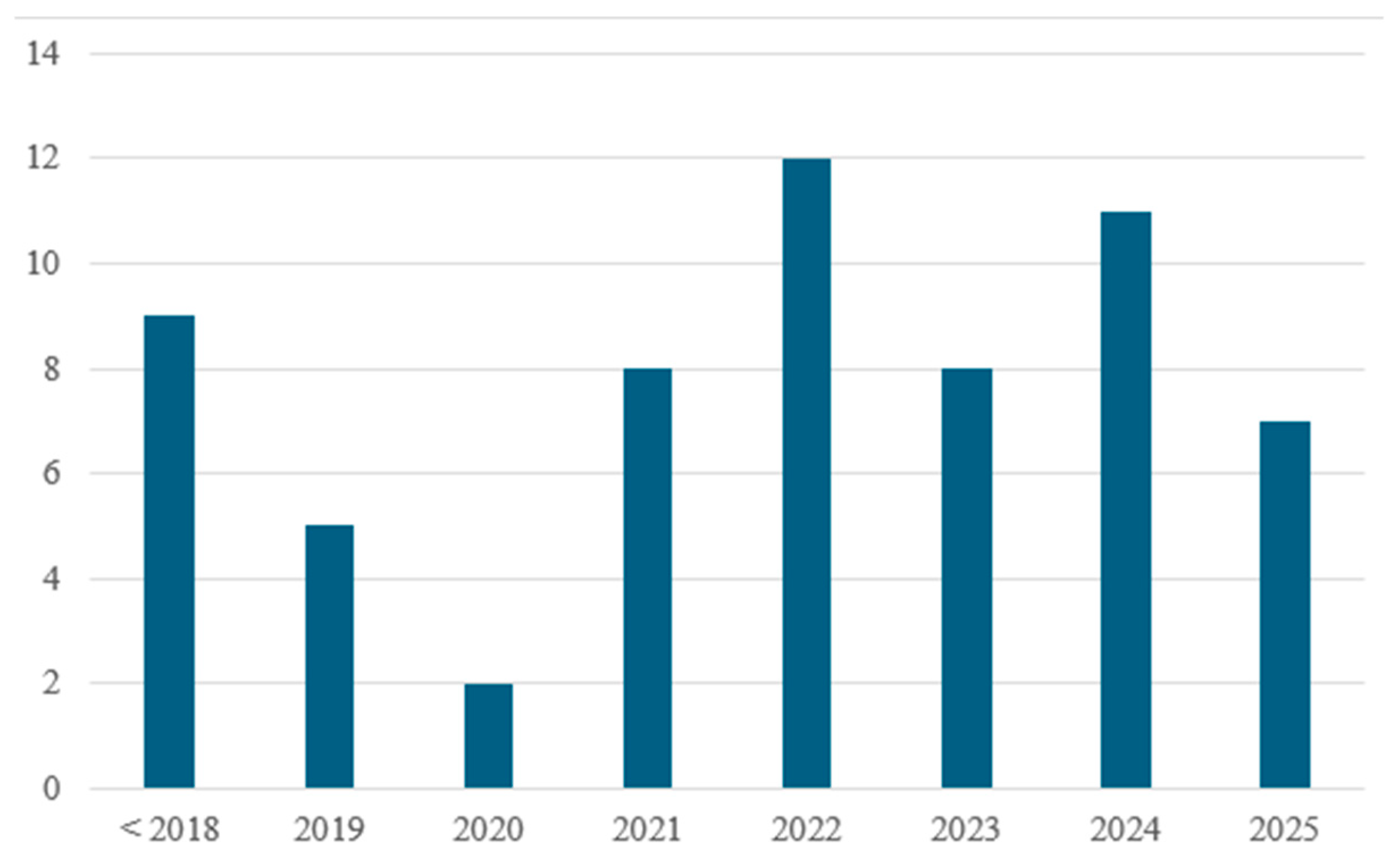
To ensure comprehensive coverage of the topic, the literature review was conducted using the Web of Science database. The search strategy employed a combination of keywords related to hydrogen-enriched natural gas and residential combustion systems. Specifically, the following terms were used in various Boolean combinations: Topic—AND: hydrogen, natural gas, combustion, burner; Topic—NOT: engine, turbine, syngas, ammonia, catalytic, catalyst, reformer. A total of 450 results were shown, which were further filtered manually. Topics excluded during manual filtering include the following: studies focused on large industrial applications with irrelevant operating conditions, studies that focused only on oxy-combustion, and studies where hydrogen and natural gas were not mixed. Papers focusing on domestic appliances or phenomena related to HENG combustion were then selected for analysis. A chart showing the year of publication for the filtered articles is presented in Figure 2. Papers that were published before 2018 consist mainly of research regarding the general interaction between hydrogen and natural gas, while the works published after 2018 consist mostly of research that also considers the implementation of HENG (hydrogen-enriched natural gas) as a fuel for domestic applications.
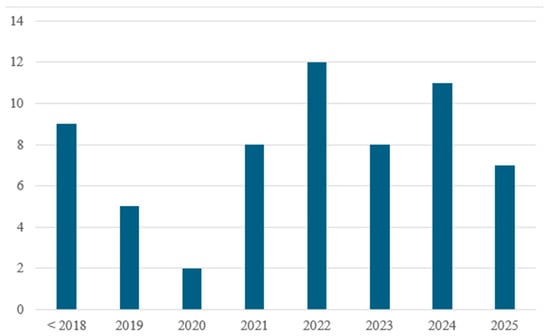
Figure 2.
Publication year of the selected articles.
2. Studies Regarding the Flashback Phenomenon
The transition toward hydrogen-enriched natural gas (HENG) raises important safety challenges, among which the flashback phenomenon is one of the most critical. Flashback occurs when the flame propagates upstream into the burner or mixing zone, and its likelihood increases significantly as hydrogen concentration rises due to the higher burning velocity and enhanced diffusivity of H2. Understanding the mechanisms that govern flashback, ranging from flammability limits and burner wall temperature to preferential diffusion effects, is essential for ensuring the safe operation of domestic and industrial appliances.
Hydrogen’s Lewis number (the ratio of thermal to mass diffusivity; around 0.3–0.4) causes its mass diffusivity to exceed its thermal diffusivity, enriching the reaction zone in H2 relative to the bulk mixture. This preferential diffusion accelerates the local burning velocity near the wall and promotes the formation of a thermo-diffusive flame front capable of penetrating the boundary layer. As the burner surface temperature rises, the thermal boundary layer becomes thinner, and preheating of the unburned mixture intensifies, further increasing the local flame speed. The combined effect is a positive feedback loop: higher wall temperature enhances preferential-diffusion-driven flame acceleration, which in turn increases heat flux to the wall. Flashback therefore does not occur simply because the bulk flow velocity falls below the laminar burning velocity, but also because the effective burning velocity in the near-wall region, amplified by both preferential diffusion and boundary-layer heating, exceeds the local flow gradient [17,18,19].
This chapter reviews the main analytical, experimental, and numerical studies that investigate flashback behavior in HENG systems, highlighting the key parameters that influence stability and the implications for burner design, appliance certification, and hydrogen integration limits.
Analytical and experimental studies have shown that the flashback phenomenon occurs in domestic gas-fired appliances when the volumetric concentration of hydrogen is between 20% and 25% for cooking devices [10,20,21], while in the case of a boiler, the safety limit identified was up to 40% [22,23,24].
The study conducted by Miao et al. [25] investigated the flammability limits of hydrogen-enriched natural gas using a constant-volume combustion chamber and optical visualization techniques. The results showed that as the hydrogen fraction increases, the flammability limits widen significantly: the lower limit remains nearly constant at around 4–5% by volume, whereas the upper limit increases from approximately 16% for natural gas to over 70% for pure hydrogen.
Aniello’s study [26] analyzed the substitution of methane with hydrogen in commercial premixed burners and the resulting effects on blow-off and flashback limits. The results indicate that adding H2 enhances blow-off resistance but simultaneously increases the thermal load on burner walls and promotes flashback. It was demonstrated that simply comparing the flow velocity at the burner exit with the laminar burning velocity is insufficient to predict flashback. The determining factor is the thermal state of the burner, specifically the temperature of the metallic surface. Transient analyses revealed a direct correspondence between the onset of flashback and the attainment of a critical wall temperature for a given mixture composition. The main conclusion is that although hydrogen improves blow-off stability, it restricts the operating range due to an increased risk of overheating and flashback, requiring a redesign of burners to ensure compatibility with hydrogen-rich mixtures.
Klymaz [27] highlights that burner wall temperature plays a critical role: at 600 K, the risk of flashback is significantly higher than at 300 K due to modifications in the thermal boundary layer (Table 1).

Table 1.
Experimental and calculated flashback limits [27,28,29,30,31].
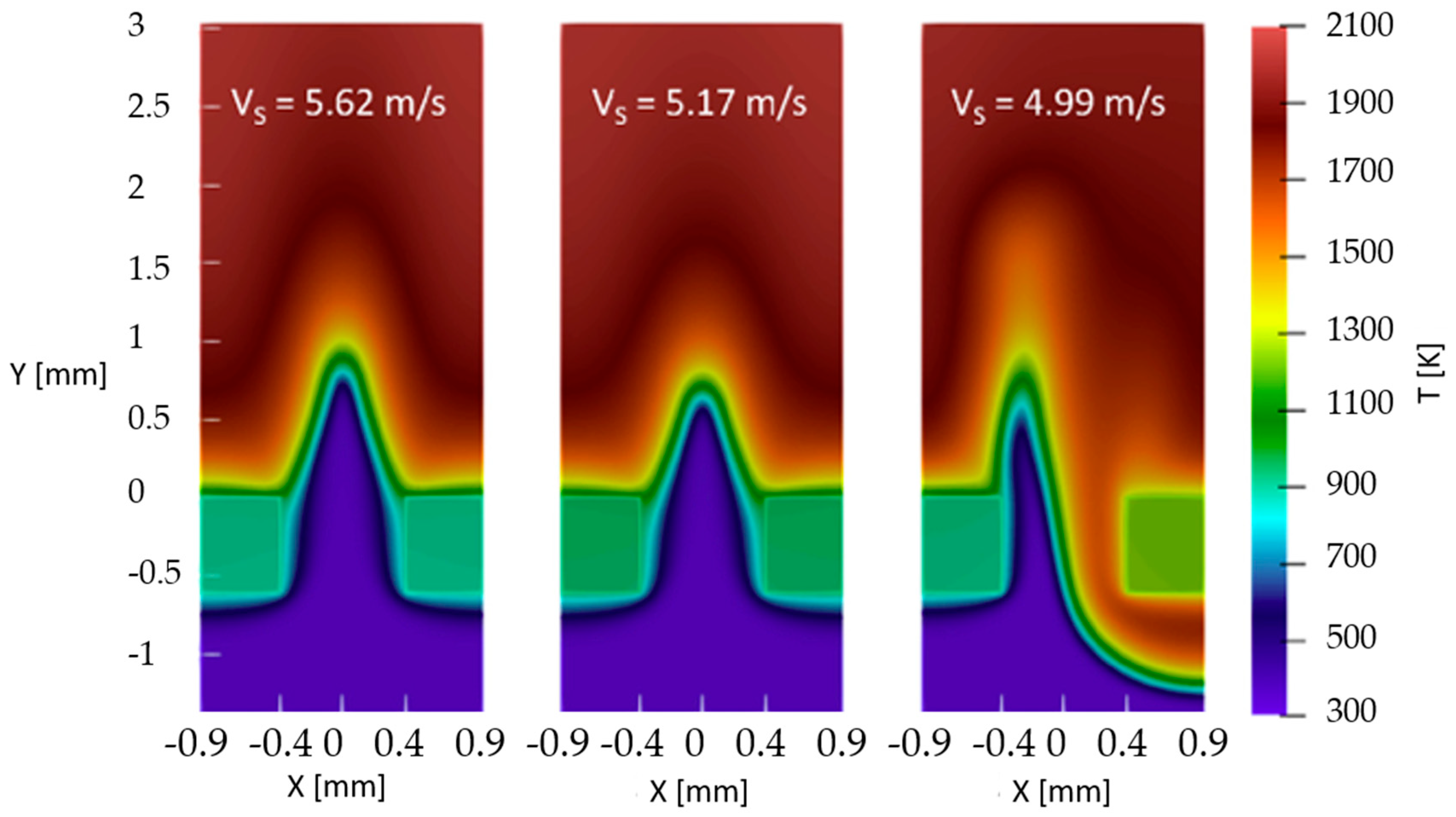
Enrique et al. [17] and Fruzza et al. [18,19] numerically investigated the influence of hydrogen enrichment on premixed flames, focusing on flashback limits and flame behavior. Their results reveal the existence of two distinct flashback regimes: a symmetric regime for low H2 content (up to 75% H2 [18,19]) and an asymmetric regime (example in Figure 3) for high concentrations (>87.5% [18,19]). The critical threshold is defined by an effective Lewis number of 0.5, below which preferential diffusion of hydrogen dominates flame dynamics. The main conclusion is that flashback risk in hydrogen-containing flames is governed not only by burning velocity but also by diffusion effects, requiring careful consideration in burner design.
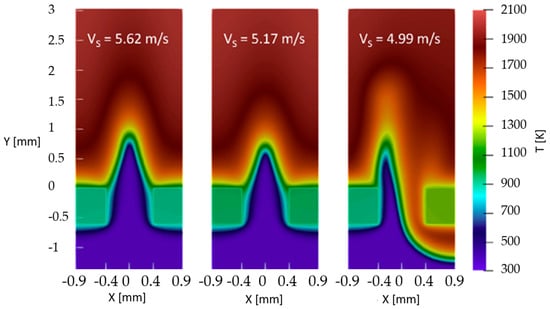
Figure 3.
Asymmetric flashback regime (reprinted with permission from [18]).
The study by Cuoci et al. [32] focused on predicting flashback limits in hydrogen–methane–air mixtures using numerical simulations. It concluded that conventional flashback models based on the critical velocity gradient become inadequate for H2 fractions exceeding 50%, highlighting the need to develop new models that incorporate preferential diffusion effects to ensure the safe operation of such systems. The authors also examined the influence of hole distribution, specifically the spacing between them. It was observed that, depending on mixture composition, this spacing has different effects. For mixtures with an equivalence ratio of ϕ > 0.50, modifying the hole spacing significantly affects the specific flashback power, whereas for mixtures with an equivalence ratio of ϕ = 0.50, the flashback limits remain unchanged.
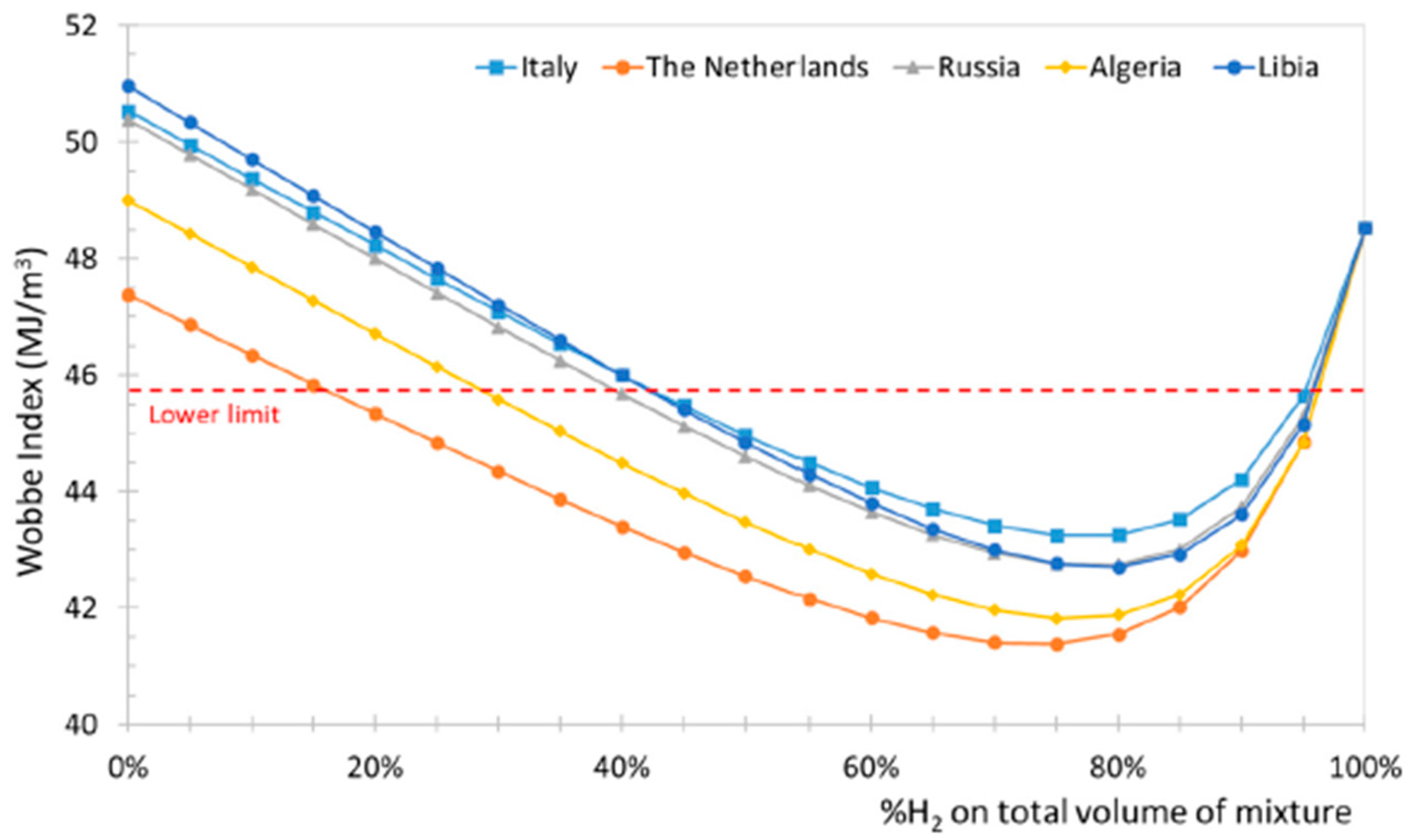
Leicher [33] also emphasizes that relying solely on the Wobbe Index is insufficient for assessing the impact of H2, and that adapting combustion technologies and certification procedures is essential for the safe integration of H2-NG blends in the residential and commercial sectors. The Wobbe Index is the main metric used to assess the interchangeability of gaseous fuels, linking heating value to density and indicating how much energy a burner receives under fixed conditions. For hydrogen–natural gas blends, it helps determine whether existing appliances can operate safely without modification. Although moderate hydrogen levels (≈20–30%, see Figure 4) typically keep the Wobbe Index within acceptable limits, the index alone cannot predict changes in flame speed, flashback risk, or NOx formation, so it must be complemented by detailed combustion analysis. Gases whose Wobbe Index differs by no more than 5–10% are considered interchangeable for most gas-fired applications. However, Xie et al. [34] demonstrated that the Wobbe Index loses applicability for HENG blends with hydrogen fractions above 20%. In atmospheric cooktop burners, for example, the Wobbe Index may remain within the nominal interchangeability band while the primary-air entrainment, flame lift, and flashback limits shift dramatically due to the higher burning velocity of H2 [10,35,36,37,38], effects that the Wobbe Index cannot capture. In partially premixed water heaters, studies show that mixtures with identical Wobbe values can exhibit very different ignition behavior, backflow tendencies and NOx formation and even affect the amount of condensable water [22,24,39,40,41,42,43], again because the index does not reflect changes in diffusivity or flame temperature. These discrepancies are directly relevant to certification frameworks such as EN 30 (domestic cooking appliances) and EN 26/EN 15502 (gas water heaters and boilers), which implicitly assume that fuels with similar Wobbe values will behave similarly in terms of stability and safety. Hydrogen-enriched mixtures violate this assumption: appliances may still “pass” Wobbe-based interchangeability criteria while failing key safety margins such as flashback resistance, material temperature limits, or CO/NOx emission thresholds. This mismatch underscores the need to revise appliance certification procedures so that they incorporate combustion-kinetic effects, particularly burning velocity, preferential diffusion, and flashback propensity, rather than relying solely on Wobbe-based equivalence.
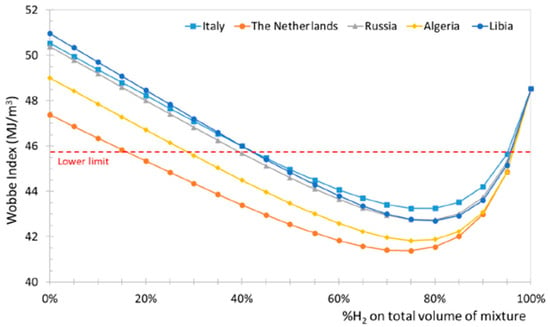
Figure 4.
Wobbe Index variation in NG-H2 blends for different NG origins (reprinted with permission from [3]).
The overarching conclusion is that flammability data obtained for methane–hydrogen mixtures can be used to characterize the behavior of hydrogen-enriched natural gas, and the extension of these limits highlights both the potential for more stable combustion and the increased explosion risks at high H2 concentrations [25,44,45,46,47,48].
3. Investigations Focused on Efficiency, Emissions, and Temperature Behavior
3.1. General Studies
Understanding how hydrogen enrichment influences combustion behavior is essential for evaluating the feasibility of hydrogen–natural gas blends in practical applications. General studies in the literature examine fundamental parameters such as laminar burning velocity, adiabatic flame temperature, radical formation, and thermal output, providing a baseline for predicting system-level performance. These investigations also assess how hydrogen affects efficiency, emissions, and flame structure across a range of conditions and appliance types. Together, they establish the foundational trends (hotter and faster flames, lower carbon-based emissions, and shifting operational limits) that guide subsequent analyses of burner design, appliance compatibility, and safe integration of hydrogen into existing gas infrastructures.
Ren et al. [49] analyzed the effect of hydrogen addition on the combustion characteristics of the main alkanes present in natural gas (CH4, C2H6, C3H8, C4H10). Three combustion mechanisms were compared against experimental data to identify the most suitable one (GRI Mech 3.0, USC Mech 2.0, San Diego). The results showed that both the laminar burning velocity and the adiabatic flame temperature increase with rising hydrogen fraction, with the strongest effect observed for methane. A strong correlation was also identified between the increased concentration of active radicals (H, O, OH) and the intensification of flame-propagation speed.
The study by Nosek et al. [50] focused on the numerical analysis of hydrogen-enriched natural gas mixtures (up to 45%) using a mathematical model. The results confirmed that the mixtures remain compatible for use (Wobbe Index preserved) but revealed an inverse correlation: at a constant volumetric flow rate, increasing H2 concentration leads to a significant reduction in thermal power output (by 44.9% at 45% H2), requiring fuel-flow adjustments to maintain performance. Environmentally, a 25% reduction in CO2 emissions was confirmed for a mixture containing 25% H2.
Wojtowicz et al. [51] conducted a study on several types of household appliances operating on natural gas, evaluating combustion quality, ignition behavior, flame stability, and thermal efficiency for different hydrogen concentrations added to the base fuel (10%, 15%, and 23%). The study found that hydrogen fractions up to 23% should not have any negative impact on appliance safety. In terms of efficiency, a maximum decrease of 5% was recorded for cooking appliances, a value that is unlikely to be noticeable to the end user.
Swirl burners have been widely shown to enhance combustion stability and efficiency by intensifying mixing, increasing residence time, and generating high shear stresses that strengthen the reaction zone. Studies on natural-gas-fired appliances demonstrate that optimized swirl angles improve flame temperature and reduce emissions, with reported efficiency gains of 2–3% for swirl angles between 20° and 56° [52,53,54]. Additional investigations confirm that swirling flow increases secondary-air entrainment, expands the stable operating range, and improves heat transfer due to the enlarged flame surface and prolonged residence time [55,56,57]. Although detailed studies specifically examining swirl burners under hydrogen-enriched natural gas (HENG) conditions were not found, the underlying mechanisms—enhanced mixing, stronger recirculation, and improved flame stabilization—are expected to remain beneficial, and may even become more important given hydrogen’s higher burning velocity and diffusivity.
The degree of premixing between hydrogen-enriched natural gas (HENG) and air plays a decisive role in flame stability, pollutant formation, and flashback propensity. Because hydrogen and methane are already fully mixed in HENG, the relevant parameter is the air–fuel premixing level inside the injector or burner head. Numerical studies show that increasing the premixing intensity enhances mixture uniformity but also increases the risk that the local fuel–air ratio will enter the flammable range inside the injector, especially at higher hydrogen fractions due to the much wider flammability limits of H2 (4–75%) compared to CH4 (5–15%). For example, Chen et al. [37] report that when the hydrogen share exceeds ~45%, the primary mixture inside the injector can fall within the ignition limits, creating a clear backfire risk even before reaching the burner ports. Conversely, insufficient premixing leads to poorer mixing quality, higher CO emissions, and reduced thermal efficiency, as observed in domestic swirl stoves where enhanced entrainment and mixing improve heat transfer and reduce CO formation [10]. Overall, the literature indicates that moderate premixing (sufficient to ensure homogeneous HENG–air mixing but avoiding flammable conditions upstream of the burner) is essential for safe and efficient operation.
The main conclusion is that hydrogen addition in premixed methane–oxygen flames leads to hotter, faster, and more compact flames, with reduced CO2 and CO emissions but an increased potential for NOx formation [58]. This highlights the need for optimizing the equivalence ratio and burner design to ensure safe and efficient operation.
3.2. Studies Focused on Cooktop Burners
Cooktop burners represent one of the most widespread domestic applications of natural gas, making them a critical test case for assessing the compatibility of hydrogen-enriched fuels. Because these appliances operate with open flames, relatively small burner geometries, and direct user interaction, their performance is highly sensitive to changes in flame temperature, burning velocity, and heat-transfer characteristics introduced by hydrogen addition. Recent studies have therefore focused on understanding how hydrogen affects flame structure, thermal efficiency, pollutant emissions, and operational stability in domestic stoves. The following works examine these aspects through experimental measurements, numerical simulations, and burner-design optimization, providing insight into the blending limits and design adaptations required for safe and efficient hydrogen use in household cooking appliances.
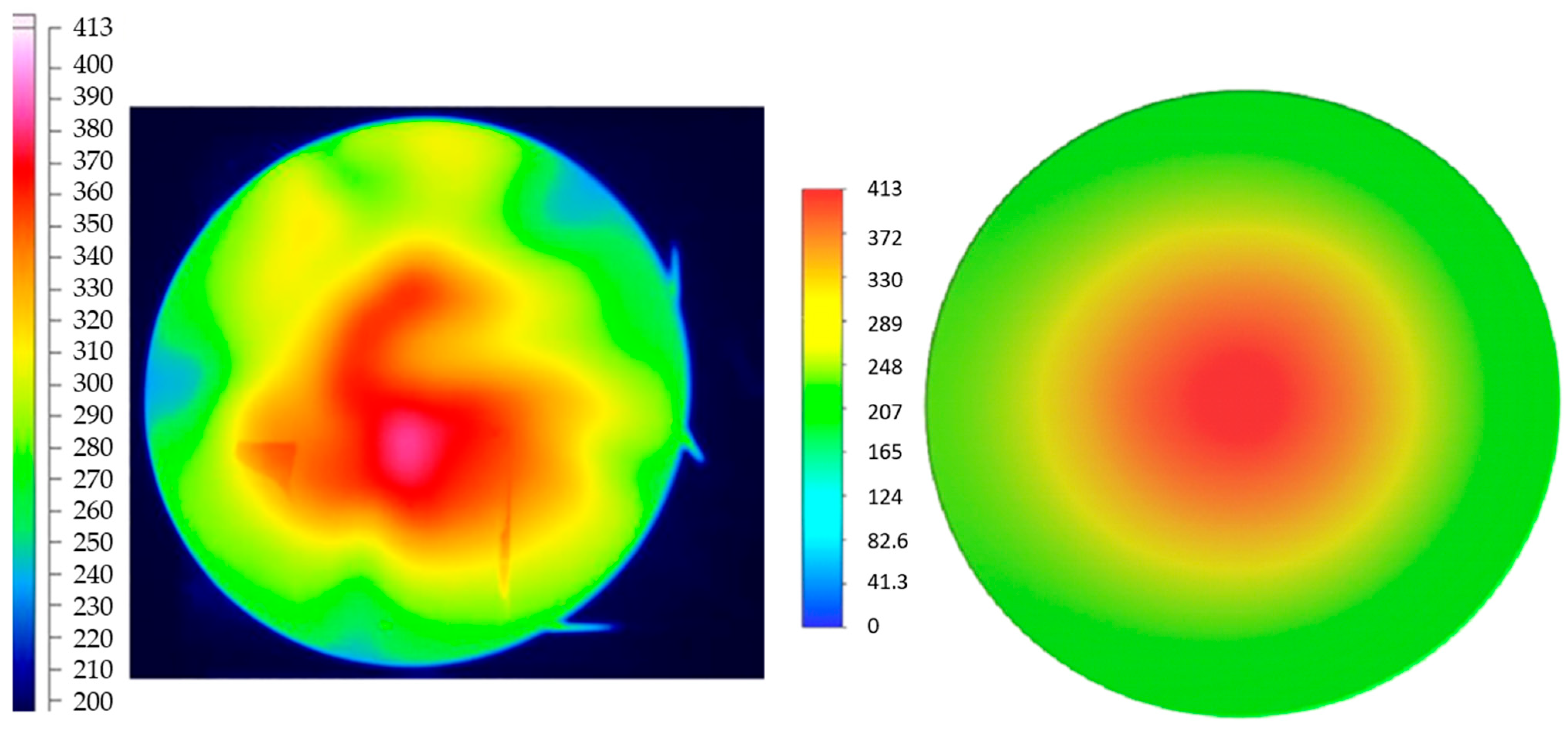
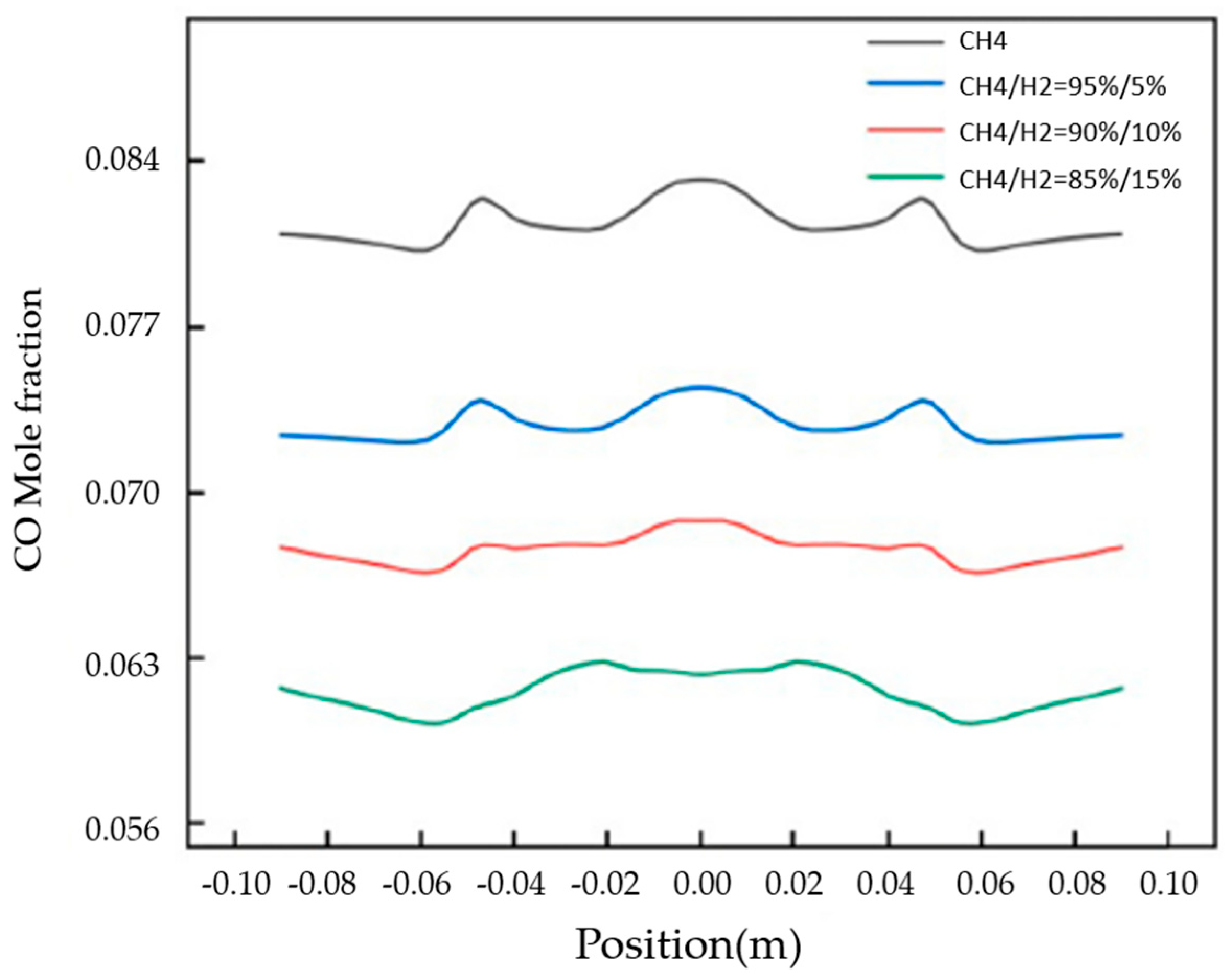
Liu et al. [10] demonstrated both experimentally and numerically that, for a domestic cooking appliance, a 15% hydrogen fraction in natural gas leads to a 6.7% decrease in the average combustion-zone temperature (Figure 5), while CO emissions are reduced by 25% (Figure 6).
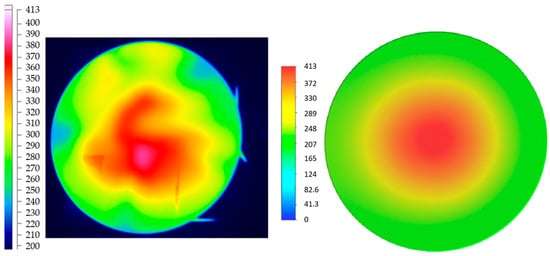
Figure 5.
Temperature (°C) distribution from experimental results (left) and CFD results (right) obtained by Liu (reprinted with permission from [10]).
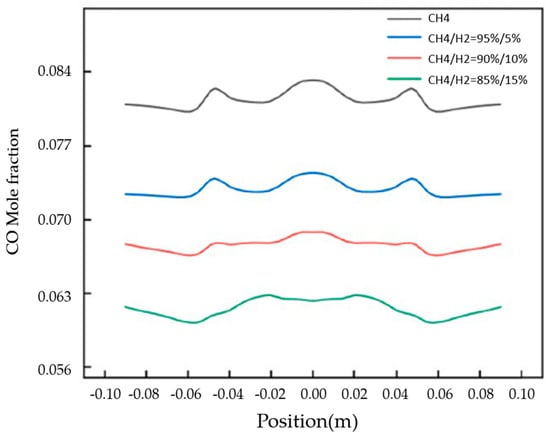
Figure 6.
CO mole fraction distribution at the exit of fire holes for different concentrations of hydrogen (Reprinted with permission from [10]).
They also experimentally investigated the behavior of a household stove fueled with an 80% natural-gas/20% hydrogen mixture, as well as geometric optimization through adjusting the wok-support height (Table 2) [38]. The results showed that the flame becomes shorter but hotter, which initially reduces heat-transfer efficiency and increases NOx emissions, while CO emissions decrease. Lowering the wok support from 7 cm to 6 cm increased thermal efficiency, reduced NOx emissions, and caused only a marginal rise in CO.

Table 2.
NOx and CO emissions at different heights of the wok stand [38].
The main conclusion is that moderate geometric adjustments (such as support height) allow the advantages of hydrogen combustion to be exploited while maintaining performance and meeting emission standards.
The study by Z. Fang et al. [35] analyzed the performance of three types of domestic burners supplied with CH4/H2 mixtures containing up to 25% hydrogen. The results show that the flame becomes shorter and more intense as the hydrogen fraction increases (See Chapter 4), and the average efficiency increases slightly for TRPGS (typical round-port gas stove) and SSPGS (swirling strip-port gas stove). For RPMGS (radiant porous-media gas stove), efficiency increases up to 15% H2 and then decreases. CO emissions decrease significantly at 25% H2, while NOx emissions remain nearly constant. Combustion remains stable up to 25% H2, although the reduced distance between the flame and burner surface may affect component durability.
Ahemat’s study [36] comparatively investigated emissions generated by butane, methane, and methane–hydrogen mixtures in domestic stove burners using EN 30-1-1-compliant experiments and CFD simulations. The main objective was to evaluate CO and CO2 levels after combustion and to determine how hydrogen affects combustion performance. The results show that butane produces the highest emissions (CO ≈ 0.30 ppm), methane generates lower values (≈0.005% CO), and the methane–hydrogen mixture further reduces CO to 0.13 ppm. Additionally, butane combustion reaches maximum temperatures of approximately 2254 K, explaining its higher emissions. The overall conclusion is that adding 50% hydrogen to methane improves combustion efficiency and significantly reduces carbon-based emissions, supporting the transition toward cleaner and more efficient cooking systems.
Chen et al. [37] proposed an optimized porous-medium domestic burner for natural-gas/hydrogen mixtures, analyzing acceptable blending limits through experimental and numerical methods. The results show that above 45% H2, flashback occurs in the injector and mixture uniformity decreases. Two combustion regimes (immersed and surface) appear in the porous zone, with transitions that cause sudden increases in CO emissions and decreases in efficiency and NOx emissions. The conclusion is that, to balance safety, emissions, and performance, the hydrogen fraction should not exceed 35%.
3.3. Studies Focused on Boilers
Boilers represent a key application area for assessing the feasibility of hydrogen–natural gas blends, as their operating conditions, combustion chamber geometry, and heat-transfer requirements differ significantly from those of cooktop burners. Compared with domestic stoves, residential boilers have been less extensively studied in the context of hydrogen enrichment, resulting in a limited pool of experimental data. For this reason, several semi-industrial and industrial boiler studies are also included in this section: although they operate at higher thermal loads, their combustion behavior, emission trends, and stability characteristics remain relevant for understanding the broader implications of hydrogen addition. Collectively, these investigations provide insight into how hydrogen affects flame temperature, pollutant formation, thermal efficiency, and operational safety across a range of boiler configurations. The following studies therefore contribute to defining practical blending limits and identifying the design and control strategies needed to ensure reliable and low-emission operation in both residential and industrial heating systems.
Mayrohfer et al. [24] investigated the use of CH4/H2 mixtures (0–40%) in heating systems and reported a 1.2% increase in efficiency and a 16.6% reduction in CO2 emissions at 40% H2, correlated with a decrease in flue-gas temperature from 582 °C to 565 °C. By analyzing two combustion modes (staged-air combustion and flameless combustion) the authors showed that NOx emissions can be kept below the TA-Luft [59] limit of 350 mg/m3: in staged-air combustion this threshold is met for burner powers above 135 kW, while in flameless combustion the values are even lower (60–200 mg/m3, depending on power and hydrogen fraction).
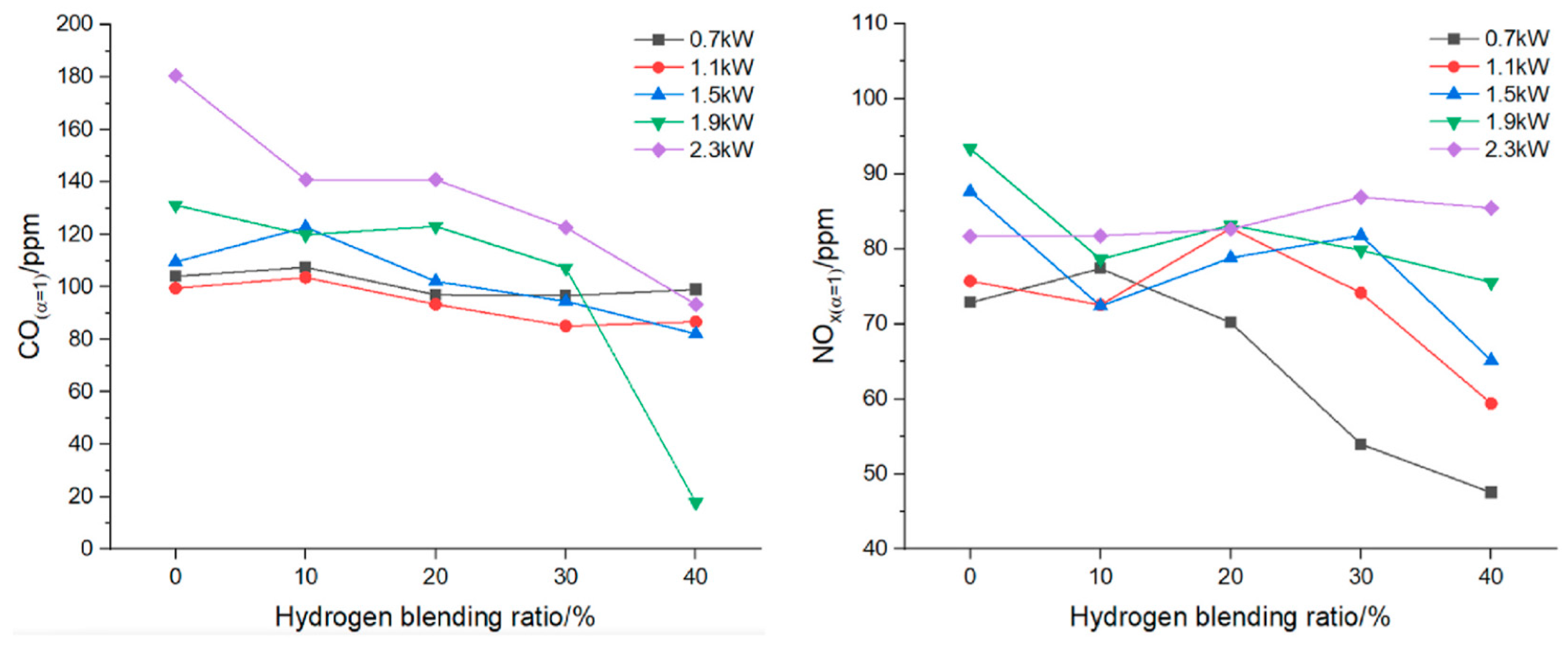
For a residential boiler, Zhan et al. [22] observed that at the same thermal input, flame temperature decreases for low H2 concentrations (10–20%) and then increases as the mixture becomes richer in hydrogen; at constant power, increasing the hydrogen fraction leads to a clear rise in temperature. CO emissions decrease slightly between 0.7 and 1.5 kW and more significantly at 1.9–2.3 kW, while NO variations remain small up to 20% H2, becoming noticeable only at higher concentrations (Figure 7).
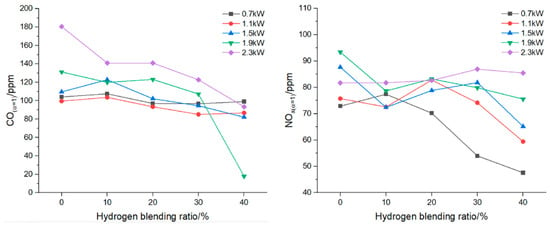
Figure 7.
CO emissions (left) and NO emissions (right) for a boiler (reprinted with permission from [22]).
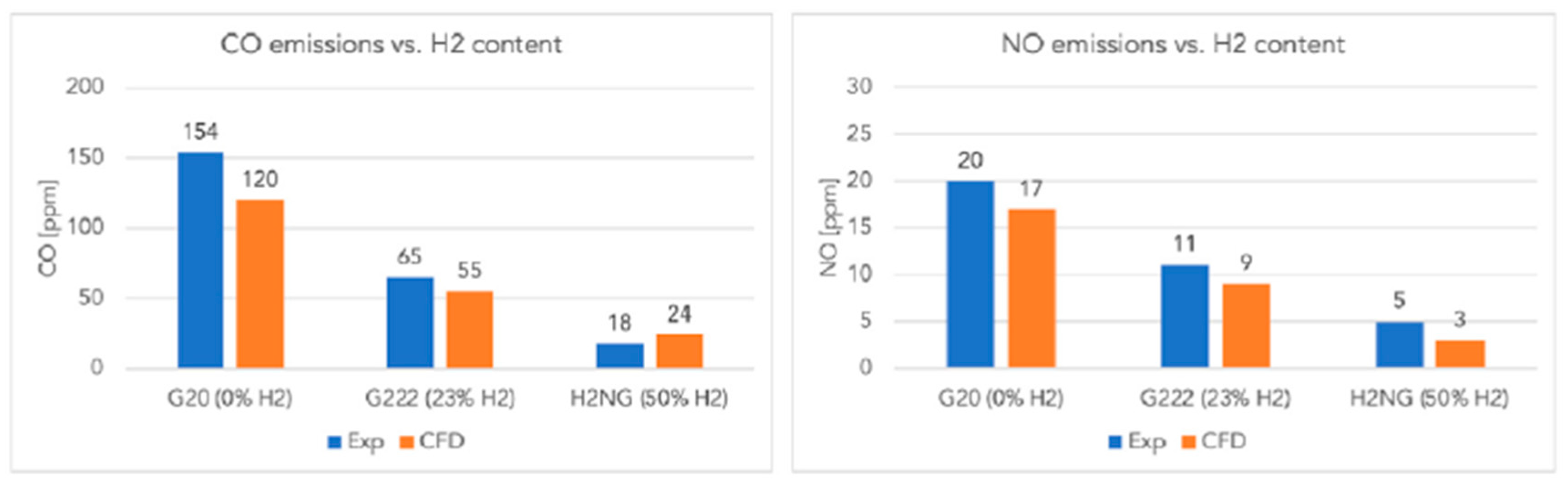
Similar results are reported by Lamioni [39], who showed, through CFD simulations validated experimentally, that hydrogen addition significantly reduces pollutant emissions in condensing boilers: CO decreases from 154 ppm (pure CH4) to 18 ppm (50% H2), and NO from 20 ppm to 5 ppm. Moreover, 23% H2 reduces CO2 emissions by about 8.7%, and 50% H2 by 24.2%, due to the more “diluted” combustion conditions (Figure 8).
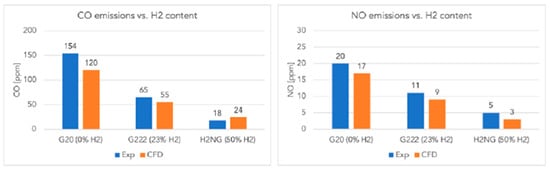
Figure 8.
CO and NO emissions in flue gases for different H2 content (reprinted with permission from [39]).
Consistent with Zhan, Siqi et al. [23] tested CH4/H2 mixtures up to 40% and observed both an increase in temperature with hydrogen fraction and a more uniform temperature distribution in the combustion chamber. The study also reports a pronounced local increase in NO emissions near the injection zone; however, the minimum NO level is obtained at 20% H2 (only a 14.7% increase compared to pure CH4), while at 30% H2 emissions rise by 47%, indicating an optimal limit around 20%.
Bălanescu et al. [40] analyzed the variation in adiabatic flame temperature as a function of hydrogen fraction and excess-air coefficient in a 25 kW condensing boiler. The results indicate that increasing both the H2 fraction and the excess air significantly reduces flame temperature, contributing to lower CO2 emissions and improved energy efficiency. Specifically, increases of up to 49% in condensate production and 47% in boiler energy savings and a 56.15% reduction in CO2 emission intensity were reported.
The study by Schiro et al. [41] shows that enriching natural gas with hydrogen reduces CO2 emissions and increases the amount of condensable water, which can enhance the efficiency of condensing boilers. However, hydrogen addition decreases volumetric heating value and requires higher fuel flow rates, while high concentrations introduce risks of flashback and undesired ignition. The main conclusion is that current boilers can operate safely and efficiently with approximately 20–23% H2, but higher proportions require design modifications to components and condensate-handling systems.
Wahiba et al. [42] investigated the performance of domestic heating systems fueled with hydrogen-enriched natural gas (HENG) and pure hydrogen. Their results show that thermal efficiency increases with hydrogen fraction due to the higher water-vapor content in the flue gases. However, excess air reduces exhaust-gas temperature and condensation efficiency. The best performance is achieved under stoichiometric combustion with pure hydrogen, though this condition increases pollutant-formation risks and requires well-controlled lean combustion.
Jankowski [43] evaluated the thermal performance and emissions of condensing boilers fueled with natural-gas/hydrogen mixtures up to 50% H2. The results show that hydrogen addition enhances convective heat transfer, flue-gas radiation, and energy recovery from water-vapor condensation (up to +6%). Overall efficiency increased by about 1.6 percentage points at 50% H2, without significant exhaust-gas losses. Pollutant emissions dropped sharply: NOx decreased to 2.7 mg/m3 and CO to 9.9 mg/m3, well below regulatory limits (33.5 mg/m3).
4. Research Examining Flame Geometry
Flame geometry is a key indicator of combustion behavior, strongly influencing heat transfer, stability, pollutant formation, and the overall performance of gas-fired appliances. Because hydrogen addition alters fundamental flame properties (such as burning velocity, diffusivity, and thermal release), its impact is often first observed through changes in flame height, shape, and attachment. Understanding these geometric transformations is therefore essential for evaluating the safe integration of hydrogen into domestic and small-scale combustion systems. The studies reviewed in this chapter investigate how hydrogen concentration, burner design, and operating conditions affect flame morphology across boilers, cooktop burners, and oven systems, providing insight into both the benefits and the operational constraints associated with hydrogen-enriched fuels.
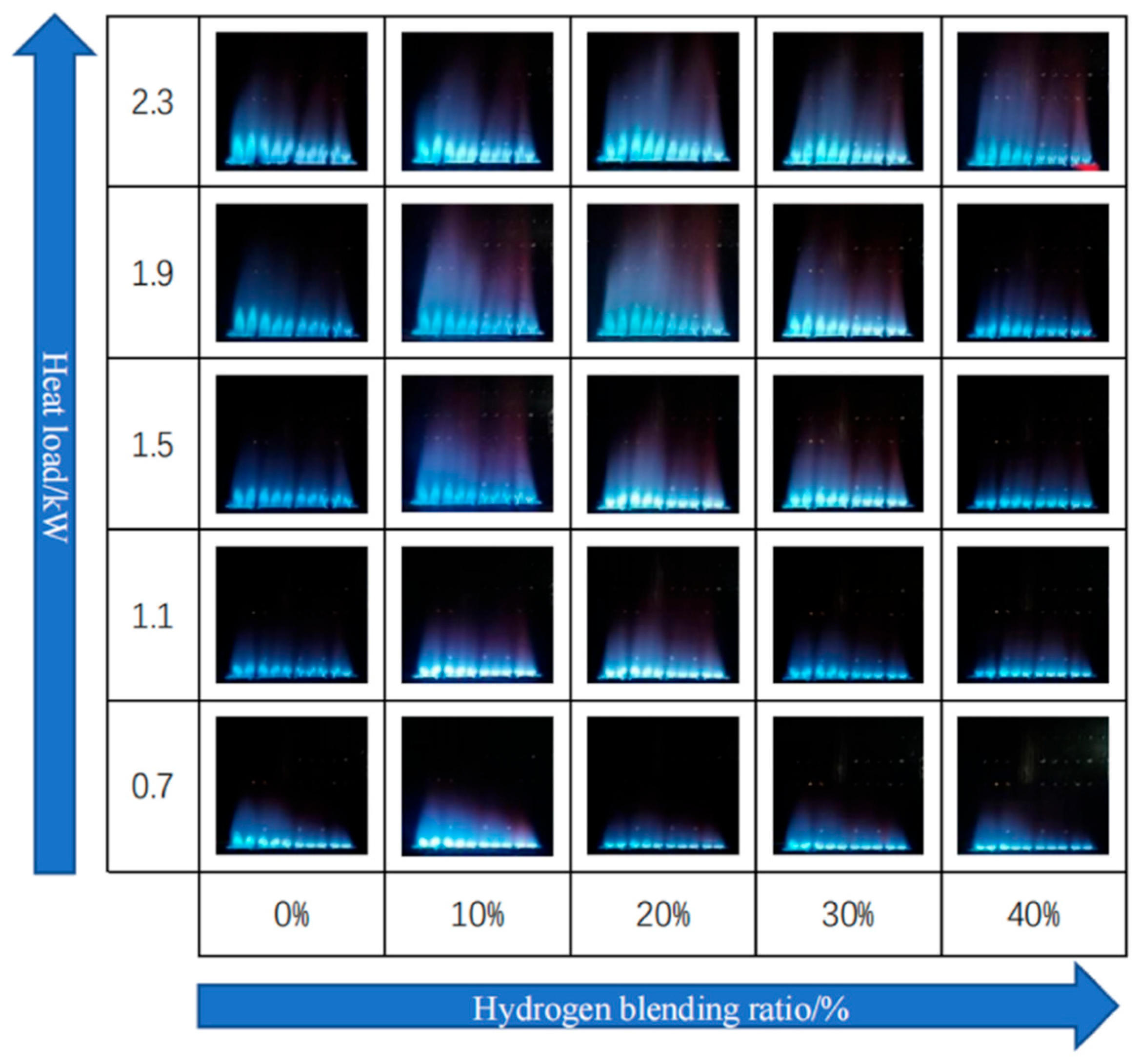
Zhan et al. [22] studied the impact of hydrogen concentrations ranging from 0 to 40% by volume in a domestic boiler operating within a thermal power range of 0.7 to 2.3 kW. They reported an initial increase followed by a decrease in flame height as the hydrogen fraction increased, for the same thermal input. Conversely, for a constant hydrogen concentration, increasing the thermal power resulted in a significant rise in flame height (Figure 9).
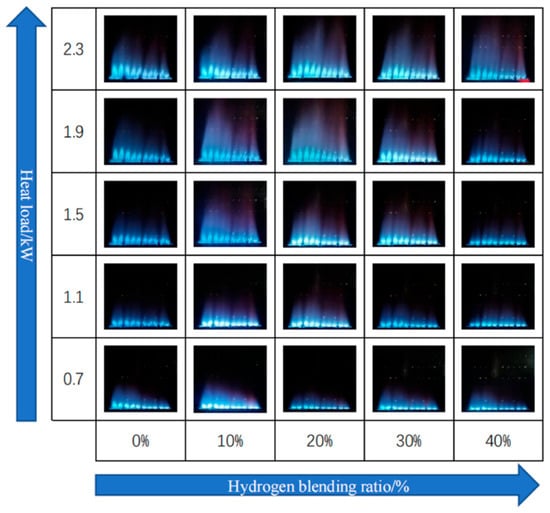
Figure 9.
Flame geometry for different thermal loads and hydrogen concentrations in a boiler (reprinted with permission from [22]).
Feith et al. [60] investigated the influence of six-burner-head geometry on flame characteristics and combustion performance in household cooking appliances operating with natural gas–hydrogen mixtures (30% H2 by volume). By testing six different burner configurations, the authors demonstrated that hydrogen addition leads to shorter and flatter flames, as well as a shift in flame color toward blue, indicating more complete and efficient combustion. Their results showed that burner heads with multiple orifices arranged both laterally and on the upper surface generate more stable and compact flames, with width-to-height ratios between 0.27 and 0.67, underscoring the importance of burner-head design in optimizing the use of hydrogen-based alternative fuels.
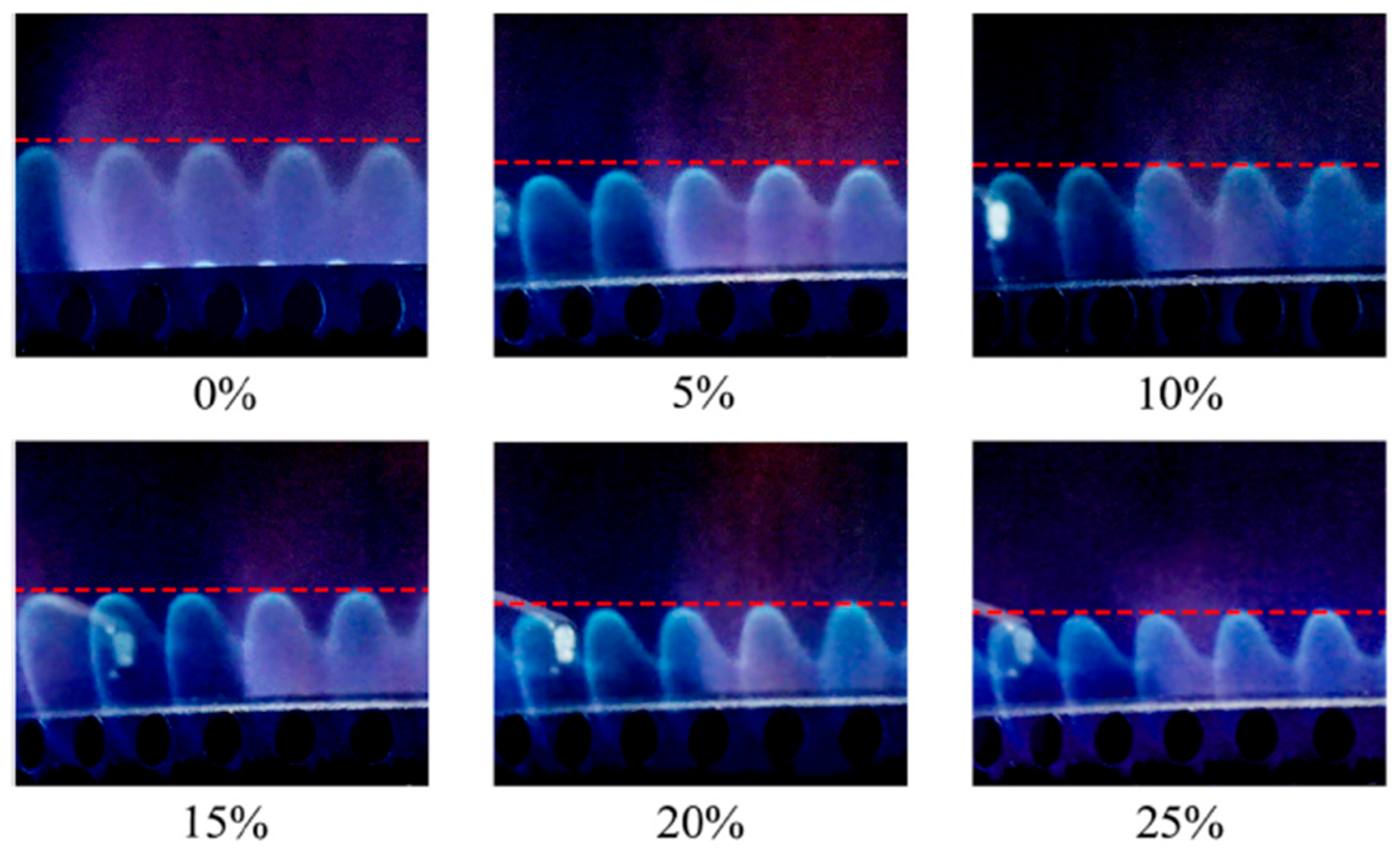
The study conducted by Zhao [44] evaluated the performance of an oven burner supplied with grid natural gas blended with hydrogen. The results showed that hydrogen addition reduces ignition time and CO emissions, but above 25% H2, the risk of flashback during ignition becomes significant. Under steady-state operation, the flame becomes shorter and stabilizes closer to the burner surface when the hydrogen fraction reaches 10%, leading to localized overheating of components (Figure 10). However, beyond this 10% threshold, the burner temperature does not increase substantially. NOx emissions remained practically unchanged, while the cyclic operating behavior of the oven produced complex temporal variations in emissions. The main conclusion is that hydrogen addition can improve ignition and reduce CO emissions, but safety constraints require that the hydrogen fraction should not exceed approximately 25% in residential applications.
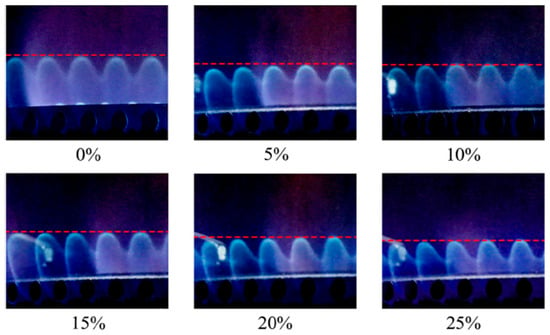
Figure 10.
Flame of TRPGS with different hydrogen addition (reprinted with permission from [35]).
Rachele et al. [61] investigated how different slit arrangements in perforated burner plates affect the structure and interaction of premixed methane–air flames in domestic condensing boilers. Using 3D CFD simulations, the authors compared a continuous series of slits with a grouped four-slit pattern and showed that slit geometry has a strong influence on flame shape and behavior. At low inlet velocities, flames remain separate and flat along each slit, while higher velocities cause neighboring flames to merge and form either a long wedge-shaped flame (continuous slits) or a single conical flame (grouped slits). These results demonstrate that burner slit design plays a crucial role in flame stabilization and pollutant formation, offering guidance for optimizing boiler performance under varying fuel compositions.
Restrepo et al. [62] examined the flame morphology of non-premixed natural gas–hydrogen mixtures, varying the hydrogen content from 0 to 100% and the air preheating temperature between 25 °C and 400 °C at a constant thermal input of 0.8 kW. The results showed that visible flame height consistently decreases with increasing hydrogen fraction, while air preheating produces a non-linear effect for blends up to 75% H2 and a linear height increase for pure hydrogen. Overall, hydrogen addition up to 50% does not significantly alter visible flame morphology compared to pure natural gas.
5. Other Studies
Beyond the core research areas addressed in previous sections, a broader set of experimental and numerical investigations provides additional insight into how hydrogen enrichment influences combustion behavior across diverse heating and industrial systems. These studies examine aspects that do not fit neatly into the categories of residential appliances, yet they offer valuable complementary evidence regarding the practical implications of hydrogen addition. The works reviewed here explore heating performance, fuel consumption, NOx formation mechanisms, turbulence effects, model validation, and the combined influence of hydrogen with oxygen enrichment or swirl-stabilized flow. Although many of these investigations involve semi-industrial or industrial-scale equipment, their findings remain relevant for understanding the fundamental combustion trends that also govern residential appliances. Collectively, they highlight both the benefits—such as improved stability, reduced carbon-based emissions, and enhanced heat-transfer rates—and the challenges—including increased NOx formation and higher component temperatures—that accompany the use of hydrogen-enriched natural gas in real-world combustion systems.
Fatih et al. [63] evaluated the heating time required to raise the temperature of 5 kg of water to 60 °C. Two identical burners were used: one supplied with natural gas and the other with three gas cylinders containing natural-gas mixtures with 10%, 20%, and 30% hydrogen. For pure natural gas, the measured heating time was 632 s. Adding 10% hydrogen increased the heating time by 13.4% (717 s). Increasing the hydrogen fraction to 20% and 30% did not lead to a linear rise in heating time, resulting instead in increases of only 15.8% (732 s) and 16.7% (738 s). The study also showed that using a mixture containing 20% hydrogen can reduce natural-gas consumption by 8% and improve burner efficiency by up to 44.4%.
Sun et al. [64] showed that hydrogen-enriched natural gas in industrial boilers introduces a practical balance between efficiency and NOx control. Field tests up to 9.7% H2 and simulations up to 30% H2 demonstrate that increasing both hydrogen content and excess air lowers theoretical flame temperature and reduces NOx (e.g., from 118 mg/m3 to 112 mg/m3, a 5% drop in tests, with larger reductions numerically). This comes at the cost of slightly higher flue-gas temperatures and a small efficiency loss (<1%). CO emissions remained within regulatory limits. Schwarz et al. [65] found that higher hydrogen fractions raise peak furnace temperature and elongate the flame, improving energy efficiency by up to 5.5%, but also increasing NOx by 167%. Salci et al. [66] reported that adding up to 20% H2 reduces CO2 by ~5.8%, enhances flame stability, and increases flame temperature, velocity, and pressure, while NO rises due to stronger OH-radical chemistry; maintaining λ = 1.3–1.4 is required for low-NOx operation. Yamei et al. [67] observed flame-temperature increases of +47 K, +52 K, +72 K, and +91 K for 15–60% H2, along with intensified turbulence and significant NO growth (+155% in-chamber, +146% at the outlet), while CO2 and CO fell by 37.5% and 70%. Kislinger [68] showed that achieving ~1060 °C requires less fuel with hydrogen (70 kW vs. 94 kW for methane), yielding ~13% faster heating due to stronger convection. Zouhaier [69] demonstrated that oxygen enrichment (20–50%) and hydrogen addition (≤15%) expand the stable-flame domain and suppress blow-off; hydrogen increases fuel-jet velocity (~15.6 m/s), improving mixing, while NOx rises sharply with oxygen (from ~90 ppm to ~331 ppm). Finally, simulations of NG/H2 combustion in a swirl-stabilized chamber [70] show that higher H2 fractions raise average and peak temperatures, reduce CO, and increase NO—especially at high primary-air ratios—while secondary-air injection effectively limits NO formation, even for pure hydrogen.
Weidinger [71] used experimentally validated CFD to assess NOx prediction accuracy for NG/H2 mixtures in a 358 kW forced-draft burner (λ = 1.2). The Steady Diffusion Flamelet model performed well up to 70% H2, whereas for 100% H2, the Flamelet Generated Manifold gave the best agreement, with only 1.3% deviation from measurements. The simulations also captured the rise in burner and flame-tube temperatures (~920–930 K), which drives thermal-NOx formation and poses potential material-integrity concerns.
Du et al. [72] used both experiments and CFD to study NG/H2 combustion in swirl burners and showed that increasing the swirl angle to 45° improved flame stability, produced a more uniform temperature field, and lowered CO and NO emissions. Also, extending the recirculation-zone length from 6 mm to 12 mm reduced NO by up to 36%.
Across all reviewed industrial-scale investigations, a consistent picture emerges: hydrogen enrichment simultaneously enhances combustion reactivity, flame speed, and thermal efficiency, while also intensifying temperature-driven NOx formation. Studies show that even moderate H2 fractions increase flame temperature, modify flame geometry, accelerate heat transfer, and reduce CO and CO2 emissions—yet they also raise NOx unless excess-air levels, staging, or swirl-induced mixing are carefully controlled. These works also highlight the importance of aerodynamics (swirl, jet velocity gradients, turbulence intensity), premixing quality, and burner geometry in stabilizing H2-containing flames and preventing flashback.
Although these studies focus on industrial burners, their conclusions remain highly relevant for domestic HENG appliances. Domestic cooktops, boilers, and ovens operate at lower thermal loads but rely on the same fundamental mechanisms: preferential diffusion, altered flame speed, boundary-layer heating, and sensitivity to air–fuel mixing. The industrial findings reinforce the need for precise control of primary-air entrainment, burner temperature, and mixing quality in household systems, especially as hydrogen fractions approach the practical limits (20–30%). They also support the central argument of this research: safe and efficient HENG operation in domestic appliances requires burner designs that explicitly account for hydrogen-induced changes in flame stabilization, flashback propensity, and NOx formation pathways.
6. Discussion
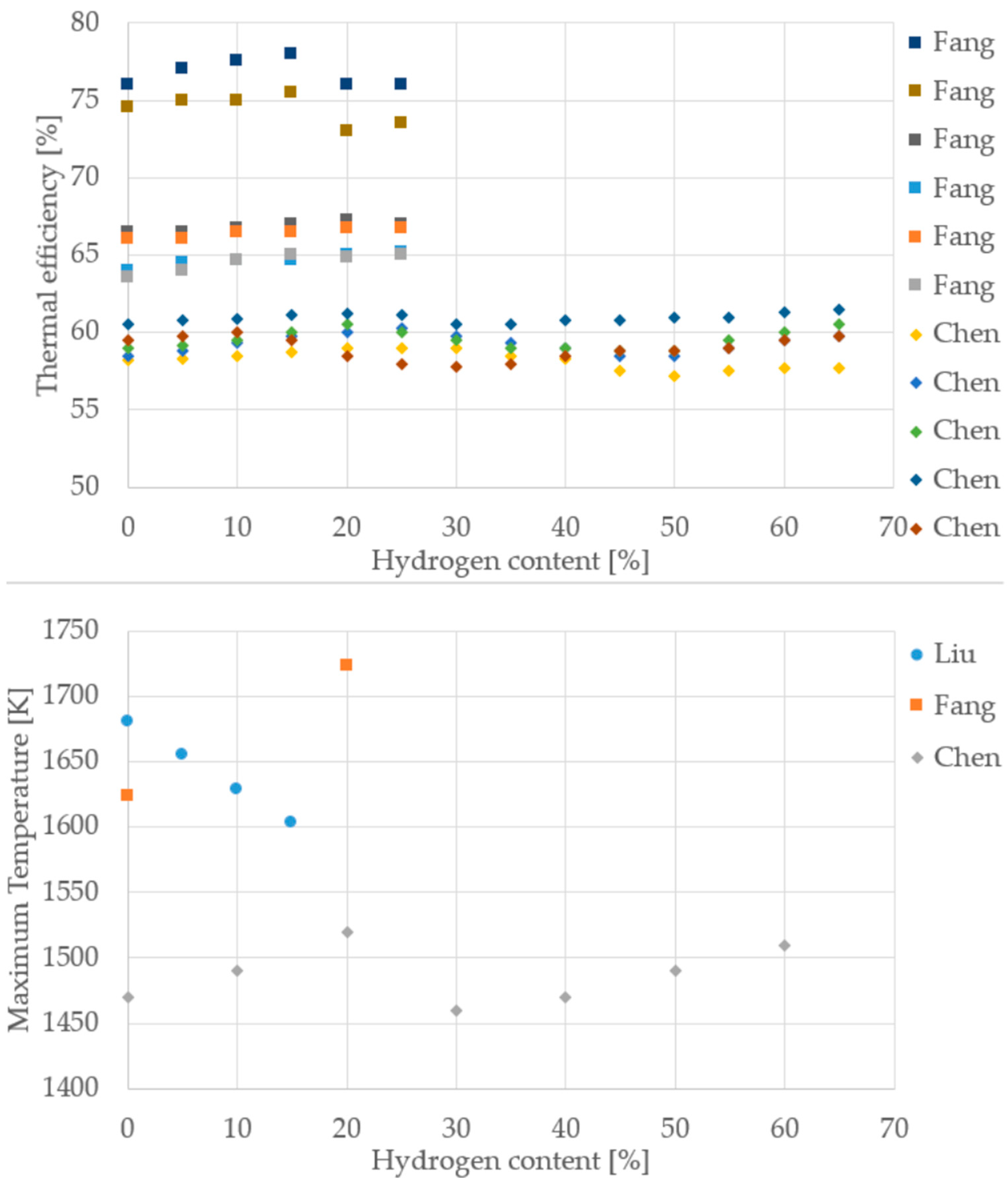
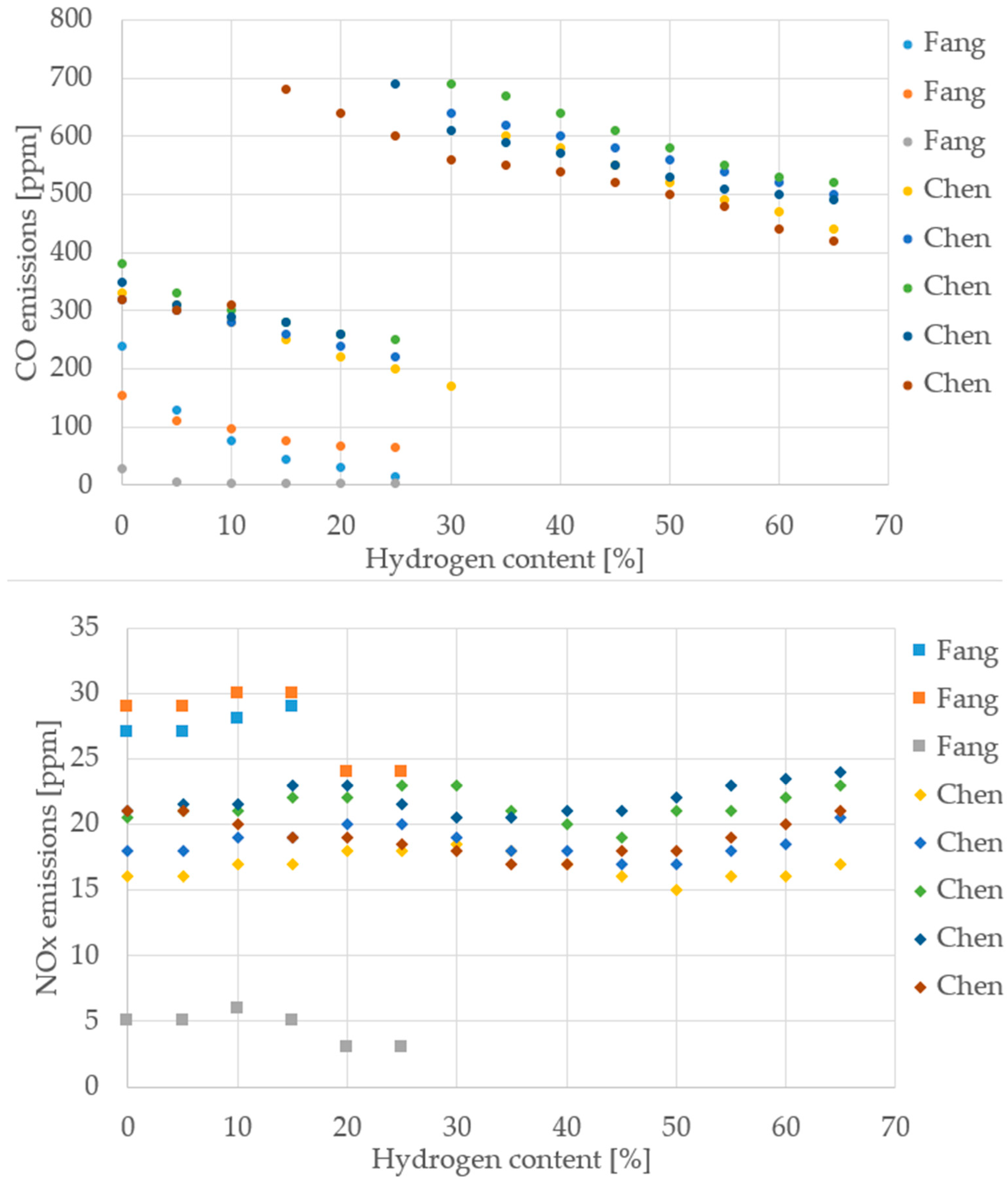
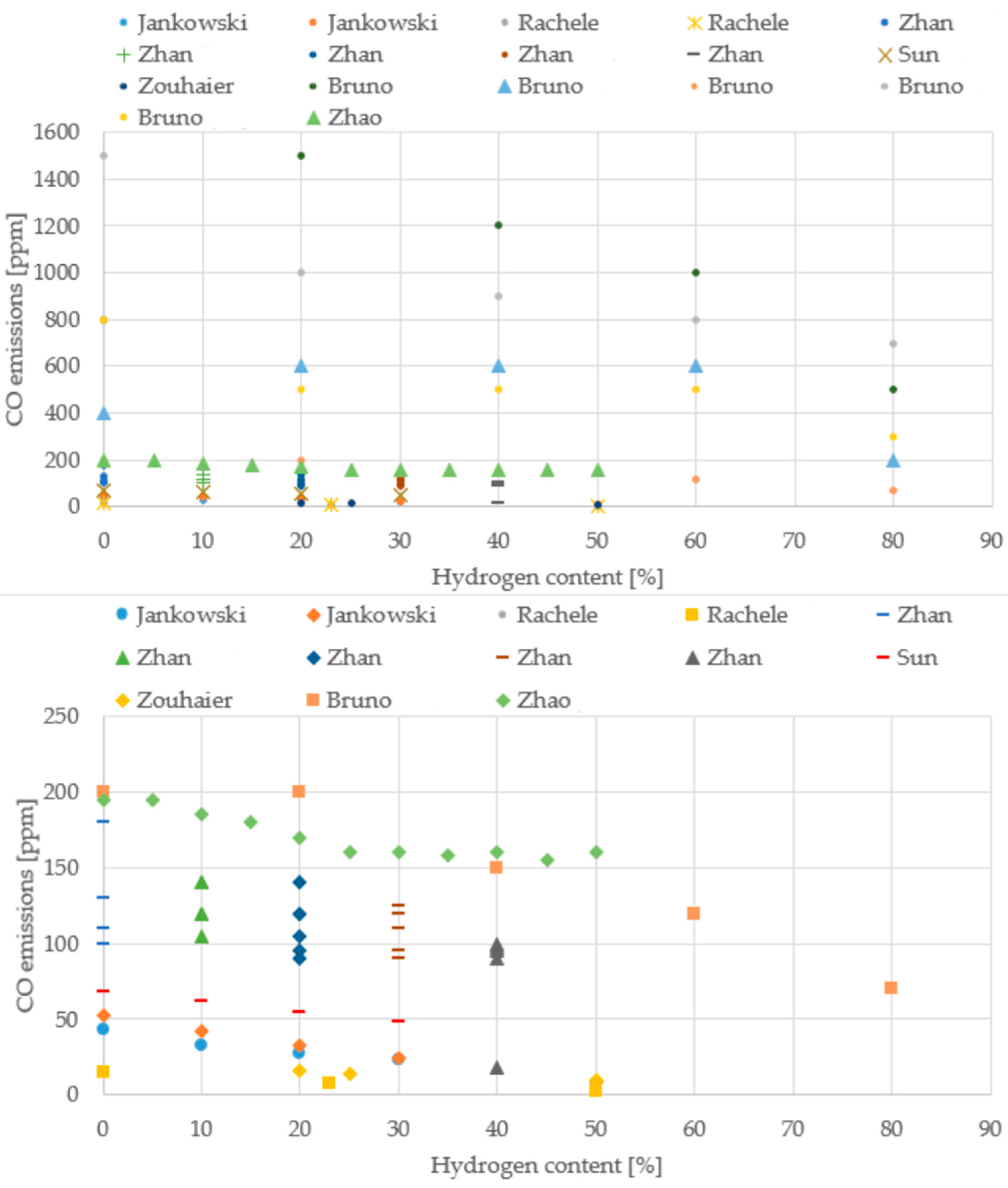
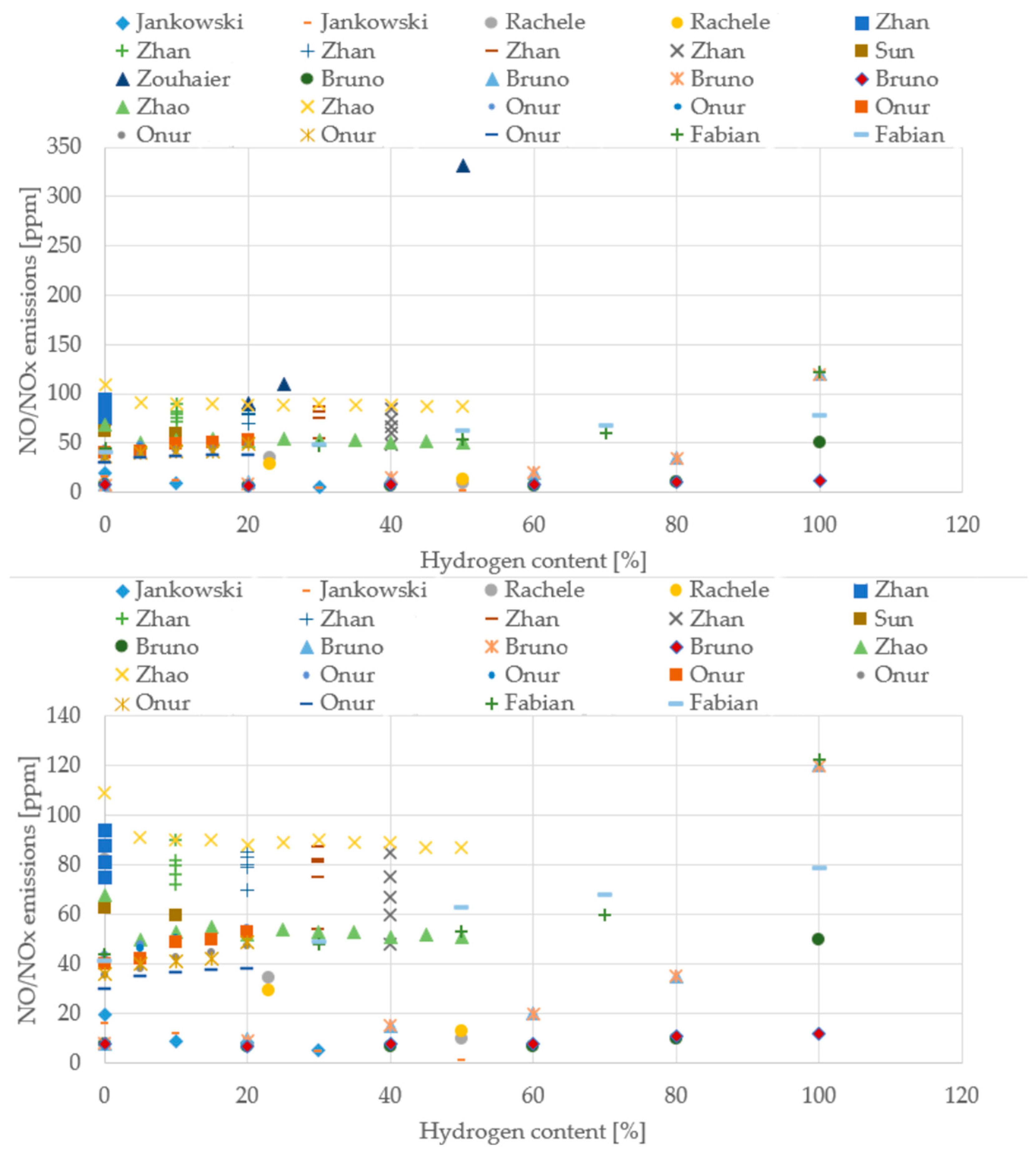
To move beyond qualitative statements and provide a robust foundation for the conclusions, quantitative trends were extracted from the literature data compiled in this review, with a focus on the changes in thermal efficiency, CO emissions, and NO/NOx emissions as a function of the hydrogen content in the fuel blend. The resulting normalized datasets are presented visually in Figure 11, Figure 12, Figure 13 and Figure 14 to allow for a direct, quantitative comparison of performance and emission metrics across different experimental setups and research groups.
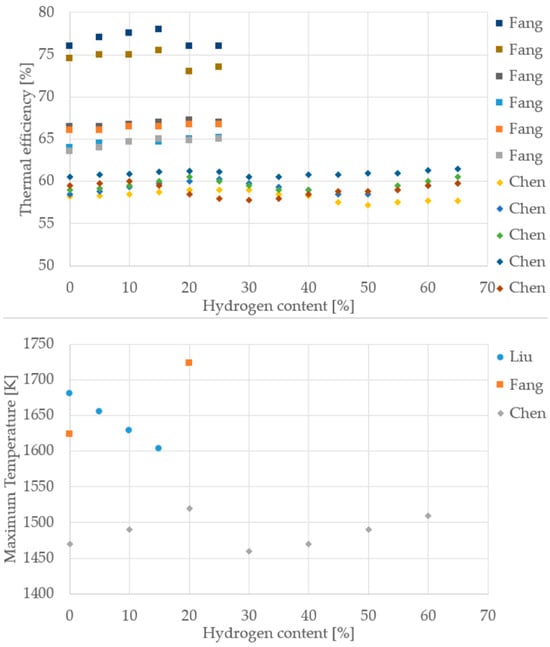
Figure 11.
Thermal efficiency and maximum temperature from different cooktop burner studies (Data from [10,35,37]).
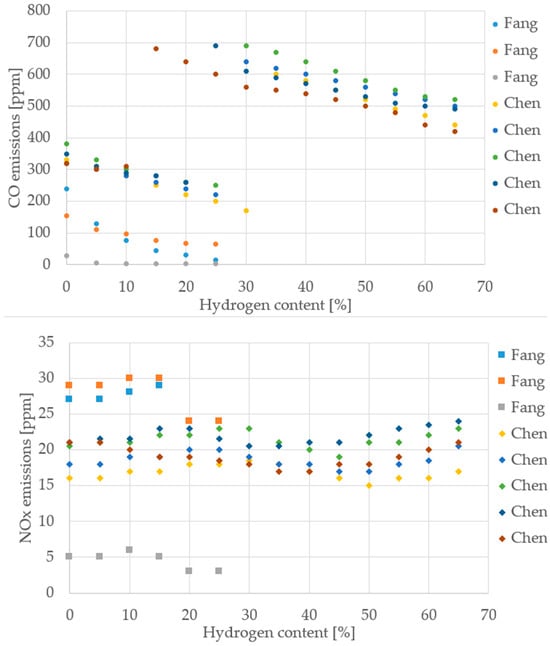
Figure 12.
CO and NOx emissions from different cooktop burner studies (Data from [35,37]).
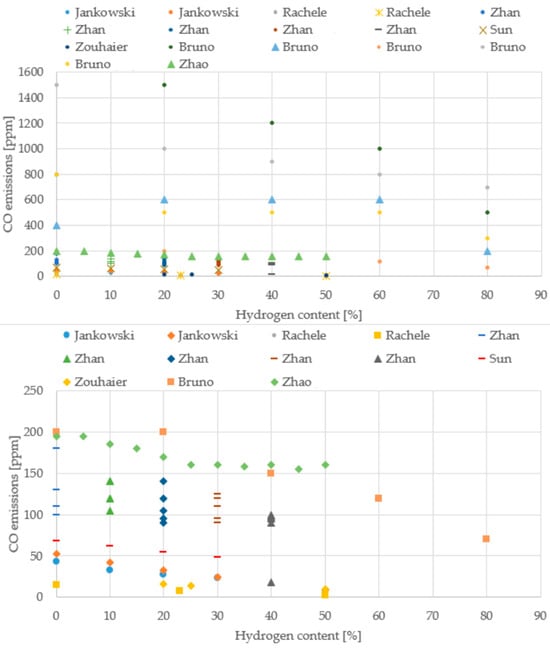
Figure 13.
CO emissions from different boiler studies (Data from [20,22,39,43,64,69,70].
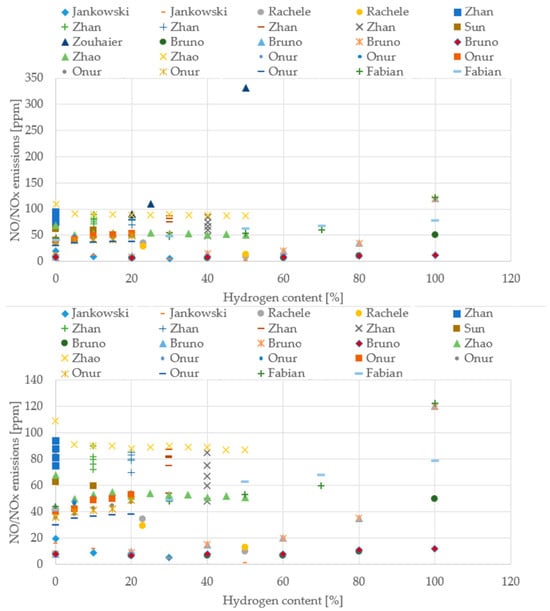
Figure 14.
NO/NOx emissions from different boiler studies (Data from [20,22,39,43,64,66,69,70,71]).
It is important to note the limitations regarding data availability for specific applications. A particular gap was observed concerning cooktop burners, where the number of available studies providing detailed, quantifiable trends for emissions and efficiency was significantly low. Furthermore, for both cooktop burners and boilers, the extraction of consistent temperature trends proved challenging. This difficulty stemmed from the wide variance in reported temperature values across different studies, primarily due to the non-standardized measurement methodologies. Each researcher typically selected the measurement location (e.g., flame core, near the injector, or exhaust gas stream) based on the specific focus of their application, leading to values that differed substantially and made direct, normalized comparison challenging.
Despite differences in burner design, measurement protocols, and operating conditions, the reviewed cooktop studies consistently show that increasing hydrogen content improves thermal efficiency and reduces CO emissions. This trend is evident across both Fang [35] and Chen [37], with thermal efficiency rising steadily up to 70% H2 and CO emissions dropping sharply. NOx behavior is more variable: while some datasets suggest a slight increase, others show stable or even reduced values, indicating that NOx sensitivity depends heavily on burner geometry and air–fuel mixing. For maximum temperature, the data are harder to interpret due to inconsistent measurement points and methods, as previously discussed. Nonetheless, the overall direction remains clear: hydrogen enrichment enhances combustion performance and lowers carbon emissions.
Across the boiler studies, the experimental setups vary widely (different burner geometries, firing rates, excess-air levels, and measurement bases), but the overall behavior with hydrogen addition remains remarkably consistent. CO emissions steadily decline as the hydrogen share increases, even though the absolute values span a broad interval, typically between 10 and 200 ppm, depending on the burner and operating point. In contrast, NO/NOx emissions cluster within a much narrower band, generally around 40–90 ppm, and show no single universal trend: some configurations rise slightly with hydrogen, others remain flat, and a few even decrease. This variability reflects the strong influence of local flame temperature, mixing intensity, and air staging. Attempts to extract a clear temperature trend are limited by the fact that each study measures temperature at different locations or using different definitions, making direct comparison difficult. Even so, the collective evidence points toward cleaner and more efficient combustion as hydrogen content increases.
7. Conclusions
This review consolidates current findings on the behavior of hydrogen-enriched natural gas in residential combustion systems, revealing both the opportunities and the technical challenges associated with hydrogen integration.
- Flashback remains the primary safety constraint.
Hydrogen’s high burning velocity and diffusivity significantly increase flashback risk, with domestic appliances typically tolerating 20–25% H2 and boilers up to ~40% under controlled conditions. Studies consistently show that burner wall temperature, preferential diffusion effects, and mixture composition play decisive roles, underscoring the need for improved predictive models and burner redesigns.
- Efficiency and emissions exhibit clear but non-linear trends.
Hydrogen addition generally produces hotter, faster, and more compact flames, improving thermal efficiency and reducing CO and CO2 emissions. However, NOx formation tends to rise with increasing hydrogen fraction due to elevated flame temperatures, unless mitigated through excess air, staged combustion, or optimized burner geometry.
- Flame geometry is highly sensitive to hydrogen content.
Across boilers, cooktop burners, and oven systems, hydrogen consistently shortens and intensifies the flame, alters attachment behavior, and increases the risk of component overheating. Burner design, particularly orifice distribution, swirl intensity, and recirculation-zone length, plays a critical role in stabilizing H2-enriched flames.
- Additional experimental studies confirm broader system-level impacts.
Research on heating performance, condensate production, oxygen enrichment, and swirl-stabilized combustion shows that hydrogen can enhance efficiency and reduce carbon-based emissions, but may require higher fuel flow rates, improved cooling, and stricter control of air distribution to maintain safe operation.
- Practical blending limits for current residential appliances lie around 20–30% H2.
Most studies converge on the conclusion that existing domestic systems can safely and efficiently operate with moderate hydrogen fractions, while higher concentrations demand targeted design modifications, updated certification procedures, and more advanced combustion-control strategies.
Overall, hydrogen-enriched natural gas represents a viable transitional pathway toward low-carbon residential energy systems, provided that safety constraints, especially flashback and NOx formation, are addressed through burner optimization, improved modeling, and updated regulatory frameworks. Continued research is essential to refine blending limits, develop hydrogen-ready appliance designs, and support the broader decarbonization of residential heating and cooking. Future research should prioritize predictive flashback models that incorporate preferential diffusion and wall-temperature coupling, hydrogen-ready burner designs validated under real-appliance operating conditions, updated interchangeability metrics beyond the Wobbe Index, and harmonized standards for testing and certifying H2-NG appliances.
Author Contributions
Conceptualization, methodology, T.M.S., C.E.I. and T.P.; software, T.M.S. and C.E.I.; validation, T.M.S. and C.E.I.; formal analysis, T.M.S. and C.E.I.; investigation, T.M.S. and C.E.I.; resources, T.M.S. and C.E.I.; data curation, T.M.S. and C.E.I.; writing—original draft preparation, T.P. and C.E.I.; writing—review and editing, T.P.; visualization, T.M.S. and C.E.I.; supervision, T.P.; project administration, T.P.; funding acquisition, T.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the project “Hub-ul Român de Hidrogen și Noi Tehnologii Energetice—Ro-HydroHub,” contract no. G 2024-81692/390006/13.11.2024, SMIS code 351358 (304724), funded by the European Regional Development Fund under the Program for Intelligent Growth, Digitization, and Financial Instruments.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
During the preparation of this manuscript, the authors used AI-assisted language tools (Microsoft Copilot, https://copilot.microsoft.com/) for the purposes of text refinement, including grammar correction, clarity improvements, and rephrasing. The authors have reviewed and edited all AI-generated output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CFD | Computational Fluid Dynamics |
| CH4 | Methane |
| CO | Carbon Monoxide |
| CO2 | Carbon Dioxide |
| H2 | Hydrogen |
| HENG | Hydrogen Enriched Natural Gas |
| NFPA | National Fire Protection Association |
| NG | Natural Gas |
| NOx | Nitrogen Oxides |
| OH* | Excited Hydroxyl Radical |
| RPMGS | Radiant Porous Media Gas Stove |
| SSPGS | Swirling Strip Port Gas Stove |
| TRPGS | Typical Round Port Gas Stove |
| WTC | Well to Combustion |
| WI | Wobbe Index (Fuel Interchangeability Index) |
References
- United Nations. Paris Agreement, United Nations Framework Convention on Climate Change. 2015. Available online: https://unfccc.int/process-and-meetings/the-paris-agreement/the-paris-agreement (accessed on 31 December 2025).
- Wright, M.L.; Lewis, A.C. Emissions of NOx from blending of hydrogen and natural gas in space heating boilers. Elem. Sci. Anthr. 2022, 10, 00114. [Google Scholar] [CrossRef]
- Franco, A.; Rocca, M. Industrial Decarbonization through Blended Combustion of Natural Gas and Hydrogen. Hydrogen 2024, 5, 519–539. [Google Scholar] [CrossRef]
- British Petroleum Report, Statistical Review of World Energy. 70th Edition. 2021. Available online: https://www.energyinst.org/statistical-review (accessed on 31 December 2025).
- Quinn, A.K.; Bruce, N.; Puzzolo, E.; Dickinson, K.; Sturke, R.; Jack, D.W.; Mehta, S.; Shankar, A.; Sherr, K.; Rosenthal, J.P. An Analysis of Efforts to Scale up Clean Household Energy for Cooking around the World. Energy Sustain. Dev. 2018, 46, 1−10. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, W.; Yang, T.; Yu, Y.; Liu, C.; Li, B. Numerical and Experimental Investigation on Heat Transfer Enhancement by Adding Fins on the Pot in a Domestic Gas Stove. Energy 2022, 239, 122439. [Google Scholar] [CrossRef]
- Zhao, N.; Li, B.; Li, H.; Ahmad, R.; Peng, K.; Chen, D.; Yu, X.; Zhou, Y.; Dong, R.; Wang, H.; et al. Field-Based Measurements of Natural Gas Burning in Domestic Wall-Mounted Gas Stove and Estimates of Climate, Health and Economic Benefits in Rural Baoding and Langfang Regions of Northern China. Atmos. Environ. 2020, 229, 117454. [Google Scholar] [CrossRef]
- Popkova, E.G.; Sergi, B.S. Energy Efficiency in Leading Emerging and Developed Countries. Energy 2021, 221, 119730. [Google Scholar] [CrossRef]
- Acharya, R.H.; Sadath, A.C. Energy Poverty and Economic Development: Household-Level Evidence from India. Energy Build. 2019, 183, 785−791. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, G.; Asim, T.; Mishra, R. Combustion characterization of hybrid methane-hydrogen gas in domestic swirl stoves. Fuel 2023, 333, 126413. [Google Scholar] [CrossRef]
- Elasu, J.; Ntayi, J.; Adaramola, M.S.; Buyinza, F. Drivers of Household Transition to Clean Energy Fuels: A Systematic Review of Evidence. Renew. Sustain. Energy Transit. 2023, 3, 100047. [Google Scholar] [CrossRef]
- Lebel, E.D.; Finnegan, C.J.; Ouyang, Z.; Jackson, R.B. Methane and NOx Emissions from Natural Gas Stoves, Cooktops, and Ovens in Residential Homes. Environ. Sci. Technol. 2022, 56, 2529−2539. [Google Scholar] [CrossRef]
- Haeseldonckx, D.; Dhaeseleer, W. The use of the natural-gas pipeline infrastructure for hydrogen transport in a changing market structure. Int. J. Hydrogen Energy 2007, 32, 1381–1386. [Google Scholar] [CrossRef]
- Field, R.A.; Derwent, R.G. Global warming consequences of replacing natural gas with hydrogen in the domestic energy sectors of future low-carbon economies in the United Kingdom and the United States of America. Int. J. Hydrogen Energy 2021, 46, 30190–30203. [Google Scholar] [CrossRef]
- Di Lullo, G.; Oni, A.O.; Kumar, A. Blending blue hydrogen with natural gas for direct consumption: Examining the effect of hydrogen concentration on transportation and well-to combustion greenhouse gas emissions. Int. J. Hydrogen Energy 2021, 46, 19202–19216. [Google Scholar] [CrossRef]
- Gao, W.; Hu, Y.; Yan, R.; Yan, W.; Yang, M.; Miao, Q.; Yang, L.; Wang, Y. Comprehensive Review on Thermal Performance Enhancement of Domestic Gas Stoves. ACS Omega 2023, 8, 26663–26684. [Google Scholar] [CrossRef] [PubMed]
- Flores-Montoya, E.; Aniello, A.; Schuller, T.; Selle, L. Predicting flashback limits in H2 enriched CH4/air and C3H8/air laminar flames. Combust. Flame 2023, 258, 113055. [Google Scholar] [CrossRef]
- Fruzza, F.; Lamioni, R.; Tognotti, L.; Galletti, C. Flashback of H2-enriched premixed flames in perforated burners: Numerical prediction of critical velocity. Int. J. Hydrogen Energy 2023, 48, 31790–31801. [Google Scholar] [CrossRef]
- Fruzza, F.; Lamioni, R.; Mariotti, A.; Salvetti, M.V.; Galletti, C. Flashback propensity due to hydrogen blending in natural gas: Sensitivity to operating and geometrical parameters. Fuel 2024, 362, 130838. [Google Scholar] [CrossRef]
- Zhao, Y.; Mcdonell, V.; Samuelsen, S. Influence of hydrogen addition to pipeline natural gas on the combustion performance of a cooktop burner. Int. J. Hydrogen Energy 2019, 44, 12239–12253. [Google Scholar] [CrossRef]
- Sun, M.; Huang, X.; Hu, Y.; Lyu, S. Effects on the performance of domestic gas appliances operated on natural gas mixed with hydrogen. Energy 2021, 244, 122557. [Google Scholar] [CrossRef]
- Zhan, X.; Chen, Z.; Qin, C. Effect of hydrogen-blended natural gas on combustion stability and emission of water heater burner. Case Stud. Therm. Eng. 2022, 37, 102246. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Jin, H.; Liu, Y.; Wu, Y. A Numerical Simulation Study on the Combustion of Natural Gas Mixed with Hydrogen in a Partially Premixed Gas Water Heater. Energies 2024, 17, 4069. [Google Scholar] [CrossRef]
- Mayrhofer, M.; Koller, M.; Seemann, P.; Prieler, R.; Hochenauer, C. Assessment of natural gas/hydrogen blends as an alternative fuel for industrial heat treatment furnaces. Int. J. Hydrogen Energy 2021, 46, 21672–21686. [Google Scholar] [CrossRef]
- Miao, H.; Lu, L.; Huang, Z. Flammability limits of hydrogen-enriched natural gas. Int. J. Hydrogen Energy 2011, 36, 6937–6947. [Google Scholar] [CrossRef]
- Aniello, A.; Poinsot, T.; Selle, L.; Schuller, T. Hydrogen substitution of natural-gas in premixed burners and implications for blow-off and flashback limits. Int. J. Hydrogen Energy 2022, 47, 33067–33081. [Google Scholar] [CrossRef]
- Kıymaz, T.B.; Böncü, E.; Güleryüz, D.; Karaca, M.; Yılmaz, B.; Allouis, C.; Gökalp, I. Numerical investigations on flashback dynamics of premixed methane-hydrogen-air laminar flames. Int. J. Hydrogen Energy 2022, 47, 25022–25033. [Google Scholar] [CrossRef]
- Lewis, B.; von Elbe, G. Stability and structure of burner flames. J. Chem. Phys. 1943, 11, 75–97. [Google Scholar] [CrossRef]
- Putnam, A.A.; Jensen, R.A. Application of dimensionless numbers to flash-back and other combustion phenomena. Symp. Combust. Flame Explos. Phenom. 1948, 3, 89–98. [Google Scholar] [CrossRef]
- Hoferichter, V.; Hirsch, C.; Sattelmayer, T. Prediction of boundary layer flashback limits of laminar premixed jet flames. In Proceedings of the ASME Turbo Expo 2018: Turbomachinery Technical Conference and Exposition, Oslo, Norway, 11–15 June 2018. [Google Scholar] [CrossRef]
- de Vries, H.; Mokhov, A.V.; Levinsky, H.B. The impact of natural gas/hydrogen mixtures on the performance of end-use equipment: Interchangeability analysis for domestic appliances. Appl. Energy 2017, 208, 1007, 19. [Google Scholar] [CrossRef]
- Cuoci, A.; Frassoldati, A.; Cozzi, F. Numerical predictions of flashback limits of H2-enriched methane/air premixed laminar flames. Proc. Combust. Inst. 2024, 40, 105696. [Google Scholar] [CrossRef]
- Leicher, J.; Schaffert, J.; Cigarida, H.; Tali, E.; Burmeister, F.; Giese, A.; Albus, R.; Görner, K.; Carpentier, S.; Milin, P. The Impact of Hydrogen Admixture into Natural Gas on Residential and Commercial Gas Appliances. Energies 2022, 15, 777. [Google Scholar] [CrossRef]
- Xie, Y.; Qin, C.; Chen, Z.; Duan, P.; Guo, S. The Impact of Hydrogen Addition to Natural Gas on Flame Stability. Int. J. Hydrogen Energy 2022, 47, 35851−35863. [Google Scholar] [CrossRef]
- Fang, Z.; Zhang, S.; Huang, X.; Hu, Y.; Xu, Q. Performance of three typical domestic gas stoves operated with methane-hydrogen mixture. Case Stud. Therm. Eng. 2023, 41, 102631. [Google Scholar] [CrossRef]
- Öztürk, A.T.; Akal, D.; Akyol, U. Investigation of the emission values of butane, methane and methane-hydrogen mixtures used in household stove burners. Int. J. Hydrogen Energy 2025, 141, 88–98. [Google Scholar] [CrossRef]
- Chen, Y.; Niu, J.; Liu, W.; Long, L.; Huang, T.; Sun, Y.; Wan, Z.; Yu, B. Experimental analysis and modeling on the blending limit of domestic burner with porous media for hydrogen enriched natural gas. Int. J. Hydrogen Energy 2024, 88, 1321–1331. [Google Scholar] [CrossRef]
- Liu, X.Y.; Huang, G.L.; Zhen, H.S.; Wei, Z.L. Experimental testing and geometric optimization of a domestic cooker burning 80%Natural-Gas+20%Hydrogen. Int. J. Hydrogen Energy 2024, 85, 281–286. [Google Scholar] [CrossRef]
- Lamioni, R.; Bronzoni, C.; Folli, M.; Tognotti, L.; Galletti, C. Impact of H2-enriched natural gas on pollutant emissions from domestic condensing boilers: Numerical simulations of the combustion chamber. Int. J. Hydrogen Energy 2023, 48, 19686–19699. [Google Scholar] [CrossRef]
- Bălănescu, D.T.; Homutescu, V.M. Effects of hydrogen-enriched methane combustion on latent heat recovery potential and environmental impact of condensing boilers. Appl. Therm. Eng. 2021, 197, 117411. [Google Scholar] [CrossRef]
- Schiro, F.; Stoppato, A.; Benato, A. Modelling and analyzing the impact of hydrogen enriched natural gas on domestic gas boilers in a decarbonization perspective. Carbon Resour. Convers. 2020, 3, 122–129. [Google Scholar] [CrossRef]
- Yaïci, W.; Longo, M. Performance analysis of domestic boilers fuelled with hydrogen-enriched natural gas blends and pure hydrogen. Energy 2025, 322, 135536. [Google Scholar] [CrossRef]
- Rimar, M.; Kizek, J.; Varga, A.; Fedák, M.; Jablonský, G. The influence of hydrogen concentration in natural gas on heat flows in a thermal aggregate. MM Sci. J. 2022, 2022, 6225–6232. [Google Scholar] [CrossRef]
- Zhao, Y.; Mcdonell, V.; Samuelsen, S. Experimental assessment of the combustion performance of an oven burner operated on pipeline natural gas mixed with hydrogen. Int. J. Hydrogen Energy 2019, 44, 26049–26062. [Google Scholar] [CrossRef]
- Jones, D.; Al-Masry, W.; Dunnill, C. Hydrogen-enriched natural gas as a domestic fuel: An analysis based on flash-back and blow-off limits for domestic natural gas appliances within the UK. Sustain. Energy Fuels 2017, 2, 710–723. [Google Scholar] [CrossRef]
- Makaryan, I.A.; Sedov, I.V.; Salgansky, E.A.; Arutyunov, A.V.; Arutyunov, V.S. A Comprehensive Review on the Prospects of Using Hydrogen-Methane Blends: Challenges and Opportunities. Energies 2022, 15, 2265. [Google Scholar] [CrossRef]
- Yilmaz, H.; Schröder, L.; Hillenbrand, T.; Brüggemann, D. Effects of Hydrogen Addition on Combustion and Flame Propagation Characteristics of Laser Ignited Methane/Air Mixtures. Int. J. Hydrogen Energy 2023, 48, 17324−17338. [Google Scholar] [CrossRef]
- Yang, X.; Wang, T.; Zhang, Y.; Zhang, H.; Wu, Y.; Zhang, J. Hydrogen Effect on Flame Extinction of Hydrogen-Enriched Methane/Air Premixed Flames: An Assessment from the Combustion Safety Point of View. Energy 2022, 239, 122248. [Google Scholar] [CrossRef]
- Ren, F.; Chu, H.; Xiang, L.; Han, W.; Gu, M. Effect of hydrogen addition on the laminar premixed combustion characteristics the main components of natural gas. J. Energy Inst. 2019, 92, 1178–1190. [Google Scholar] [CrossRef]
- Nosek, R.; Zvada, B.; Ďurčanský, P.; Kantová, N.Č.; Mičko, P. Numerical Analysis of Hydrogen-Enriched Natural Gas on Combustion and Emission Characteristics. Arab. J. Sci. Eng. 2025, 50, 13745–13754. [Google Scholar] [CrossRef]
- Wojtowicz, R. An analysis of the effects of hydrogen addition to natural gas on the work of gas appliances. Naft.-Gaz 2019, 75, 465–473. [Google Scholar] [CrossRef]
- Deymi-Dashtebayaz, M.; Rezapour, M.; Afshoun, H.R.; Sheikhani, H.; Barzanooni, V. Optimum swirl angle of natural gas combustion in domestic cooker burner with various output port. Proc. Inst. Mech. Eng. J. Mech. Eng. Sci. 2022, 236, 11571–11585. [Google Scholar] [CrossRef]
- Singh, A.; Yadav, V.K.; Amardeep; Maddheshiya, M.K.; Sharma, S.; Jha, M.; Singh, P. Experimental and Computational Analysis of Household Cook Stoves: A Review. In Recent Trends in Thermal Engineering; Springer: Singapore, 2022; pp. 89–101. [Google Scholar] [CrossRef]
- Hou, S.S.; Chou, C.H. Parametric Study of High-Efficiency and Low-Emission Gas Burners. Adv. Mater. Sci. Eng. 2013, 2013, 154957. [Google Scholar] [CrossRef]
- Zhen, H.S.; Leung, C.W.; Wong, T.T. Improvement of domestic cooking flames by utilizing swirling flows. Fuel 2014, 119, 153–156. [Google Scholar] [CrossRef]
- Matthujak, A.; Wichangarm, M.; Sriveerakul, T.; Sucharitpwatskul, S.; Phongthanapanich, S. Numerical investigation on the influences of swirling flow to thermal efficiency enhancement of an LPG-energy saving burner. Case Stud. Therm. Eng. 2021, 28, 101466. [Google Scholar] [CrossRef]
- Kotb, A.; Saad, H. Case study for co and counter swirling domestic burners. Case Stud. Therm. Eng. 2018, 11, 98–104. [Google Scholar] [CrossRef]
- Xin, Y.; Wang, K.; Zhang, Y.; Zeng, F.; He, X.; Takyi, S.A.; Tontiwachwuthikul, P. Numerical Simulation of Combustion of Natural Gas Mixed with Hydrogen in Gas Boilers. Energies 2021, 14, 6883. [Google Scholar] [CrossRef]
- Bundesministerium für Umwelt, Naturschutz und Reaktorsicherheit. Technische Anleitung zur Reinhaltung der Luft (TA Luft); Gemeinsames Ministerialblatt; Bundesministerium für Umwelt, Naturschutz und Reaktorsicherheit: Berlin, Germany, 2022.
- Sorgulu, F.; Ozturk, M.; Javani, N.; Dincer, I. Effect of burner head geometry on flame dispersion in gas stoves with hydrogen and natural gas blends. Process Saf. Environ. Prot. 2024, 183, 1135–1151. [Google Scholar] [CrossRef]
- Lamioni, R.; Bronzoni, C.; Folli, M.; Tognotti, L.; Galletti, C. Effect of slit pattern on the structure of premixed flames issuing from perforated burners in domestic condensing boilers. Combust. Theory Model. 2023, 27, 218–243. [Google Scholar] [CrossRef]
- Restrepo, A.; Viana, M.; Colorado, A.; Amell, A. Experimental investigation of hydrogen enriched natural gas diffusion reactions using preheated air in a hot coflow burner. Int. J. Hydrogen Energy 2021, 48, 337–349. [Google Scholar] [CrossRef]
- Sorgulu, F.; Ozturk, M.; Javani, N.; Dincer, I. Experimental investigation for combustion performance of hydrogen and natural gas fuel blends. Int. J. Hydrogen Energy 2023, 48, 34476–34485. [Google Scholar] [CrossRef]
- Sun, C.; Wang, T.; Wang, P.; Zhang, Y.; Cui, C.; Lu, Y.; Liu, W.; Zhang, Y.; Zhang, Y. Numerical Simulation and Field Experimental Study of Combustion Characteristics of Hydrogen-Enriched Natural Gas. Processes 2024, 12, 1325. [Google Scholar] [CrossRef]
- Schwarz, S.; Daurer, G.; Gaber, C.; Demuth, M.; Hochenauer, C. Experimental investigation of hydrogen enriched natural gas combustion with a focus on nitrogen oxide formation on a semi-industrial scale. Int. J. Hydrogen Energy 2024, 63, 173–183. [Google Scholar] [CrossRef]
- Salcı, O.; Öztuna, S. Modeling of hydrogen blending natural gas combustion characteristics and emission analyses in industrial burners. Int. J. Hydrogen Energy 2025, 144, 782–797. [Google Scholar] [CrossRef]
- Lan, Y.; Wang, Z.; Xu, J.; Yi, W. The Impact of Hydrogen on Flame Characteristics and Pollutant Emissions in Natural Gas Industrial Combustion Systems. Energies 2024, 17, 4959. [Google Scholar] [CrossRef]
- Kislinger, C.; Daurer, G.; Schwarz, S.; Demuth, M.; Gaber, C.; Hochenauer, C. CFD study of hydrogen combustion effects on the heat-up characteristics of steel samples using a low-swirl burner: A comparative analysis with methane. Appl. Therm. Eng. 2025, 261, 125105. [Google Scholar] [CrossRef]
- Riahi, Z.; Mergheni, M.A.; Sautet, J.-C.; Ben Nasrallah, S. Experimental study of natural gas flame enriched by hydrogen and oxygen in a coaxial burner. Appl. Therm. Eng. 2016, 108, 287–295. [Google Scholar] [CrossRef]
- Pinto, B.M.; Pacheco, G.P.; Mendes, M.A.A.; Coelho, P.J. Numerical Simulation of Natural Gas/Hydrogen Combustion in a Novel Laboratory Combustor. Appl. Sci. 2025, 15, 7123. [Google Scholar] [CrossRef]
- Weidinger, F.; Aichinger, G.; Dreizler, D.; Hochenauer, C. The impact of hydrogen on forced-draft gas burners: A numerical investigation of nitrogen oxide emissions. Int. J. Hydrogen Energy 2025, 143, 582–595. [Google Scholar] [CrossRef]
- Du, W.; Zhou, S.; Qiu, H.; Zhao, J.; Fan, Y. Experiment and numerical study of the combustion behavior of hydrogen-blended natural gas in swirl burners. Case Stud. Therm. Eng. 2022, 39, 102468. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.