Abstract
A comprehensive study of the structural–phase transformations and hydrogen desorption kinetics in the Mg56Al44 system was conducted using a multistage approach combining thermodynamic modeling CALPHAD, Thermo-Calc 2025a, mechanical synthesis (MS), spark plasma sintering (SPS), and subsequent dispersion treatment. Thermodynamic modeling revealed a stable existence region of the intermetallic compound Mg17Al12, exhibiting Cp-T anomalies at 303 and 351 °C that closely corresponded to the experimental DSC/TGA results. Microstructural analysis showed that varying the ball-to-powder ratio BPR 20:1 and BPR 30:1 determines the defect density, crystallite size 25–45 nm, and lattice strain 1.5–3.0 × 10−3, all of which directly influence the hydrogen desorption kinetics. For the samples synthesized at BPR 30:1, the onset temperature of hydrogen release decreased to 180–200 °C while maintaining a hydrogen storage capacity of 4.9 wt.%, accompanied by a reduction in the apparent activation energy of desorption from 92 to 74 kJ·mol−1 according to the Kissinger method. The dispersion stage partially disrupted and redistributed the surface MgO layer, leading to a reduction in its overall contribution and improvement in structural homogeneity, rather than complete oxide removal. The combined MS-SPS-dispersion processing route enabled controlled nanostructure formation, reduced the hydrogen desorption temperature by approximately 100 °C compared to conventional MgH2-based materials, and significantly enhanced the thermokinetic performance. These findings demonstrate that Mg-Al alloys are promising candidates for solid-state hydrogen storage systems with improved desorption kinetics and reduced activation barriers.
1. Introduction
Magnesium-based materials have long been considered promising candidates for hydrogen storage due to their high theoretical gravimetric capacity (7.6 wt.%), low cost, and environmental safety [,,]. However, their practical application is hindered by the high hydrogen desorption temperature (above 300 °C) and the sluggish kinetics of sorption processes [,,]. Over the past decades, various approaches have been explored to overcome these drawbacks, including the introduction of catalytic additives (Ti, V, Ni, Fe) to accelerate kinetics [,,,], nanostructuring to increase the reactive surface area [,], the use of carbon matrices to stabilize cycling [,], and the development of Mg-based composites with transition metals []. Nevertheless, most of these strategies remain limited by high cost, scaling challenges, and reduced cyclic stability under repeated use.
Among alternative solutions, the Mg-Al system has attracted particular interest. The interaction of magnesium with aluminum leads to the formation of intermetallic phases such as Mg17Al12 and Mg2Al3 [,,,]. Although their hydrogen capacities are lower than that of Mg 4.44 wt.% and 3.02 wt.%, respectively [], the presence of these compounds reduces the hydrogen desorption temperature and improves thermal conductivity, which is crucial for uniform heat transfer during sorption-desorption cycling [,]. The evolution of phase composition depends strongly on the Mg:Al stoichiometry, ranging from Mg(Al) + Mg17Al12 to Mg2Al3 + Al(Mg) [].
MS is one of the most effective methods for producing such materials, as it enables intensive particle refinement followed by the formation of nanostructured intermetallides [,,]. Prolonged milling for up to 20 h can lower the desorption temperature by 50–70 °C [,,], but it requires high energy input and complicates the control of phase transformations [,,]. To stabilize the resulting structure, spark plasma sintering has recently been employed, enabling rapid powder densification and fixation of the phase composition through fast heating.
In this work, the formation of the Mg-Al system is considered as a sequential evolution of structural and phase transformations. In the first stage, mechanical synthesis initiates the formation of intermetallic phases and defect structures. In the second stage, spark plasma sintering stabilizes and densifies the material. Finally, post-synthesis dispersion is applied to increase particle dispersity, increase the reactive surface area, and enhance heat transfer during sorption processes. This approach reduces particle agglomeration and improves hydrogen sorption kinetics [,].
Previous studies [,,,,] on Mg-Al systems for hydrogen storage have mainly relied on experimental approaches, focusing on alloy composition, catalytic additives, or nanostructuring to enhance sorption properties. Although thermodynamic modeling based on the CALPHAD approach has been successfully applied to systems such as Mg-Ni, Mg-Ca, and Mg-Y for predicting hydride phase stability and transformation temperatures [,,], comparable studies for the Mg-Al system remain limited.
In the present work, the CALPHAD method is employed not only to establish phase equilibria and determine the stability range of Mg17Al12, but also to correlate these predictions with experimental DSC/TGA results.
It is worth noting that the introduction of a post-synthesis dispersion stage represents a methodological advancement compared with traditional mechanical synthesis and sintering. This step provides a controllable means to reduce surface oxides, enhance reactive surface activity, and optimize hydrogen desorption kinetics [].The novelty of this work lies in the implementation of a sequential three-step processing route—MS, SPS, and dispersion—which enables a direct linkage between structural evolution and hydrogen sorption behavior. Unlike previous Mg-Al studies [,,], where the effect of milling energy was analyzed without subsequent consolidation or dispersion, the proposed combined approach allows for controlled adjustment of the intermetallic phase fraction and microstructure. This correlation between milling intensity, phase evolution, and hydrogen desorption kinetics has not been previously reported for the Mg-Al syste. Table 1 summarizes the abbreviations and symbols employed in this study

Table 1.
Symbols and abbreviations used throughout the study.
2. Materials and Methods
2.1. Thermodynamic Modeling
In this work, thermodynamic modeling of the Mg-Al system was performed using the Thermo-Calc 2025a software package with the TCAL9 thermodynamic database, which is parameterized for light-metal alloys and includes Mg-Al-H interactions. The objective of the calculations was to predict the phase composition and stability of hydride and intermetallic compounds over a wide range of Mg–Al–H ratios. Based on the equilibrium phase diagram, the stability regions of MgH2, Mg17Al12, and solid solution phases were identified, which allowed us to determine the temperature and concentration conditions for their existence. The obtained results were further used to justify the choice of the Mg56Al44 stoichiometry and the optimized sintering regime at 350 °C.
To evaluate the thermodynamic feasibility of the phases, the Gibbs free energy was calculated as a function of temperature using the approximation:
where G0 is the reference energy at 0 K, and a and b are empirical coefficients fitted within the database.
G(T) = G0 + aT + bTln(T),
Based on these calculations, 3D heat capacity models Cp(T) were constructed at selected points of the phase diagram to identify temperatures corresponding to minimum thermodynamic potentials. This allowed us to determine the critical temperatures for the decomposition of metastable phases and the evolution of stable intermetallics. The modeling was carried out in the temperature range of 25–600 °C with a composition step of 1 at.% under a hydrogen partial pressure of 0.1 MPa. The modeling thus served as the basis for selecting the temperature regimes for the sorption–desorption experiments.
2.2. Material Preparation (MS, SPS, and Post-Synthesis Dispersion)
Commercial magnesium powder (99%, 120–180 μm) and aluminum powder (99.99%, 70 μm) were used as starting materials. MS was carried out in a planetary ball mill (Retsch PM100) at 400 rpm for 5 h under a high-purity argon atmosphere. Two ball-to-powder ratios BPR 20:1 and 30:1 were selected to compare the effect of milling energy on microstructural evolution and hydrogen sorption behavior. To minimize agglomeration and cold welding, stearic acid was added as a process control agent (15 wt.% relative to the powder mixture). The selected milling parameters were chosen based on previous reports [,,].
SPS was performed in a graphite die (Ø25 mm) at a pressure of 12.96 MPa and a temperature of 350 °C with a holding time of 5 min. The temperature was monitored by a pyrometer, and the control accuracy was within ±5 °C.
The selected parameters were based on both CALPHAD thermodynamic modeling and preliminary optimization experiments. The modeling predicted the stability region of the equilibrium Mg + Mg17Al12 phase field near 350 °C, which was experimentally confirmed as the temperature providing sufficient diffusion bonding without excessive grain growth. Additional SPS trials performed in the temperature range of 300–400 °C with holding times of 3–10 min showed that at lower temperatures (<320 °C) incomplete densification and weak interparticle bonding occurred, while above 380 °C partial melting and increased oxidation were observed. Therefore, the SPS regime of 350 °C for 5 min was determined to be the optimal condition ensuring phase stabilization and microstructural integrity of the Mg56Al44 system.
The consolidated compacts were subsequently subjected to low-intensity dispersion in an inert atmosphere at 200 rpm for 20 min in order to refine the particle size, increase the active surface area, and reduce agglomeration after SPS [].
2.3. Materials Characterization
A set of complementary techniques was employed to analyze the phase composition, microstructure, and thermal properties of the samples. X-ray diffraction (XRD) was carried out on a D6 Phaser diffractometer using Cu-Kα radiation at 45 kV and 40 mA. Data were collected in the 2θ range of 5–153° with a step size of 0.013° and a counting time of 30.6 s per step. The diffraction patterns were processed using the Diffrac-EVA Ver.7 software package with the PDF-4 AXIOM database, and phase identification was performed by peak integration followed by Rietveld refinement.
To differentiate the contributions of crystallite size and lattice strain to the observed peak broadening in Figures 5 and 6, a Williamson–Hall analysis was performed according to the relation
where β is the full width at half maximum (FWHM), θ is the Bragg angle, λ = 1.5406 Å is the wavelength of the Cu Kα radiation, D is the crystallite size, and ε is the microstrain.
β cos θ = (κλ/D) + 4ε sin θ,
Surface morphology and microstructural features were examined using a TM4000 Plus scanning electron microscope operated in BSE contrast mode. Elemental distribution was determined by elemental mapping.
Thermal stability of the samples was evaluated by combined thermogravimetric analysis and TGA/DSC. Powder specimens of 10 mg were heated in corundum crucibles in the temperature range of 30–400 °C at a heating rate of 10 °C/min under a high-purity argon atmosphere (99.999%), with an empty crucible used as reference.
2.4. Sorption-Desorption Experiments
For the hydrogen sorption–desorption experiments with Mg56Al44 samples, a custom-designed experimental device (ED) was fabricated from stainless steel tubing, equipped with a crucible holder and an internal type-K thermocouple. The crucible was made of 12Cr18Ni10T stainless steel and fixed on a 1 mm diameter steel wire, which allowed accurate positioning of the specimen inside the ED [,,,,,,]. Heating was provided by a resistive heater wound around the outer surface of the device. A photograph of the ED without the heating element is shown in Figure 1.
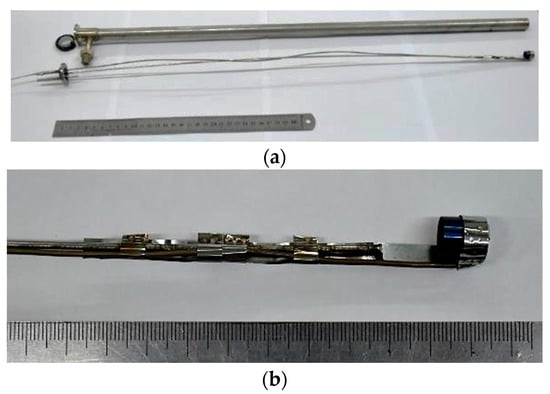
Figure 1.
Experimental device for hydrogen sorption–desorption measurements. (a) Components of the experimental device (ED). (b) Crucible mounting unit with internal thermocouple.
Sorption-desorption experiments were conducted using a TURBOLAB 90i vacuum station (Leybold GmbH, Cologne, Germany), additionally equipped with vacuum valves, pressure gauges, and a quadrupole mass spectrometer RGA-100 (Stanford Research Systems, Sunnyvale, CA, USA) for gas composition analysis. Prior to testing, the powder specimens were degassed at 350 °C for 10 min following a 30 min evacuation to a total pressure of 0.44 Pa, with the partial pressure of water vapor reduced to 3.9·10−4 Pa. Subsequently, the samples were exposed to hydrogen sorption at 300 °C and 0.1 MPa for 15 min. The desorption stage was carried out after re-evacuation of the chamber (0.073 Pa) under linear heating from 50 to 350 °C at a rate of 10 °C/min. The gas composition was continuously monitored in the mass-to-charge ratio range of 1–50 amu with a scanning step of 24 s, ensuring detailed tracking of hydrogen release kinetics. Each full cycle consisted of degassing, sorption, and desorption stages, and two consecutive cycles were performed to verify reproducibility.
The hydrogen absorption properties were evaluated using the pressure-composition-temperature (PCT) method with an automated H-Sorb system. Measurements were carried out at 250 °C over a pressure range of 0 to 2 MPa. The following parameters were determined: maximum achievable hydrogen capacity (wt.% H2), plateau pressures for hydrogenation/dehydrogenation, absorption–desorption hysteresis, and the saturation rate (t%).
For quantitative evaluation of hydrogen desorption kinetics, the activation energy () was determined using the Kissinger method based on DSC data obtained at different heating rates ( = 5, 10, 15, and 20 °C/min). The method is based on the shift in the desorption peak temperature () with increasing heating rate and is expressed by the equation:
where is the heating rate, is the peak temperature, is the activation energy, and is the universal gas constant.
3. Results
3.1. Thermodynamic Calculations
To investigate the phase evolution and determine the optimal synthesis parameters in the Mg-Al system, thermodynamic modeling was carried out using the CALPHAD approach. Particular attention was paid to compositions close to the Mg56Al44 stoichiometry, which is considered promising for hydrogen storage materials []. Figure 2 shows an isothermal section of the Mg-Al-H system. The model predicts that, in the Mg-enriched region (40–60 at.% Mg), the stable phase field corresponds to the three-phase equilibrium of Mg + Mg17Al12 + H2.
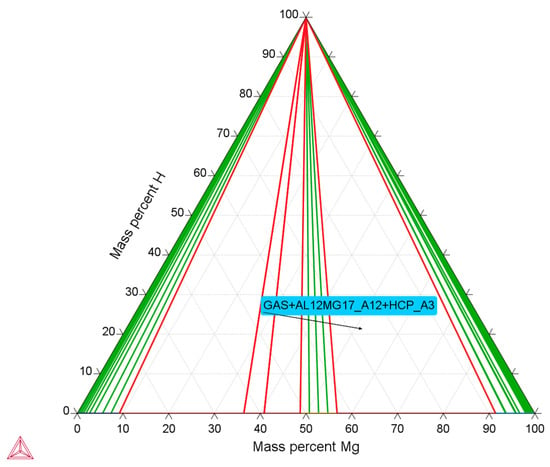
Figure 2.
Isothermal section of the Mg-Al-H system predicted by CALPHAD modeling.
In the diagram, the arrow indicates the stability region of the phase assemblage comprising the hexagonal solid solution (HCP_A3), the intermetallic Mg17Al12 (AL12MG17_A12), β-Al(Mg), ε-AlMg, and the gas phase H2. The presence of hydrogen in the stable field highlights the potential for reversible interaction with the material and confirms its suitability for hydrogen storage applications []. An important conclusion is that the Mg17Al12 intermetallic is stabilized in the system over a relatively wide compositional range. The simultaneous presence of Mg ensures a balance between thermodynamic stability and hydrogen storage capacity []. Thus, the model provides a rationale for selecting the Mg56Al44 composition as optimal for further investigations.
3.2. Microstructural Evolution After MS
For the experimental validation of the selected composition, Mg56Al44 powders were synthesized using mechanical alloying. By varying BPR of 20:1 and 30:1, it was possible to evaluate and optimize the degree of refinement and powder morphology, both of which influence the hydrogen absorption capacity of the system. Microstructural analysis (Figure 3) revealed pronounced differences in morphology and elemental distribution in the Mg56Al44 powders depending on the milling regime. For the BPR of 20:1 (Figure 3a,b), the powders exhibited a more homogeneous structure with fine, rounded particles ranging from submicron sizes to several micrometers.
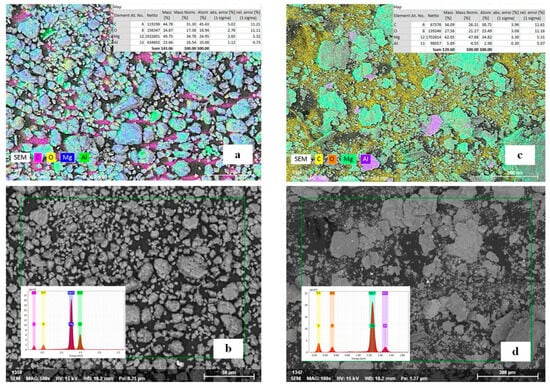
Figure 3.
SEM images and elemental mapping of the Mg56Al44 system after mechanical alloying at different BPR ratios: (a,b) 20:1; (c,d) 30:1.
According to the EDS analysis (Figure 3a,b) of the sample synthesized at BPR 20:1, a uniform distribution of Mg and Al was observed, with a relatively low oxygen content, indicating limited surface oxidation. Such particle refinement and rounding are consistent with previously reported optimal conditions of mechanical alloying [,]. A similar morphological outcome was noted in [,], where lower BPR values prevented excessive defect accumulation and promoted the formation of uniform fine particles.
In the case of BPR 30:1 (Figure 3c,d), a more heterogeneous structure was observed, characterized by pronounced agglomeration and irregular flaky-shaped particles. Elemental mapping revealed a higher oxygen content compared to the BPR 20:1 sample, indicating enhanced surface oxidation. These effects may be associated with the excessive energy input during mechanical treatment, where high-intensity milling accelerates defect formation in the crystal lattice [,,]. Similar trends of agglomeration and the growth of oxygen-containing phases at elevated BPR were also reported in studies of Mg-Ni and Mg-Al powders [,,].
3.3. Microstructure After SPS
At the stage of mechanical alloying, the key factors were particle refinement and surface activation, whereas after spark plasma sintering the decisive role is played by the nature of densification and the diffusion interaction between the components. A comparison of the microstructure of the sintered compacts (Figure 4) reveals how mechanical alloying at different BPR values affects the density, phase distribution, and oxidation level of the samples.
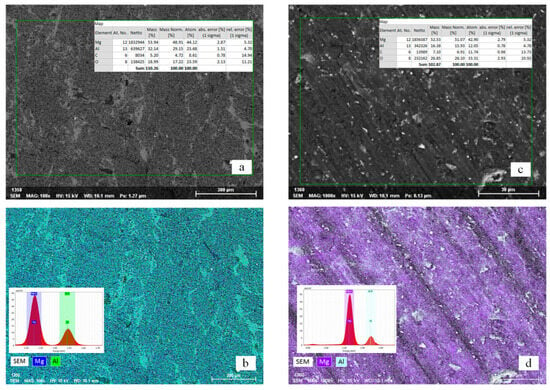
Figure 4.
Microstructure and elemental distribution of sintered Mg56Al44 samples obtained after SPS at 350 °C: (a,b) sample after MA at BPR 20:1; (c,d) sample after MA at BPR 30:1.
The BPR 20:1 sample (Figure 4a,b) exhibits a dense two-phase structure formed as a result of eutectoid transformation during SPS. Areas highlighted in green BSE contrast and continuous interparticle necks indicate well-developed diffusion bridges and good densification []. EDS analysis shows a Mg-to-Al ratio close to the nominal composition and a significantly lower oxygen background compared to the Mg56Al44 sample synthesized at BPR 30:1. Such a level of oxidation is typical for Mg powders subjected to MS under moderate energy input, where thin MgO films are partially redistributed during SPS without forming oxide stringers along grain boundaries [].
The Mg56Al44 sample obtained at BPR 30:1 (Figure 4c,d) reveals pronounced oriented banding, which may indicate local gradients of composition and density caused by non-uniform distribution of current and heat, as well as directed powder flow under load in the graphite die []. Elemental mapping reveals higher oxygen content, which correlates with the higher BPR and impact energy during MS. This leads to enhanced surface activation, particle agglomeration [], and defect accumulation [], and consequently, to a higher fraction of MgO.
The SPS cycle initiates the formation of the Mg17Al12 phase along interparticle contacts, while fine dispersion and short dwell time suppress the growth of large intermetallic colonies [].
3.4. XRD Changes After Dispersion of MS-SPS-Dispersion Compacts
For optimal hydrogen exchange, it is important not only to form the appropriate phases but also to ensure a suitable particle size and distribution, as well as minimal surface oxidation []. To address this, a dispersion stage was introduced after SPS, which had not previously been applied in the Mg-Al system.
The introduction of this stage was justified by the need to enhance material dispersity, increase the specific reactive surface area, and reduce the extent of agglomeration formed after MS and SPS []. In addition, dispersion helps to lower the oxidation level by disrupting surface oxide films and partially redistributing oxides, which is particularly important for Mg-containing systems due to their high tendency toward oxidation [].
Figure 5 presents the XRD patterns of samples synthesized at BPR 20:1 after MS, SPS, and subsequent dispersion.
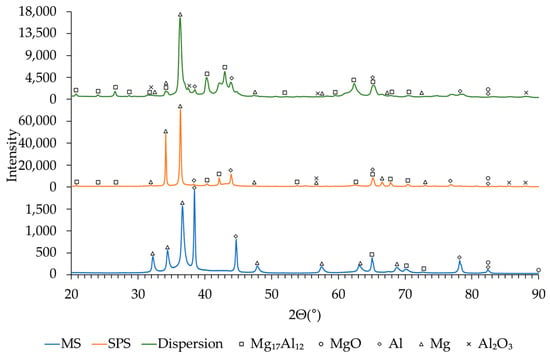
Figure 5.
XRD patterns (bottom to top) of the sample after MS at BPR 20:1, after SPS at 350 °C, and after 20 min of dispersion.
According to the XRD patterns (Figure 5), at the stage of MS (blue curve), diffraction peaks corresponding to Mg, Al, and the intermetallic Mg17Al12 are observed, along with minor reflections of MgO. After SPS, the intensity of the Mg and Mg17Al12 peaks increases, which is associated with diffusion reactions and the formation of stable intermetallic compounds []. At the final dispersion stage, the peaks of Mg and Mg17Al12 become less intense and broadened, indicating enhanced dispersion and a reduction in crystallite size [], as confirmed by the data in Table 2.

Table 2.
Average crystallite sizes of various phases after MS at BPR 20:1, SPS at 350 °C, and 20 min of dispersion.
At the same time, the contribution of MgO decreases, which confirms the partial disruption of surface oxide films and the redistribution of oxygen within the material. Similar trends were observed for the samples synthesized at BPR 30:1, as shown in Figure 6.
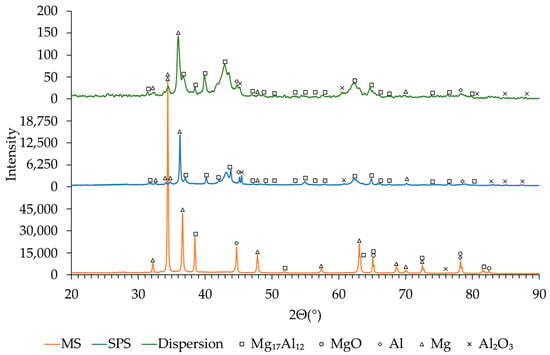
Figure 6.
XRD patterns of the Mg56Al44 sample (from bottom to top) after MS at BPR 30:1, after SPS at 350 °C, and after 20 min of post-synthesis dispersion.
The XRD patterns presented in Figure 6 illustrate the phase evolution of Mg56Al44 after MS, SPS, and subsequent dispersion. Following MS, diffraction peaks corresponding to Mg, Al, and Mg17Al12 were identified, along with minor contributions from MgO and Al2O3, indicating partial surface oxidation during milling []. After SPS, the peaks associated with Mg17Al12 became more intense, reflecting the progress of intermetallic formation and stabilization through diffusion [], while the residual peaks of Al diminished. In addition, oxide phases became more pronounced, which can be attributed to partial oxidation at particle boundaries during high-temperature processing. After dispersion, the diffraction patterns corresponding to Mg and Mg17Al12 broadened and decreased in intensity, indicating crystallite refinement as summarized in Table 3 and increased lattice strain [].

Table 3.
The average crystallite sizes of the different phases after MS at BPR 30:1, after SPS at 350 °C, and after 20 min of post-synthesis dispersion.
At the same time, the fraction of oxide phases (MgO and Al2O3) decreased, indicating partial removal or redistribution of surface oxide layers during mechanical processing [].
These observations demonstrate that dispersion effectively reduces agglomeration, enhances phase homogeneity, and increases the available surface area, thereby creating favorable conditions for improving the hydrogen sorption-desorption kinetics [].
To quantitatively evaluate the effects of grain refinement and internal distortions after the MS, SPS, and dispersion stages, the lattice strain (ε) values for the main phases of the Mg56Al44 system were calculated using the Williamson–Hall method, as presented in Table 4.

Table 4.
Lattice strain (ε) values of Mg56Al44 phases after MS, SPS, and dispersion, calculated using the Williamson–Hall method.
The Williamson–Hall analysis confirmed that the peak broadening observed in Figure 5 and Figure 6 is attributed to both grain refinement and an increase in lattice strain. The calculated microstrain (ε) values increased from approximately 1.5–1.9 × 10−3 after SPS to 2.5–3.0 × 10−3 after dispersion, which is consistent with the reduction in crystallite size from 40–45 nm to 25–30 nm. This strain development is associated with an increased defect density and lattice distortion induced by mechanical shear during the dispersion stage, which also facilitates hydrogen diffusion and enhances desorption kinetics [].
3.5. Thermal Stability
Since the structure and phase composition of the Mg56Al44 system directly determine its ability for reversible hydrogen absorption and release, thermal stability becomes a key parameter []. For materials intended for hydrogen storage, it is particularly important to establish the temperature ranges at which degassing, decomposition of metastable phases, and the formation of stable intermetallics occur. These processes define the operating temperature of sorption–desorption cycles and the long-term durability of the material [].
TGA and DSC are among the most informative techniques for investigating such characteristics. The combined recording of mass change and heat flow makes it possible to identify the onset of degassing and the decomposition of intermediate compounds []. Figure 7 presents the results of thermodynamic modeling of the heat capacity of the Mg56Al44 system (a) and the experimental TGA/DSC curves (b), which enable direct comparison of the calculated predictions with the processes of formation and decomposition of the Mg17Al12 phase.
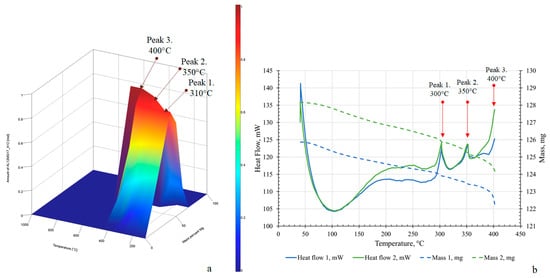
Figure 7.
Thermodynamic modeling of heat capacity (a) and experimental TGA/DSC curves (b) for Mg56Al44 samples: green—after MS at BPR 20:1 followed by SPS at 350 °C and 20 min dispersion; blue—after MS at BPR 30:1 followed by SPS at 350 °C and 20 min dispersion.
In Figure 7a, three characteristic thermal anomalies are observed at 310 °C, 350 °C, and 400–450 °C. The first peak at 310 °C is interpreted as the onset of metastable phase decomposition, accompanied by mass loss as confirmed by TGA data []. The second peak at 350 °C corresponds to the formation of stable intermetallics such as Mg17Al12 and associated structural relaxations [], resulting in a pronounced endothermic effect. The third peak at 400–450 °C is associated with the oxidation of residual organics originating from the process control agent, as well as with the dehydroxylation and decomposition of surface carbonates formed during brief exposure to air [].
Figure 7b shows the experimental TGA/DSC curves for Mg56Al44 samples after dispersion, obtained under different MS conditions (BPR 20:1 and BPR 30:1). The absolute mass values presented in the graphs correspond to the balance sensor signal and include the crucible contribution. For the analysis, the relative mass loss of the sample (10 mg) was used.
The experimental data are in good agreement with the results of thermodynamic modeling: the temperature ranges of the three characteristic peaks coincide with the calculated values, with minimal deviations not exceeding ±5 °C for the anomalies at 303 °C and 351 °C. Repeated measurements (n = 3) demonstrated good reproducibility of the thermal effects, with a standard deviation of peak positions of ±3 °C.
Both samples synthesized at BPR 20:1 and BPR 30:1 demonstrate a comparable total mass loss. The differences in thermal curves reflect variations in the microstructural state of the material.
Analysis of the temperature dependence of heat flow for Mg56Al44 samples after dispersion MS at BPR 20:1 and BPR 30:1 revealed two endothermic effects at 303 °C and 351 °C. The BPR 20:1 sample is characterized by smoothed peaks, indicative of a homogeneous structure, whereas the BPR 30:1 sample exhibits sharper and more intense peaks, associated with localized degassing, oxide decomposition, and increased defect density [].
To quantitatively assess the kinetic enhancement observed for the BPR 30:1 sample, the apparent activation energy (Ea) of hydrogen desorption was evaluated using the Kissinger approach. The relationship between ln(β/Tp2) and 1/Tp yielded linear trends consistent with a single-step desorption mechanism. The calculated Ea values were 92 kJ·mol−1 for the BPR 20:1 sample and 74 kJ·mol−1 for the BPR 30:1 sample, indicating an approximately 20% reduction in activation energy.
3.6. Hydrogen Sorption Behavior
After two sorption-desorption cycles, a decrease in the intensity of the observed thermal effects was recorded; however, their temperature positions and overall profiles remained unchanged. This may indicate partial changes in the composition or degree of structural ordering of the material as a result of interaction with hydrogen, while preserving the general nature of the thermal processes [].
At the first stage, experiments were performed with Mg56Al44 samples. The sorption stage at 300 °C and 0.1 MPa was not accompanied by gas release; therefore, mass spectrometry was not employed. A slight pressure drop (from 1.0 × 105 to 9.97 × 104 Pa over 15 min) can be attributed either to hydrogen uptake or to thermodynamic effects, such as gas volume changes during cooling and stabilization of temperature gradients [].
The desorption stage represents the main scientific interest, where the composition of the released gases was monitored during heating. Figure 8 presents the kinetic profiles of gas release from Mg56Al44 samples prior to hydrogen saturation.
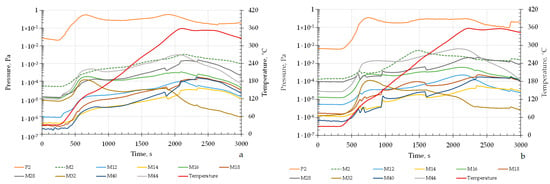
Figure 8.
Kinetic profiles of gas release from Mg56Al44 samples prior to hydrogen saturation: (a) after MS at BPR 20:1 followed by SPS and dispersion; (b) after MS at BPR 30:1 followed by SPS and dispersion.
The mass-to-charge ratios in the spectra correspond to the following positively charged ions: M2–H2+; M12–C+; M14–C2H42+, CH2+, and N2+/N; M16–O22+ and O+; M17–OH+; M18–H2O+; M28–CO+; M32–O2+; M40–Ar+; M44–CO2+.
In the case of the sample shown in Figure 8a, hydrogen release begins at 200 °C and exhibits a gradual increase in partial pressure with heating. The maximum release is observed in the 330–370 °C range, reaching a pressure of about 10−3–10−2 Pa. The kinetics are characterized by a smooth profile without pronounced fluctuations, reflecting a uniform distribution of defects and a more stable matrix state after mechanical synthesis at the lower-intensity BPR 20:1.
For the sample in Figure 8b, hydrogen release starts earlier, at 180 °C, and proceeds more intensively. In the 300–380 °C range, the partial pressure of H2 rises to 10−2–10−1 Pa, i.e., an order of magnitude higher compared to the sample in Figure 8a. The release curve exhibits distinct peak-like features, indicating localized degassing centers and increased structural defectiveness [].
Figure 9 presents the kinetic profiles of gas release from Mg56Al44 samples after hydrogen saturation, obtained during linear heating at 10 °C/min up to 350 °C.
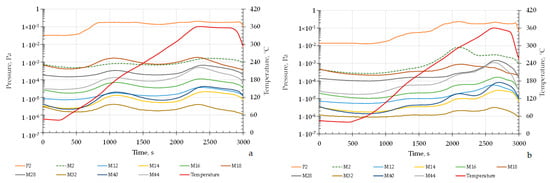
Figure 9.
Kinetic profiles of gas release from Mg56Al44 samples after hydrogenation: (a) after MS at BPR 20:1 followed by SPS and dispersion; (b) after MS at BPR 30:1 followed by SPS and dispersion.
For both samples shown in Figure 9a stepped hydrogen release is observed, which is associated with the sequential dehydrogenation of different hydride phases and the weakening of interphase boundaries []. At the initial stage, below 200 °C, only a minor gas evolution occurs, attributed to hydrogen desorption from the surface and near-surface defect regions []. In the temperature range of 200–300 °C, intensive hydrogen release takes place due to the decomposition of metastable MgH2 hydride phases formed during hydrogenation. In this same interval, dehydrogenation of hydride-like regions near the Mg17Al12 phase also contributes to the overall release [].
Differences in the kinetics between the samples in Figure 9 are manifested in both the intensity and the temperature of hydrogen desorption peaks. For sample 9a, which exhibits lower dispersion and defect density, the main desorption peak is shifted toward higher temperatures, in the range of 280–300 °C. In contrast, sample 9b shows an accelerated onset of hydrogen release and a more pronounced low-temperature peak at approximately 250 °C, which is associated with the increased defect density and the shortened diffusion path for hydrogen escape [,,,,,,,].
Hydrogen storage properties of the Mg56Al44 samples were further evaluated using the pressure-composition-temperature (PCT) method after hydrogenation. For sample 9a after mechanical synthesis at BPR 20:1 followed by SPS and dispersion, the maximum hydrogen capacity was measured at 4.2 wt.% H2, whereas sample 9b (after mechanical synthesis at BPR 30:1 followed by SPS and dispersion exhibited a higher capacity of 4.9 wt.% H2. These results are consistent with previously reported data [], confirming that increased milling intensity enhances defect density and dispersion, thereby facilitating hydrogen absorption.
4. Discussion
The obtained results demonstrate that the phase evolution, thermal stability, and hydrogen sorption-desorption behavior of the Mg56Al44 system strongly depend on the BPR during MS, followed by SPS and post-dispersion treatment. Thermodynamic modeling using the CALPHAD approach confirmed the stabilization of the Mg17Al12 intermetallic in the Mg-rich region. Previous studies [,,,,,] have highlighted the dual role of the Mg17Al12 phase: it lowers the desorption temperature and enhances thermal conductivity, but excessive amounts reduce the overall hydrogen storage capacity [].
According to the obtained data, the fraction of Mg17Al12 was approximately 28% for samples synthesized at BPR 20:1 and increased to 42% at BPR 30:1. This increase was accompanied by a rise in hydrogen capacity from 4.2 to 4.9 wt.% and an acceleration of desorption, indicating the catalytic role of the intermetallic phase in hydrogen transport processes.
The summarized data are presented in Table 5, showing the correlation between the Mg17Al12 phase fraction, hydrogen capacity, and desorption temperatures for samples synthesized under different BPR conditions.

Table 5.
Correlation between the Mg17Al12 phase content, milling conditions, and hydrogen characteristics of Mg56Al44 samples.
However, as noted in previous studies [,,], a further increase in the Mg17Al12 fraction may reduce the overall hydrogen capacity of the system due to a decrease in the proportion of active hydride MgH2. Future work will aim to establish a quantitative correlation between the Mg17Al12 phase fraction, determined from Rietveld refinement, and hydrogen capacity to identify the optimal phase ratio.
Our results are consistent with these observations: the sample processed at BPR 20:1, SPS at 350 °C, and 20 min of post-dispersion exhibited moderate Mg17Al12 formation with smoother desorption peaks at 280–300 °C, whereas the sample prepared at BPR 30:1, SPS at 350 °C, showed a higher fraction of this phase, resulting in earlier hydrogen release at 180–200 °C, but with more pronounced oxidative degradation.
The dispersion stage plays a crucial role in reducing the MgO content and enhancing the surface reactivity of the Mg-Al system. The observed decrease in the intensity of MgO and Al2O3 reflections after dispersion, as shown in Figure 5 and Figure 6, can be attributed to mechanical fragmentation of surface oxide films and redistribution of oxygen within the bulk []. During dispersion, repeated impacts cause partial delamination and cracking of MgO layers, exposing fresh metallic surfaces and promoting the reformation of Mg and Al bonds on the nanoscale [].
This process effectively minimizes the thickness of oxide shells surrounding the particles and improves hydrogen access to active metallic regions. EDS mapping confirmed this trend—the oxygen signal became more homogeneous, and its average intensity decreased by approximately 15 ± 3% compared with the SPS-treated sample. Quantitative evaluation based on EDS supports the conclusion that dispersion partially removes or redistributes surface oxides rather than forming new oxide phases.
Microstructural characterization confirmed that lower milling energy at BPR 20:1 promotes the formation of uniform fine-grained particles with limited oxidation, in agreement with previous studies on Mg-Al and Mg-Ni alloys [,]. In contrast, the sample milled at BPR 30:1 and SPS at 350 °C exhibited strong agglomeration, surface oxidation, and heterogeneous morphology, similar to observations reported for mechanically over-milled Mg-based systems [,,,,,]. These structural differences explain the distinct hydrogen desorption kinetics observed in Figure 8 and Figure 9.
TGA/DSC analysis revealed characteristic endothermic peaks at 303 °C and 351 °C, corresponding to the decomposition of metastable hydrides and the stabilization of Mg17Al12, respectively. The desorption temperatures measured in this study 250–300 °C, depending on BPR are consistent with previous reports for Mg-Al alloys obtained via MS and SPS [], although our post-dispersion step further reduced peak intensities and improved the homogeneity of desorption profiles. Notably, the combination of SPS and dispersion suppressed grain coarsening and improved phase distribution, thereby accelerating hydrogen release compared to conventional sintering of Mg-Al alloys [,].
Gas analysis via mass spectrometry further highlighted differences between the two BPR regimes. The BPR 20:1 sample released hydrogen more gradually, whereas the BPR 30:1 sample exhibited higher desorption rates with sharp localized peaks, indicative of heterogeneous pathways caused by defects. Such behavior has previously been linked to reduced long-term stability under cycling [,].
Comparison with literature data [,] shows that the obtained samples exhibit significantly higher hydrogen absorption efficiency than pure intermetallic phases. For MgH2, the practically achievable capacities at 300–350 °C do not exceed 5.5–6.0 wt.% due to incomplete reversibility of the reaction and sluggish kinetics. For the Mg17Al12 phase, experimental capacities are in the range of 0.4–0.5 wt.% []. Thus, the observed values of 4.2 wt.% (BPR 20:1) and 4.9 wt.% (BPR 30:1) correspond to 65–70% of the capacity of pure MgH2, while occurring at significantly lower desorption temperatures (180–300 °C). This confirms the positive effect of Al content and the Mg17Al12 intermetallic phase on the thermokinetics of hydrogen release.
The observed hydrogen capacity of 4.9 wt.% for the BPR 30:1 sample slightly exceeds the theoretical limit of 4.43 wt.% calculated under the assumption of complete conversion of Mg to MgH2. This discrepancy can be explained by the temporary trapping of hydrogen in defect-rich and interfacial regions generated during intensive milling. It is well known that mechanical synthesis (MS) produces a high density of dislocations and grain boundaries capable of accommodating hydrogen beyond the equilibrium solubility of MgH2 []. In addition, the Mg17Al12 phase may enhance hydrogen solubility at Mg/Mg17Al12 interfaces, as supported by thermodynamic modeling and previously reported for Mg-Al-Ni systems [].
It should also be noted that a small systematic overestimation of the measured capacity (up to ±0.2 wt.%) may result from baseline drift and buoyancy effects typical of bulk PCT measurements*.
Therefore, the measured value of 4.9 wt.% lies within the experimental uncertainty and can be rationalized by additional hydrogen trapping within defected regions and interfacial boundaries formed during mechanical synthesis.
The Williamson–Hall analysis confirmed that the broadening of diffraction peaks is associated with both grain refinement and increased lattice strain []. After dispersion, the microstrain increased from approximately 1.5–1.9 × 10−3 to 2.5–3.0 × 10−3, accompanied by a reduction in crystallite size to 25–30 nm and a higher defect density, which facilitates hydrogen diffusion. The thermoanalytical data are consistent with the Thermo-Calc calculations and reveal successive endothermic effects corresponding to the decomposition of metastable phases, the formation of Mg17Al12, and the degassing of residual organics. The decrease in hydrogen desorption activation energy from 92 to 74 kJ·mol−1 for the BPR 30:1 sample confirms the improvement in kinetics due to structural strengthening and defect formation induced by mechanical dispersion [].
Although the BPR 30:1 regime provides higher hydrogen capacity and faster desorption kinetics, it also leads to increased defect density and partial surface oxidation, which may limit cyclic stability during long-term operation. For practical applications and upscaling of hydrogen storage materials, a combined approach is recommended—performing mechanical synthesis at intermediate BPR values (e.g., 15:1 or 25:1) or reducing milling time, followed by a dispersion stage that compensates for excessive defect density and lowers oxide content.
The calculated activation energy values obtained using the Kissinger method quantitatively confirm that the BPR 30:1 sample exhibits enhanced hydrogen desorption kinetics due to the increased lattice defect density and refined microstructure.
Overall, this study demonstrates that the proposed technological sequence MS → SPS → dispersion enables controlled structural and phase evolution and the formation of an optimal fraction of intermetallic compounds. This approach opens pathways for the targeted design of Mg-Al systems with enhanced sorption properties and reduced hydrogen desorption temperatures. Future research should focus on systematic cycling tests, quantitative evaluation of activation energies, and the integration of protective coatings or catalysts (e.g., Ti, Ni, or rare-earth additives) to further reduce oxidation and improve cyclic durability.
5. Conclusions
In the Mg56Al44 system, following the MS, SPS, and dispersion stages, an increase in lattice microstrain to 2.5–3.0 × 10−3, a reduction in crystallite size to 25–30 nm, and a decrease in hydrogen desorption activation energy from 92 to 74 kJ·mol−1 were observed.
The results confirmed the stabilization of the Mg17Al12 phase, with anomalous Cp-T features at 303 °C and 351 °C correlating with DSC/TGA data. A clear dependence of hydrogen desorption kinetics on MS conditions was established. The sample synthesized at BPR 20:1 and subjected to SPS at 350 °C formed homogeneous powders and exhibited smooth hydrogen desorption in the 280–300 °C range. Hydrogen storage measurements using the PCT method revealed that the maximum hydrogen capacity strongly depends on MS conditions: the sample synthesized at BPR 20:1 and subjected to SPS and dispersion exhibited a capacity of 4.2 wt.% H2, while the sample processed at BPR 30:1 achieved 4.9 wt.% H2.
In contrast, the sample processed at BPR 30:1 and SPS at 350 °C showed an accelerated onset of hydrogen release at 180–200 °C. An additional dispersion step refined particle size and improved kinetics by reducing MgO content. The Kissinger analysis showed that the hydrogen desorption activation energy decreased by approximately 20% for the sample with BPR 30:1, providing quantitative confirmation of the observed improvement in hydrogen release kinetics.
Overall, the sequential MS-SPS-dispersion route effectively tailors phase composition and microstructure, lowering the desorption temperature by up to 100 °C compared to conventional MgH2 systems and offering a scalable pathway for advanced hydrogen storage materials.
Author Contributions
Conceptualization, T.R.T. and Z.N.O.; methodology, O.O.; software, R.Y.Z.; validation, A.Z.M., N.M.M. and B.Y.B.; formal analysis, G.K.U.; investigation, I.A.S.; resources, A.Z.M.; data curation, N.M.M.; writing—original draft preparation, Z.N.O.; writing—review and editing, T.R.T.; visualization, B.Y.B.; supervision, I.A.S.; project administration, N.M.M.; funding acquisition, A.Z.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been carried out with the financial support of the Science Committee of the Ministry of Science and Higher Education of the Republic of Kazakhstan as part of the grant financing by MSHE RK for the AP19574566 project on the topic “Development of Hydrogen Battery Materials Based on Mg-Ni-Ce”.
Data Availability Statement
The data supporting the findings of this study, including the Thermo-Calc simulation files as well as the experimental XRD and SEM datasets, are available from the corresponding author upon reasonable request. All computational and experimental data used in this work were generated and analyzed by the authors.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Li, Z.; Zhang, M.; Xu, H. Revealing the Roles of Heat Transfer, Thermal Dynamics, and Reaction Kinetics in Hydrogenation/Dehydrogenation Processes for Mg-Based Metal Hydride Hydrogen Storage. Energies 2025, 18, 2924. [Google Scholar] [CrossRef]
- Chibani, A.; Boucetta, C.; Haddad, M.A.N.; Boukhari, A.; Fourar, I.; Merouani, S.; Badji, R.; Adjel, S.; Belkhiria, S.; Boukraa, M.; et al. A novel metal hydride reactor design: The effect of using copper, AlN and AlSi10Mg composite fins on the dehydrogenation process of LaNi5-Metal alloy. Int. J. Hydrogen Energy 2025, 141, 118–132. [Google Scholar] [CrossRef]
- Tuluhong, A.; Chang, Q.; Xie, L.; Xu, Z.; Song, T. Current Status of Green Hydrogen Production Technology: A Review. Sustainability 2024, 16, 9070. [Google Scholar] [CrossRef]
- Jiang, H.; Ding, Z.; Li, Y.; Lin, G.; Li, S.; Du, W.; Chen, Y.; Shaw, L.L.; Pan, F. Hierarchical interface engineering for advanced magnesium-based hydrogen storage: Synergistic effects of structural design and compositional modification. Chem. Sci. 2025, 16, 7610–7636. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhou, Y.; Li, Y.; Hao, Y.; Wu, P.; Ding, Z. Recent Advances in the Preparation Methods of Magnesium-Based Hydrogen Storage Materials. Molecules 2024, 29, 2451. [Google Scholar] [CrossRef] [PubMed]
- Parviz, R.; Heydarinia, A.; Khosravi, M.; Nili-Ahmadabadi, M. New Mg-based composite with layered-porous structure for enhanced hydrogen storage. J. Energy Storage 2025, 117, 116145. [Google Scholar] [CrossRef]
- Sun, Z.; Lu, X.; Nyahuma, F.M.; Yan, N.; Xiao, J.; Su, S.; Zhang, L. Enhancing Hydrogen Storage Properties of MgH2 by Transition Metals and Carbon Materials: A Brief Review. Front. Chem. 2020, 8, 552. [Google Scholar] [CrossRef]
- Sünbül, S.E.; Öztürk, S.; İçin, K. Structural and Hydrogen Storage Properties of Mg60-Ni40 and Mg80-Ni20 Alloys Prepared by Planar Flow Casting. J. Mater. Eng. Perform. 2020, 29, 6101–6107. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Shao, H.; Li, W.; Lin, H. Catalysis and Downsizing in Mg-Based Hydrogen Storage Materials. Catalysts 2018, 8, 89. [Google Scholar] [CrossRef]
- Lyu, J.; Elman, R.R.; Svyatkin, L.A.; Kudiiarov, V.N. Theoretical and experimental research of hydrogen solid solution in Mg and Mg–Al system. Materials 2022, 15, 1667. [Google Scholar] [CrossRef]
- Jangir, M.; Jain, I.P.; Gattia, D.M. Effect of Ti-based additives on the hydrogen storage properties of MgH2: A review. Hydrogen 2023, 4, 523–541. [Google Scholar] [CrossRef]
- Hanada, N.; Ichikawa, T.; Fujii, H. Catalytic effect of nanoparticle 3d transition metals on hydrogen storage properties in MgH2 prepared by mechanical milling. J. Phys. Chem. B 2005, 109, 7188–7194. [Google Scholar] [CrossRef]
- Schur, D.V.; Veziroglu, A.; Zaginaychenko, S.Y.; Matysina, Z.A.; Veziroglu, T.N.; Gabdullin, M.T.; Ramazanov, T.S.; Zolonarenko, A.D.; Zolonarenko, A.D. Theoretical studies of lithium–aluminum amid and ammonium as prospective hydrogen storage materials. Int. J. Hydrogen Energy 2019, 44, 24810–24820. [Google Scholar] [CrossRef]
- Matysina, Z.A.; Zaginaichenko, S.Y.; Schur, D.V.; Veziroglu, T.N.; Veziroglu, A.; Gabdullin, M.T.; Zolotarenko, A.D.; Zolotarenko, A.D. The mixed lithium–magnesium imide Li2Mg(NH)2 as a promising hydrogen storage material. Int. J. Hydrogen Energy 2018, 43, 16092–16106. [Google Scholar] [CrossRef]
- Liu, L.; Ren, D.; Liu, F. A Review of Dissimilar Welding Techniques for Magnesium Alloys to Aluminum Alloys. Materials 2014, 7, 3735–3757. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Jia, X.; Qin, Z.; Ding, X.; Li, Y. Enhancements in hydrogen storage properties of magnesium hydride supported by carbon fiber. Inorganics 2024, 12, 273. [Google Scholar] [CrossRef]
- Mintz, M.; Gavra, Z.; Kimmel, G.; Hadari, Z. The reaction of hydrogen with magnesium alloys and magnesium intermetallic compounds. J. Less Common Met. 1980, 74, 263–270. [Google Scholar] [CrossRef]
- Ren, L.; Li, Y.; Zhang, N.; Li, Z.; Lin, X.; Zhu, W.; Lu, C.; Ding, W.; Zou, J. Nanostructuring of Mg-Based Hydrogen Storage Materials: Recent Advances for Promoting Key Applications. Nano-Micro Lett. 2023, 15, 93. [Google Scholar] [CrossRef] [PubMed]
- Cermak, J.; Kral, L.; Roupcova, P. Improved hydrogen sorption kinetics in Mg modified by chosen catalysts. Int. J. Hydrogen Energy 2019, 44, 8315–8324. [Google Scholar] [CrossRef]
- Zaluska, A.; Zaluski, L.; Ström-Olsen, J.O. Structure, catalysis and atomic reactions on the nanoscale: A systematic approach to metal hydrides for hydrogen storage. Appl. Phys. A 2001, 72, 157–165. [Google Scholar] [CrossRef]
- Bouaricha, S.; Dodelet, J.P.; Guay, D.; Huot, J.; Boily, S.; Schulz, R. Hydriding behavior of Mg–Al and leached Mg–Al compounds prepared by high-energy ball milling. J. Alloys Compd. 2000, 297, 282–293. [Google Scholar] [CrossRef]
- Takamura, H.; Miyashita, T.; Kamegawa, A.; Okada, M. Grain size refinement in Mg–Al-based alloy by hydrogen treatment. J. Alloys Compd. 2003, 356, 804–808. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Ohotnicky, P.P.; Adams, J.B.; Rohrer, C.L.; Hyland, R.W. Anisotropic surface segregation in Al–Mg alloys. Surf. Sci. 1997, 373, 357–370. [Google Scholar] [CrossRef]
- Lyu, J.; Elman, R.; Svyatkin, L.; Kudiiarov, V. Theoretical and experimental research of hydrogen storage properties of Mg and Mg–Al hydrides. J. Alloys Compd. 2022, 907, 168618. [Google Scholar] [CrossRef]
- Martinez-Garcia, A.; Estrada-Guel, I.; Reguera, E.; Amaro-Hernandez, R.; González, S.; Garay-Reyes, C.G.; Martínez-Sánchez, R. Design and mechanosynthesis of low-weight high-entropy alloys with hydrogen storage potential properties. Hydrogen 2024, 5, 670–684. [Google Scholar] [CrossRef]
- Skakov, M.; Kozhakhmetov, Y.; Mukhamedova, N.; Miniyazov, A.; Sokolov, I.; Urkunbay, A.; Zhanbolatova, G.; Tulenbergenov, T. Effect of high-temperature treatment on structural-phase state and mechanical properties of IMC of the Ti-25Al-25Nb at.% System. Materials 2022, 15, 5560. [Google Scholar] [CrossRef] [PubMed]
- Kabirian, F.; Mahmudi, R. Impression creep behavior of a cast AZ91 magnesium alloy. Metall. Mater. Trans. 2009, 40, 116–127. [Google Scholar] [CrossRef]
- Schmid-Fetzer, R.; Gröbner, J. Thermodynamic Database for Mg Alloys—Progress in Multicomponent Modeling. Metals 2012, 2, 377–398. [Google Scholar] [CrossRef]
- Matysina, Z.; Zolotarenko, A.; Kartel, M.; Veziroglu, A.; Veziroglu, T.; Gavrylyuk, N.; Schur, D.; Gabdullin, M.; Akhanova, N.; Ramazanov, T.; et al. Hydrogen in magnesium alanate Mg(AlH4)2, aluminum and magnesium hydrides. Int. J. Hydrogen Energy 2023, 48, 2271–2293. [Google Scholar] [CrossRef]
- Nadaraia, V.; Suchkov, S.N.; Imshinetskiy, I.M.; Mashtalyar, D.V.; Kosianov DYu Belov, E.A.; Sinebryukhov, S.L.; Gnedenkov, S.V. New superhydrophobic composite coatings on Mg-Mn-Ce magnesium alloy. J. Magnes. Alloys 2023, 11, 1721–1739. [Google Scholar] [CrossRef]
- Baklanov, V.; Zhanbolatova, G.; Skakov, M.; Miniyazov, A.; Sokolov, I.; Tulenbergenov, T.; Kozhakhmetov, Y.; Bukina, O.; Orazgaliev, N. Study of the temperature dependence of a carbidized layer formation on tungsten under plasma irradiation. Mater. Res. Express 2022, 9, 016403. [Google Scholar] [CrossRef]
- Crivello, J.-C.; Nobuki, T.; Kato, S.; Abe, M.; Kuji, T. Hydrogen absorption properties of the γ-Mg17Al12 phase. J. Adv. Sci. 2008, 19, 88–96. [Google Scholar] [CrossRef]
- Crivello, J.-C.; Nobuki, T.; Kato, S.; Abe, M.; Kuji, T. Hydrogen absorption properties of the γ-Mg17Al12 phase and its Al-richer domain. J. Alloys Compd. 2007, 446–447, 157–161. [Google Scholar] [CrossRef]
- Han, G.; Lu, Y.; Jia, H.; Ding, Z.; Wu, L.; Shi, Y.; Wang, G.; Luo, Q.; Chen, Y.; Wang, J.; et al. Magnesium-based energy materials: Progress, challenges, and perspectives. J. Magnes. Alloys 2023, 11, 3896–3925. [Google Scholar] [CrossRef]
- Mukhamedova, N.M.; Miniyazov, A.Z.; Zhanbolatova, G.K.; Ospanova, Z.N.; Sabyrtayeva, A.A.; Shaikieva, K.S. Evolution of phase transformations in the Mg–Ni–Ce system after mechanical synthesis and spark plasma sintering. Materials 2025, 18, 2131. [Google Scholar] [CrossRef]
- Aguey-Zinsou, K.F.; Ares-Fernandez, J.R. Hydrogen in magnesium: New perspectives toward functional stores. Energy Environ. Sci. 2010, 3, 526–543. [Google Scholar] [CrossRef]
- Wang, X.L.; Tu, J.P.; Zhang, P.L.; Zhang, X.B.; Chen, C.P.; Zhao, X.B. Hydrogen storage properties of the mechanically alloyed Mg–Mg17Al12 composite. Int. J. Hydrogen Energy 2007, 32, 2134–2142. [Google Scholar] [CrossRef]
- Ning, H.; Wei, G.; Chen, J.; Meng, Z.; Wang, Z.; Lan, Z.; Huang, X.; Chen, J.; Qing, P.; Liu, H.; et al. Investigation on hydrogenation performance of Mg17Al12 by adding Y. Sci. Rep. 2024, 14, 18115. [Google Scholar] [CrossRef]
- Andreasen, A. Hydrogenation properties of Mg–Al alloys. Int. J. Hydrogen Energy 2008, 33, 7489–7497. [Google Scholar] [CrossRef]
- Peng, W.; Lan, Z.; Wei, W.; Xu, L.; Guo, J. Investigation on preparation and hydrogen storage performance of Mg17Al12 alloy. Int. J. Hydrogen Energy 2016, 41, 1759–1765. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, R.; Li, L.; Xiao, H.; Jiang, Y. Microstructure and Properties of Semi-Solid ZCuSn10P1 Alloy Processed with an Enclosed Cooling Slope Channel. Metals 2018, 8, 275. [Google Scholar] [CrossRef]
- Ryabicheva, M.; Zhigalenok, Y.; Abdimomyn, S.; Skakov, M.; Miniyazov, A.; Zhanbolatova, G.; Mukhamedova, N.; Ospanova, Z.; Djenizian, T.; Malchik, F. From lab to market: Economic viability of modern hydrogen evolution reaction catalysts. Fuel 2025, 395, 135227. [Google Scholar] [CrossRef]
- Lucaci, M.; Biris, A.R.; Orban, R.L.; Sbarcea, G.B.; Tsakiris, V. Effects of mechanical alloying on the hydrogen storage properties of the Mg76Ti12Fe12−xNix (x = 4, 8) materials. J. Alloys Compd. 2009, 484, 89–94. [Google Scholar] [CrossRef]
- Pasquini, L. The Effects of Nanostructure on the Hydrogen Sorption Properties of Magnesium-Based Metallic Compounds: A Review. Crystals 2018, 8, 106. [Google Scholar] [CrossRef]
- Mukhamedova, N.; Miniyazov, A.; Sabyrtayeva, A.; Tulenbergenov, T.; Oken, O. Dispersion of Sintered Mg-Ni-Ce Materials for Efficient Hydrogen Storage. Crystals 2025, 15, 743. [Google Scholar] [CrossRef]
- Batyrbekov, E.; Khasenov, M.; Gordienko, Y.; Samarkhanov, K.; Ponkratov, Y. Optical radiation from the sputtered species under gas excitation by the products of the 6Li(n,α)3H nuclear reaction. J. Lumin. 2020, 220, 116973. [Google Scholar] [CrossRef]
- Batyrbekov, E.; Khasenov, M.; Gordienko, Y.; Samarkhanov, K.; Kenzhina, I.E.; Kotlyar, A.; Miller, A.; Tskhe, V.; Bochkov, V. Experimental Facility to Study the Threshold Characteristics of Laser Action at the p-s-Transition of Noble Gas Atom upon Excitation by 6Li(n,α)3H Nuclear Reaction Products. Appl. Sci. 2022, 12, 12889. [Google Scholar] [CrossRef]
- Batyrbekov, E.; Khasenov, M.; Skakov, M.; Gordienko, Y.; Samarkhanov, K.; Kotlyar, A.; Bochkov, V. High-Energy Tritium Ion and α-Particle Release from the Near-Surface Layer of Lithium During Neutron Irradiation in the Nuclear Reactor Core. Fusion Sci. Technol. 2023, 80, 520–529. [Google Scholar] [CrossRef]
- Ponkratov, Y.; Batyrbekov, E.; Khasenov, M.; Samarkhanov, K.; Chikhray, Y. Application of High-Energy Tritium Ions and Alpha Particles Formed in 6Li(n,α)T Nuclear Reaction to Excite the Luminescence of Inert Gas Mixtures. Fusion Sci. Technol. 2021, 77, 327–332. [Google Scholar] [CrossRef]
- Bochkov, V.; Ponkratov, Y.; Nikitenkov, N.; Baklanova, Y.; Gordienko, Y.; Tulubayev, Y.; Samarkhanov, K.; Karambayeva, I. Determination of Thermophysical Properties of Prototypes of Tin-Lithium Alloy by Differential Scanning Calorimetry. J. Phys. Conf. Ser. 2022, 2155, 012016. [Google Scholar] [CrossRef]
- Kulsartov, T.; Kenzhina, I.; Ponkratov, Y.; Gordienko, Y.; Zaurbekova, Z.; Samarkhanov, K.; Askerbekov, S.; Kenzhin, Y.A.; Yelishenkov, A.B. Investigation of the Interaction of Deuterium with Sn73Li27 Tin-Lithium Alloy. Nucl. Mater. Energy 2024, 41, 101825. [Google Scholar] [CrossRef]
- Kenzhin, Y.; Kenzhina, I.; Kulsartov, T.; Zaurbekova, Z.; Askerbekov, S.; Ponkratov, Y.; Gordienko, Y.; Yelishenkov, A.; Udartsev, S. Study of Hydrogen Sorption and Desorption Processes of Zirconium Beryllide ZrBe2. Nucl. Mater. Energy 2024, 39, 101634. [Google Scholar] [CrossRef]
- Shi, R.; Luo, A.A. Applications of CALPHAD modeling and databases in advanced lightweight metallic materials. Calphad 2018, 62, 1–17. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Calka, A.; Wexler, D. Mechanical milling assisted by electrical discharge. Nature 2002, 419, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Miniyazov, A.; Skakov, M.; Tulenbergenov, T.; Sokolov, I.; Mukhamedova, N.; Agatanova, A.; Sabyrtaeva, A.; Akhmedi, T. Structural evolution of carbon from methane pyrolysis in microwave plasma. Carbon Trends 2025, 21, 100552. [Google Scholar] [CrossRef]
- Malik, S.; Zhumadil, K.; Avchukir, K.; Skakov, M.; Miniyazov, A.; Mukhamedova, N.; Zhanbolatova, G.; Malchik, F. Enhancing electrochemical performance of LaNi5 anodes using MXene as a multifunctional additive for Ni-MH batteries. J. Electroanal. Chem. 2025, 996, 119409. [Google Scholar] [CrossRef]
- Abdimomyn, S.; Zhigalenok, Y.; Skakov, M.; Miniyazov, A.; Mukhamedova, N.; Malchik, F. Fundamental aspects and electrochemical investigation of metal hydride electrodes: Principles, methods, and practical insights. Applied Physics Reviews. Appl. Phys. Rev. 2025, 12, 031331. [Google Scholar] [CrossRef]
- Huot, J.; Liang, G.; Boily, S.; Van Neste, A.; Schulz, R. Structural study and hydrogen sorption kinetics of ball-milled magnesium hydride. J. Alloys Compd. 1999, 293–295, 495–500. [Google Scholar] [CrossRef]
- Kang, Y.; Zhang, K.; Lin, X. Surface Modifications of Magnesium-Based Materials for Hydrogen Storage and Nickel–Metal Hydride Batteries: A Review. Coatings 2023, 13, 1100. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, Y.; Li, Y.; Hao, Y.; Wu, P.; Ding, Z. Magnesium-Based Hydrogen Storage Alloys: Advances, Strategies, and Future Outlook for Clean Energy Applications. Molecules 2024, 29, 2525. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, J.; Xiao, H.; Shen, H.; Xie, L.; Sun, G.; Zu, X. A First-Principles Study of Hydrogen Desorption from High Entropy Alloy TiZrVMoNb Hydride Surface. Metals 2021, 11, 553. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, J.; Bowman, R.C., Jr.; Fang, Z.Z. Roles of Ti-Based Catalysts on Magnesium Hydride and Its Hydrogen Storage Properties. Inorganics 2021, 9, 36. [Google Scholar] [CrossRef]
- Skakov, M.K.; Kabdrakhmanova, S.K.; Akatan, K.; Zhilkashinova, A.M.; Shaimardan, E.; Beisebekov, M.M.; Nurgamit, K.; Baklanov, V.V.; Koyanbayev, Y.T.; Miniyazov, A.Z.; et al. La2CuO4 Electrode Material For Low Temperature Solid Oxide Fuel Cells. ES Mater. Manuf. 2023, 22, 969. [Google Scholar] [CrossRef]
- Mukhamedova, N.; Kozhakhmetov, Y.; Skakov, M.; Kurbanbekov, S.; Mukhamedov, N. Microstructural stability of a two-phase (O + B2) alloy of the Ti–25Al–25Nb system (at.%) during thermal cycling in a hydrogen atmosphere. AIMS Mater. Sci. 2022, 9, 270–282. [Google Scholar] [CrossRef]
- Zhu, M.; Lu, Y.; Ouyang, L.; Wang, H. Thermodynamic Tuning of Mg-Based Hydrogen Storage Alloys: A Review. Materials 2013, 6, 4654–4674. [Google Scholar] [CrossRef]
- Kozhahmetov, Y.; Mukhamedova, N.; Urkunbay, A.; Yerkezhan, T.; Yermolenko, M. Structural and mechanical properties of heat resistant titanium allows of the Ti-24.5Al-24.5Nb (at. %) system. Mater. Today Proc. 2023, 81, 1216–1222. [Google Scholar] [CrossRef]
- Leiva, D.R.; Jorge, A.M., Jr.; Ishikawa, T.T.; Botta, W.J. Hydrogen Storage in Mg and Mg-Based Alloys and Composites Processed by Severe Plastic Deformation. Mater. Trans. 2019, 60, 1561–1570. [Google Scholar] [CrossRef]
- Wan, L.F.; Liu, Y.; Cho, E.S.; Forster, J.D.; Jeong, S.; Wang, H.; Urban, J.J.; Guo, J.; Prendergast, D. Atomically thin interfacial suboxide key to hydrogen storage performance enhancements of magnesium nanoparticles encapsulated in reduced graphene oxide. Nano Lett. 2017, 17, 5540–5545. [Google Scholar] [CrossRef]
- Gavra, Z.; Hadari, Z.; Mintz, M.H. Effects of nickel and indium ternary additions on hydrogenation of Mg–Al intermetallics. J. Inorg. Nucl. Chem. 1981, 43, 1763–1768. [Google Scholar] [CrossRef]
- de Lima-Andreani, G.F.; Fazan, L.H.; Baptistella, E.B.; Oliveira, B.D.; Cardoso, K.R.; Travessa, D.N.; Neves, A.M.; Jorge, A.M. The Effect of Air Exposure on the Hydrogenation Properties of 2Mg-Fe Composite after Mechanical Alloying and Accumulative Roll Bonding (ARB). Metals 2023, 13, 1544. [Google Scholar] [CrossRef]
- Kudiiarov, V.N.; Kenzhiyev, A.; Elman, R.R.; Kurdyumov, N.; Ushakov, I.A.; Tereshchenko, A.V.; Laptev, R.S.; Kruglyakov, M.A.; Khomidzoda, P.I. The Defect Structure Evolution in MgH2-EEWNi Composites in Hydrogen Sorption–Desorption Processes. Metals 2025, 15, 72. [Google Scholar] [CrossRef]
- Shang, Y.; Pistidda, C.; Gizer, G.; Klassen, T.; Dornheim, M. Mg-based materials for hydrogen storage. J. Magnes. Alloys 2021, 9, 1837–1860. [Google Scholar] [CrossRef]
- Jain, I.P.; Lal, C.; Jain, A. Hydrogen storage in Mg: A most promising material. Int. J. Hydrogen Energy 2010, 35, 5133–5144. [Google Scholar] [CrossRef]
- Lv, P.; Guzik, M.N.; Sartori, S.; Huot, J. Effect of ball milling and cryomilling on the microstructure and first hydrogenation properties of TiFe + 4 wt.% Zr alloy. J. Mater. Res. Technol. 2019, 8, 1828–1834. [Google Scholar] [CrossRef]
- Lass, E.A. Hydrogen storage measurements in novel Mg-based nanostructured alloys produced via rapid solidification and devitrification. Int. J. Hydrogen Energy 2011, 36, 10787–10796. [Google Scholar] [CrossRef]
- Williams, M.; Nechaev, A.N.; Lototskyy, M.V.; Yartys, V.A.; Solberg, J.K.; Denys, R.V.; Pineda, C.; Li, Q.; Linkov, V.M. Influence of aminosilane surface functionalization of rare earth hydride-forming alloys on palladium treatment by electroless deposition and hydrogen sorption kinetics of composite materials. Mater. Chem. Phys. 2009, 115, 136–141. [Google Scholar] [CrossRef]
- Parambhath, V.B.; Nagar, R.; Ramaprabhu, S. Effect of Nitrogen Doping on Hydrogen Storage Capacity of Palladium Decorated Graphene. Langmuir 2012, 28, 7826–7833. [Google Scholar] [CrossRef]
- Charbonnier, M.; Romand, M.; Goepfert, Y.; Leonard, D.; Bessueille, F.; Bouadi, M. Palladium(+2) reduction: A key step for the electroless Ni metallization of insulating substrates by a tin-free process. Thin Solid Film. 2006, 515, 1623–1633. [Google Scholar] [CrossRef]
- Shan, X.; Payer, J.H.; Jennings, W.D. Mechanism of increased performance and durability of Pd-treated metal hydriding alloys. Int. J. Hydrogen Energy 2009, 34, 363–369. [Google Scholar] [CrossRef]
- Ding, Z.; Li, Y.; Yang, H.; Lu, Y.; Tan, J.; Li, J.; Li, Q.; Chen, Y.; Shaw, L.L.; Pan, F. Tailoring MgH2 for hydrogen storage through nanoengineering and catalysis. J. Magnes. Alloys 2022, 10, 2946–2967. [Google Scholar] [CrossRef]
- Li, F.; Liu, D.; Sun, K.; Yang, S.; Peng, F.; Zhang, K.; Guo, G.; Si, Y. Towards a Future Hydrogen Supply Chain: A Review of Technologies and Challenges. Sustainability 2024, 16, 1890. [Google Scholar] [CrossRef]
- Habibi, M.; Hosseini, M.G.; Wang, K. Toward sustainable energy: A comprehensive review of hydrogen production, storage, and utilization. Renew. Sustain. Energy Rev. 2025, 226, 116193. [Google Scholar] [CrossRef]
- Konovalov, D.; Tolstorebrov, I.; Iwamoto, Y.; Lamb, J.J. Hydrogen and Japan’s Energy Transition: A Blueprint for Carbon Neutrality. Hydrogen 2025, 6, 61. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, X.; Li, Y.; Zhao, Y.; Shu, Y.; Zhang, G.; Yang, T.; Liu, Y.; Wu, P.; Ding, Z. Rare-Earth Metal-Based Materials for Hydrogen Storage: Progress, Challenges, and Future Perspectives. Nanomaterials 2024, 14, 1671. [Google Scholar] [CrossRef]
- Garbiec, D.; Leshchynsky, V.; García-Junceda, A.; Swadźba, R.; Siwak, P.; Adamek, G.; Radwański, K. Microstructure and Mechanical Properties of Spark Plasma Sintered and Severely Deformed AA7075 Alloy. Metals 2021, 11, 1433. [Google Scholar] [CrossRef]
- Dong, X.; Li, Y.; Zhai, Y.; Liu, Z.; Zhang, G.; Yang, F. Study on Microstructure and Hydrogen Storage Properties of Mg80Ni16−xAlxY4 (x = 2, 4, 8) Alloys. Metals 2024, 14, 126. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).