Abstract
This study investigates the influence of rhizosphere dynamics and soil chemical properties on the distribution, abundance, and diversity of arbuscular mycorrhizal fungi (AMF) across two seasons (summer and winter). A total of 11 rhizospheric soil and root samples were collected from various wild plant species, including Senna italica, Cyperus laevigatus, Phragmites australis, Pelargonium peltatum, Zygophyllum simplex, Citrullus colocynthis, Malva parviflora, Zygophyllum coccineum, Calotropis procera, Solanum nigrum, and Salsola baryosm. Phragmites australis exhibited the highest AMF spore count (175 and 124/100 g dry soil in summer and winter), while Calotropis procera showed the lowest (101 and 63/100 g). AMF species identified included Glomus ambisporum, Rhizophagus intraradices, Claroideoglomus etunicatum, Diversispora globifera, Funneliformis geosporum, Funneliformis mosseae, Rhizophagus fasciculatus, and Gigaspora spp. The Shannon diversity index ranged from 0.692 (Zygophyllum simplex) to 0.653 (Salsola imbricata), and Simpson’s index from 0.498 to 0.461. Phragmites australis recorded the highest root colonization (90.5% and 84.7%), arbuscule (76% and 69.3%), and vesicle formation (36%) in summer, while Calotropis procera had the lowest. In summer, AMF spore counts showed significant correlations with soil nutrients (N, P, K), and with total organic carbon (TOC) and organic matter (OM). During winter, TOC and OM remained influential, while correlations with nutrients weakened. Soil pH, electrical conductivity (EC), and texture exhibited minimal correlation with AMF spore counts in both seasons.
1. Introduction
Arbuscular mycorrhizal fungi (AMF) are globally widespread and constitute a significant component of the soil microbial community, forming symbiotic associations with nearly 80% of wild plants and numerous agricultural crops [,]. These fungi play a critical role in ecosystem functioning, influencing nutrient uptake, productivity, and providing plants with enhanced defense mechanisms against various environmental and agricultural stresses, including drought, salinity, and acidity. Despite their ecological importance, the distribution, abundance, and diversity of AMF are influenced by various environmental and soil factors, including root density, soil physicochemical properties, and seasonal changes [,,,]. Plant root density significantly affects AMF spore counts in the soil. Researchers such as Cuenca and Lovera and Anderson et al. have reported that AMF spores may be nearly absent in soils with diminished root densities. This indicates the crucial role that root presence plays in the distribution and abundance of AMF, as these fungi rely on host plants for their development and symbiotic functions. Additionally, Brundrett highlighted that AMF spore production is influenced by plant phenology, further emphasizing the interplay between plant developmental stages and AMF dynamics [,,].
Soil characteristics, including pH, moisture content, and disturbance such as high salinity, are important determinants of AMF distribution and abundance. Soil pH has been a focus of numerous studies, with some researchers reporting no significant correlation between AMF spore counts and pH levels due to changes in soil chemistry. However, Shukla et al. found a positive correlation between AMF spore populations and soil pH, indicating that soil pH may play a more nuanced role in influencing fungal populations [,,,,].
In addition to pH, soil organic matter content is a key factor in AMF distribution. Oehl et al. suggested that a decrease in organic content may reduce AMF spore numbers and presence in the soil. Moreover, soil disturbances, such as plowing and land use changes, have been shown to negatively impact AMF spore counts [,,].
Seasonal changes significantly influence AMF colonization and spore production. Titus et al. noted that seasonal changes affect AMF colonization by influencing the developmental and physiological stages of the host plant. Khan found that AMF root colonization and endogone spore abundance fluctuate seasonally, with spore numbers peaking during the months of October to January. Similarly, Guadarrama and Álvarez-Sánchez reported that AMF species diversity and spore counts are higher during the dry season compared to the rainy season, highlighting the impact of seasonal moisture variations on AMF communities. Studies by Adil et al. also indicated that seasonal variations in temperature and soil moisture have a marked effect on AMF spore production, and the abundance of AMF populations is significantly influenced by the season. This underscores the challenge of establishing clear patterns of AMF distribution and abundance due to seasonal fluctuations [,,,].
The influence of edaphic and climatic factors on AMF dynamics is well-documented. Lingfei et al. indicated that edaphoclimatic characteristics affect both the temporal and spatial dynamics of AMF infection and spore populations. Soil moisture, pH, and depth are key factors affecting AMF spore populations with increasing soil depth. Further studies have shown that soil fungal diversity tends to be higher in environments with lower pH levels. Climate factors, including temperature, precipitation, and cold tolerance, also play significant roles in AMF distribution. The interaction between soil fungi and their environment, including competition and mutualism, may further influence AMF community structure [,,,].
Recent molecular studies using high-throughput sequencing have deepened our understanding of AMF communities, particularly in relation to rhizosphere processes and soil chemistry. For example, Zhang et al. found that rhizosphere soils often harbor greater AMF diversity than plant roots, likely due to microenvironmental factors such as root exudates and localized nutrient availability. Likewise, Luo et al. reported strong correlations between soil properties—such as pH, organic matter, phosphorus, and nitrogen—and AMF richness across arid desert landscapes. Spatial heterogeneity, driven by topography, moisture gradients, and host plant identity, further contributes to variation in the abundance of AMF genera such as Glomus and Diversispora [,].
Despite this growing body of research, knowledge of AMF dynamics in arid and semi-arid environments remains limited—particularly regarding seasonal changes in colonization and diversity in wild plant communities. In regions such as Riyadh, where extreme climate conditions prevail, such knowledge is essential for understanding plant–fungal interactions and ecosystem resilience. Therefore, this study aims to investigate how selected physical and chemical soil properties affect AMF spore abundance, species richness, and root colonization intensity. By comparing samples collected in two distinct seasons, we assess how environmental variability shapes AMF communities in the wild ecosystems surrounding the Riyadh region.
2. Materials and Methods
2.1. Sampling Collection Strategies
Eleven species of wild plants associated with AMF were targeted to study the seasonal variation in AMF such as the following: Senna italica, Cyperus laevigatus, Phragmites australis, Pelargonium peltatum, Zygophyllum simplex, Citrullus colocynthis, Malva parviflora, Zygophyllum cocceinum, Calotropis procera, Solanum nigrum, Salsola baryosm, where all samples were collected during two different seasons. Both soil and plant roots samples were gathered from the rhizosphere zone of the targeted plants at a depth of 3–10 cm. Three replicates were randomly collected per plant during summer and winter seasons, respectively, to obtain a total of 33 samples per season. All samples were put into polythene bags and labeled with data of samples such as plant name, date, and location; then, we brought them to the laboratory. In lab, root samples were thoroughly cleaned with tap water to remove adhered soil, and fine roots were carefully separated from the whole mass of roots. Fine roots were cut into 1 cm fragments and immersed in FAA solution in glass tubes and stored at 4 °C for later analysis. The soil samples were air-dried at lab temperature for 15 days then stored at 4 °C for soil physicochemical properties analysis, AMF spore counts, abundance, and diversity [].
2.2. Extraction of AMF Spores
AMF spores were extracted according to wet sieving and decanting method by Khan, and AMF spores were counted as numbers/100 g soil; spores were identified using synoptic keys provided on the INVAM website and Schenck and Perez manual (1990) [].
2.3. Measurement AMF Colonization Ratio
To measure AMF colonization ratio in roots, fresh fine roots were carefully selected, stained, and studied according to methods of Phillips and Hayman (1970) and Al Qarawi et al. (2012) [,].
2.4. Soil Physicochemical Characteristics
Soil pH and electrical conductivity (EC) were assessed using a 1:1 mixture of soil and deionized water and measured by a pH meter and an EC meter. The distribution of soil particle sizes was examined using the hydrometer technique, and soil texture was classified according to the USDA soil textural triangle. Cation exchange capacity (CEC) was evaluated through extraction with 1 N ammonium acetate (NH4OAc) at pH 7.0. Concentrations of exchangeable cations (Ca2+, Mg2+, K+, and Na+) were determined in the extract using inductively coupled plasma–optical emission spectrometry (ICP-OES). Calcium carbonate (CaCO3) content was quantified using the calcimeter technique. Soil organic matter (OM) was estimated via the Walkley–Black wet oxidation method [,].
2.5. Statistical Analyses
The acquired data were arranged, and descriptive statistical analyses including mean, minimum, maximum, standard deviation (SD), and coefficient of variance (CV) were performed using SPSS 27 (IBM Corp., New York, NY, USA) and Microsoft Excel. The figures were drawn using Microsoft Excel program. Comparison of the mean was made with Statistical Package for Social Studies (SPSS 27; IBM Corp., New York, NY, USA) using least significant difference at the risk level of 5% [].
3. Results and Discussion
The average values of physiochemical properties of soils are given in Table 1, which indicates that the soil texture was a sandy loam with an alkaline pH of 8.1, moderate salinity (EC 2.8 dS/m), and high calcium CaCO3 content of (11.91%) which is a typical of arid environments. These conditions can restrict nutrient availability and make it more difficult for AMF to colonize roots. For instance, sandy soils have low water retention, alkaline pH limits phosphorus availability, and salinity can inhibit fungal growth. Despite these challenges, many AMF species are well-adapted to such stresses and play a vital role in helping plants access nutrients in harsh environments. Therefore, these soil characteristics likely influence AMF species and how effectively they dominate. The results which are presented in Table 2, Table 3, Table 4 and Table 5 highlight the interplay between rhizosphere dynamics, soil chemical characteristics, and the distribution and abundance of arbuscular mycorrhizal fungi (AMF) across different plant species during the summer and winter seasons. The analysis underscores the critical role of the rhizosphere environment in shaping AMF colonization and community diversity, demonstrating how seasonal variations and plant-specific interactions influence fungal presence and activity.

Table 1.
Soil physicochemical characteristics.

Table 2.
Soil chemical characteristics of rhizosphere plants during the summer season.

Table 3.
Soil chemical characteristics of rhizosphere plants during the winter season.

Table 4.
Spore count and AMF colonization percentage of different plants during summer season.

Table 5.
Spore count and AMF colonization percentage of different plants during winter season.
3.1. Rhizosphere Soil Nutrients
Soil nutrient composition, as shown in Table 2 and Table 3, varied significantly among plant species and across seasons, reflecting species-specific rhizosphere dynamics. Nitrogen (N) levels, in particular, differ significantly among plant species and seasons, showing the impact of plant-specific rhizosphere dynamics. Nitrogen (N) levels were the highest in Phragmites australis during summer (437.8 mg/kg) and winter (482.5 mg/kg), likely due to its dense root system enhancing microbial activity and nutrient cycling. Conversely, Calotropis procera showed the lowest nitrogen levels (70.7 mg/kg in summer and 72 mg/kg in winter), possibly due to its adaptation to nutrient-poor soils and the inhibitory effects of its allelopathic activity on microbial and AMF colonization. These results (as given in Table 2 and Table 3) show a close link between plant traits, rhizosphere processes, and soil nutrients.
Phosphorus (P) availability was generally low in summer, ranging from 1.3 mg/kg in Phragmites australis to 0.1 mg/kg in Calotropis procera. In winter, P levels ranged from 1.5 mg/kg to 0.1 mg/kg for the same species (Table 2 and Table 3). On the other hand, the study revealed that potassium (K), sodium (Na), calcium (Ca), and magnesium (Mg) concentrations showed notable variability, reflecting differences in soil type, plant exudates, and microbial interactions, particularly, arbuscular mycorrhizal fungi (AMF) during the summer and winter seasons (Table 2 and Table 3). Both total organic carbon (TOC) and organic matter (OM) showed seasonal variations in Phragmites australis, with lower values recorded during summer (2.2% for TOC and 3.6% for OM). These values increased slightly in winter, reaching 2.1% for TOC and 3.4% for OM, possibly due to enhanced microbial activity under optimal soil temperature conditions. In contrast, Calotropis procera consistently exhibited lower TOC and OM values, with summer measurements of 0.53% and 0.5%, respectively, and a slight increase in winter to 0.6% for TOC and 1.41% for OM. This seasonal variation highlights the influence of temperature and microbial activity on soil organic content.
3.2. AMF Spore Count and Community Diversity
The results, as shown in Table 4 and Table 5, highlight the influence of seasonal variation and rhizosphere dynamics on AMF spore counts. In summer, Phragmites australis and Cyperus rotundus recorded the highest spore counts, with 175 and 162 spores/100 g of soil, respectively. In winter, their counts decreased to 124 and 116 spores/100 g. In contrast, Calotropis procera consistently showed the lowest spore counts in both seasons—101 spores/100 g in summer and 63 spores/100 g in winter.
3.3. AMF Spore Diversity
Shannon biodiversity indices of AMF spores revealed that the values ranged between 0.692 for Zygophyllum simplex and 0.653 for Salsola imbricata, whereas Simpson biodiversity indices of AMF spores recorded values that ranged between 0.498 for Zygophyllum simplex and Citrullus colocynthis and 0.461 for Salsola imbricate (Table 6). Overall, the findings concluded that a wide range of AMF spore species was identified and isolated from various wild plant rhizosphere zones, including Glomus ambisporum, Claroideoglomus etunicatum, Rhizophagus intraradices, and Diversispora globifera; in the same context, it is notable that the G. ambisporum was the most common among different plant rhizospheres which may reflect how rhizosphere dynamics and root attraction influence the arbuscular mycorrhizal fungi (AMF) community, which varied among the studied plant species, indicating differences in symbiotic associations (Table 6 and Figure 1).

Table 6.
Shannon–Simpson biodiversity index of AMF spores isolated from the rhizosphere of different plant species during the summer and winter seasons.
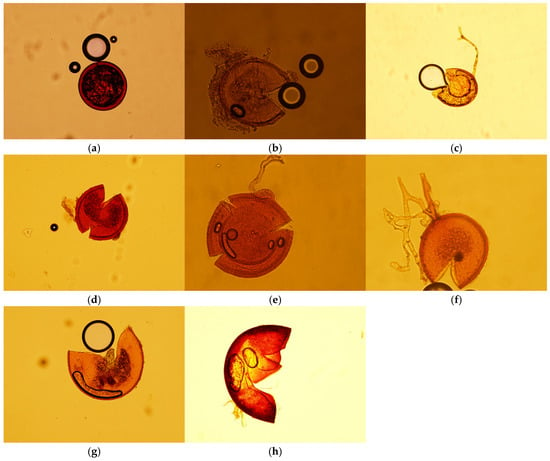
Figure 1.
AMF spores isolated from a various wild plants during summer and winter: (a) intact spore of Glomus ambisporum, (b) crashed spore of Rhizophagus intraradices, (c) crashed spore of Claroideoglomus etunicatum, (d) crashed spore of Rhizophagus fasciculatus, (e) crashed spore of Diversispora globifera, (f) crashed spore of Funneliformis geosporume, (g) crashed spore of Funneliformis mosseae, (h) crashed spore of Gigaspora spp. Scale bar: 100 µm.
3.4. AMF Structure Colonization Percentage % (Mycelium, Vesicles, and Arbuscules)
Regarding Mycelium percentage, the results revealed that the Phragmites australis recorded a higher value of Mycelium percentage at 90% compared to 57% for Calotropis procera in summer; the Mycelium percentage recorded a lower value at 84% and 55% for Phragmites australis and Calotropis procera, respectively, in winter (Table 4 and Table 5).
3.5. Vesicles and Arbuscules
The findings showed that the percentage of vesicles in Phragmites australis roots increased noticeably to 36% in summer, followed by a significant decline to 24% in winter. In contrast, Calotropis procera exhibited a consistently lower vesicle percentage, declining significantly to 22% in summer and increasing to 42% in winter. Additionally, ANOVA analysis revealed that the percentage of arbuscules in Phragmites australis increased significantly to 76% in summer, but declined to 69% in winter (Table 7). On the other hand, Calotropis procera showed a significant decrease in arbuscule percentage to 49%, followed by a noticeable increase to 52% in winter (Table 4 and Table 5). The colonization structures of arbuscular mycorrhizal fungi (AMF), including hyphae (H), arbuscules (A), vesicles (V), and spores (S), were observed in the roots of various wild plant species, as shown in Figure 2. In Phragmites australis, spores, hyphae, and vesicles were found. Citrullus colocynthis had hyphae and vesicles. Malva parviflora showed hyphae and arbuscules. Calotropis procera had spores, hyphae, and vesicles. Pelargonium peltatum had hyphae and spores, and Cyperus rotundus had hyphae and vesicles. In Solanum nigrum, spores, hyphae, and vesicles were present, while Zygophyllum simplex showed spores and hyphae.

Table 7.
Pearson correlation between soil properties and AMF spores during winter and summer seasons.
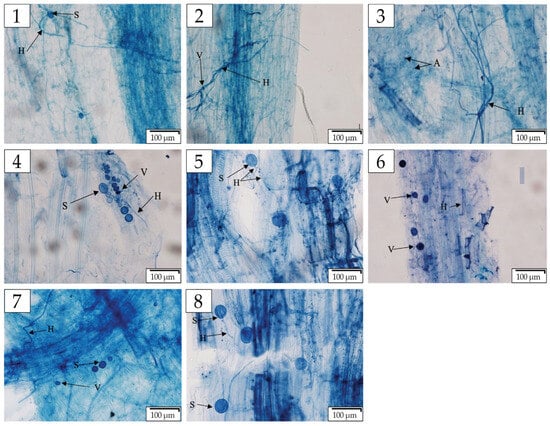
Figure 2.
Colonization structures of arbuscular mycorrhizal fungi (AMF), including hyphae, arbuscules, vesicles, and spores, observed in roots of various wild plants. AMF spores, hyphae, and spores were present in Phragmites australis roots (1); hyphae and vesicles in Citrullus colocynthis (2); hyphae and arbuscules in Malva parviflora (3); spores, hyphae, and vesicles in Calotropis procera (4); hyphae and spores in Pelagonium peltatum (5); hyphae and vesicles in Cyperus rotundus (6); spores, hyphae, and vesicles in Solanum nigrum (7); and spores and hyphae in Zygophyllum simplex (8). Scale bar: 100 µm. Abbreviations: H—Hyphae; S—Spore; V—Vesicle; A—Arbuscular.
The results showed that the number of AMF spores was strongly correlated to several soil nutrients and organic matter. Total organic carbon (TOC) and organic matter (OM) had the highest positive correlations with spore count in both summer and winter. This means that soils with more organic material tend to have more AMF spores, likely because organic matter improves soil health and supports fungal growth. Nutrients like phosphorus and nitrogen also showed strong positive correlations, especially in the summer, which may be due to higher plant activity during this time. Sodium had an unusually high correlation in summer, which could be related to how certain plants and fungi adapt to salty soils. On the other hand, factors like pH, salinity (EC), and soil texture (sand, silt, and clay) showed little or no correlation with AMF spore count. This suggests that organic matter and key nutrients are more important than soil structure in supporting AMF abundance
Our findings show that soil properties play a major role in shaping AMF spore density and distribution in the rhizosphere. These differences directly affect the chances of root infection and colonization. This agrees with Janowski and Leski (2022), who pointed out that fungal distribution in soils is largely controlled by soil chemistry, plant interactions, and dispersal pathways. We also found that nutrient availability depends not only on soil conditions but also on root system density and the presence of AMF spores and colonization. Similar observations were reported by Adil et al. (2017), who showed that both seasonal changes and host traits influence colonization intensity and spore abundance [,].
The Poaceae (Gramineae) family stood out in our study for its strong ability to form mutualistic associations with AMF. This is likely due to their dense, fibrous root systems, which create ideal conditions for fungal growth. Our results are consistent with Ahulu et al. (2005), who found that most Poaceae species are naturally mycorrhizal. In contrast, Calotropis procera consistently showed the lowest values for colonization and spore counts. This may be explained by its allelopathic properties and aggressive growth behavior, which can suppress AMF activity [].
Seasonal variation was another clear driver of AMF dynamics. Colonization rates and spore counts were lower in winter but rose sharply in summer. Warmer conditions likely provide more favorable soil temperatures and physicochemical traits for fungal activity. These results align with Tshwene-Mauchaza and Aguirre-Gutiérrez (2019), who highlighted the importance of climate—especially temperature and rainfall—in shaping AMF distribution and seasonal tolerance []. In addition, the results showed that in summer, almost all nutrients (N, P, K, Ca, Mg, Na) were strongly linked to spore counts. Sodium and total organic carbon (TOC) had the highest correlations, showing that when plants and microbes are most active, nutrients and carbon directly support spore production. Organic matter (OM) also showed a very strong relationship with spores. However, in winter, the pattern was different. TOC and OM still had strong correlations with spores, but most nutrients (especially K, Ca, and Mg) were less important. Nitrogen and phosphorus still influenced spores, but not as much as in summer. This suggests that in winter, when plant activity is lower, AMF rely more on organic matter and carbon in the soil rather than fresh nutrient availability. Overall, organic matter is important for AMF in both seasons, but nutrients play a bigger role during summer when conditions are better for growth.
Finally, our study suggests that declines in AMF colonization may also be linked to root architecture and nutrient levels at deeper soil layers. This pattern has been widely reported in earlier research (Jakobsen and Nielsen, 1983; Smith, 1978; Sutton, 1973; Sutton and Barron, 1972), which showed that both spore density and hyphal infection decrease with soil depth. Similar declines in colonized root length and spore counts were documented by Koide and Mooney (1987) and Zajicek et al. (1986). More recent studies (Higo et al., 2013; Oehl et al., 2005; Muleta et al., 2008; Säle et al., 2015) also support this trend, confirming that AMF abundance, spore density, and root colonization are generally reduced in deeper soil layers [,,,,,,,,,].
4. Conclusions
The study highlights the complex relationships between soil properties, seasonal changes, plant type, and the abundance and activity of arbuscular mycorrhizal fungi (AMF) in arid environments. Despite challenging soil physicochemical properties such as sandy loam texture, alkalinity, moderate salinity, and high CaCO3 content, the AMF were able to establish, sporulate, and form symbiotic associations with a range of local wild plants. Arid conditions usually limit nutrient availability and fungal growth, but they did not completely stop AMF activity. This shows how tough and adaptable these fungi are in harsh environments. One important finding of this study is that organic matter and soil nutrients play a major role in affecting the number of AMF spores. Both total organic carbon (TOC) and organic matter (OM) exhibited strong positive correlations with spore counts across seasons, underlining the importance of soil organic content in maintaining and enhancing fungal populations.
Nutrients like phosphorus and nitrogen, especially in summer, helped increase AMF activity. This is likely because plants grow more and have higher metabolism, which helps fungi develop. These results show that organic matter and nutrients matter more than soil texture or salinity for AMF growth in dry soils. Soil pH, electrical conductivity (EC), and texture (sand, silt, and clay) are usually important for soil microbes, but they showed little or no effect on AMF spore density here. This may imply that AMF communities in arid environments have adapted to a broad range of physical soil characteristics and are more sensitive to biological and chemical properties, especially organic inputs and nutrient cycling. Phragmites australis and Cyperus rotundus consistently supported higher nutrient levels, spore densities, and root colonization rates, positioning them as favorable partners for AMF in degraded or nutrient-deficient environments. In contrast, Calotropis procera showed the lowest AMF colonization, nutrient levels, and spore counts, potentially due to its allelopathic properties or association with less favorable soil conditions. These plant-specific differences highlight the importance of host selection in ecological restoration and sustainable land management, particularly in arid and semi-arid regions where soil fertility is a major constraint.
Seasonal variability also played a significant role in AMF dynamics, with summer months exhibiting greater AMF activity compared to winter. This is likely linked to enhanced plant growth and increased soil microbial activity under warmer conditions, which provide better opportunities for AMF sporulation and colonization. The dominance of widely distributed AMF species such as Glomus ambisporum, Rhizophagus intraradices, and Claroideoglomus etunicatum suggests that a core group of AMF taxa are capable of thriving in a range of plant–soil environments, albeit with varying levels of efficiency depending on host and season. Future research should clarify the roles of different AMF species in arid ecosystems under changing climates and diverse plants. Long-term studies can show how AMF respond to drought and heat. Molecular tools can reveal hidden diversity and functions. Studying AMF interactions with other microbes and testing organic amendments or bioinoculants may help improve soil health and sustainable farming in arid regions.
Author Contributions
Conceptualization, S.S.A., F.A. and S.N.S.; Formal analysis, S.S.A. and F.A.; Data curation, F.A., S.S.A. and S.N.S.; Writing—original draft preparation, B.A., S.S.A. and F.A.; Writing—review and editing, S.S.A., F.A., B.A. and S.S.A.; Supervision, S.S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded by Ongoing Research Funding program, King Saud University, Riyadh, Saudi Arabia. ORF-2025-866.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used in this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors extend their deep appreciation to King Saud University, Riyadh, Saudi Arabia for funding the Ongoing Research Funding program, (ORF-2025-866).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Smith, S.E.; Read, D. Mycorrhizal Symbiosis, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2008; ISBN 9780123705266. [Google Scholar]
- Brundrett, M.C.; Tedersoo, L. Evolutionary History of Mycorrhizal Symbioses and Global Host Plant Diversity. New Phytol. 2018, 220, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C. Arbuscular Mycorrhizae and Terrestrial Ecosystem Processes. Ecol. Lett. 2004, 7, 740–754. [Google Scholar] [CrossRef]
- Powell, J.R.; Rillig, M.C. Biodiversity of Arbuscular Mycorrhizal Fungi and Ecosystem Function. New Phytol. 2018, 220, 1059–1075. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A. Roles of Arbuscular Mycorrhizas in Plant Nutrition and Growth: New Paradigms from Cellular to Ecosystem Scales. Annu. Rev. Plant Biol. 2011, 62, 227–250. [Google Scholar] [CrossRef]
- Bohrer, K.E.; Friese, C.F.; Amon, J.P. Seasonal Dynamics of Arbuscular Mycorrhizal Fungi in Differing Wetland Habitats. Mycorrhiza 2004, 14, 329–337. [Google Scholar] [CrossRef]
- Cuenca, G.; Lovera, M. Seasonal Variation and Distribution at Different Soil Depths of Arbuscular Mycorrhizal Fungi Spores in a Tropical Sclerophyllous Shrubland. Botany 2010, 88, 54–64. [Google Scholar] [CrossRef]
- Anderson, E.L.; Millner, P.D.; Kunishi, H.M. Maize Root Length Density and Mycorrhizal Infection as Influenced by Tillage and Soil Phosphorus. J. Plant Nutr. 1987, 10, 1349–1356. [Google Scholar] [CrossRef]
- Brundrett, M.C. Mycorrhizal Associations and Other Means of Nutrition of Vascular Plants: Understanding the Global Diversity of Host Plants by Resolving Conflicting Information and Developing Reliable Means of Diagnosis. Plant Soil 2009, 320, 37–77. [Google Scholar] [CrossRef]
- Yang, F.Y.; Li, G.Z.; Zhang, D.E.; Christie, P.; Li, X.L.; Gai, J.P. Geographical and Plant Genotype Effects on the Formation of Arbuscular Mycorrhiza in Avena Sativa and Avena Nuda at Different Soil Depths. Biol. Fertil. Soils 2010, 46, 435–443. [Google Scholar] [CrossRef]
- Stürmer, S.L.; Siqueira, J.O. Species Richness and Spore Abundance of Arbuscular Mycorrhizal Fungi across Distinct Land Uses in Western Brazilian Amazon. Mycorrhiza 2011, 21, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Friese, C.F.; Koske, R.E. The spatial Dispersion of Spores of Vesicular-Arbuscular Mycorrhizal Fungi in a Sand Dune: Microscale Patterns Associated with the Root Architecture of American Beachgrass. Mycol. Res. 1991, 95, 952–957. [Google Scholar] [CrossRef]
- Bagyaraj, D. Ecology of Vesicular Arbuscular Mycorrhiza. In Handbook on Applied Mycology; Arora, D.K., Rai, B., Mukerji, K.G., Knudsen, G.R., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 1991; Volume 1, pp. 3–34. ISBN 0849356946. [Google Scholar]
- Shukla, A.; Vyas, D.; Jha, A. Soil Depth: An Overriding Factor for Distribution of Arbuscular Mycorrhizal Fungi. J. Soil Sci. Plant Nutr. 2013, 13, 23–33. [Google Scholar] [CrossRef]
- Oehl, F.; Sieverding, E.; Ineichen, K.; Ris, E.-A.; Boller, T.; Wiemken, A. Community Structure of Arbuscular Mycorrhizal Fungi at Different Soil Depths in Extensively and Intensively Managed Agroecosystems. New Phytol. 2005, 165, 273–283. [Google Scholar] [CrossRef]
- Land, S.; Schönbeck, F. Influence of Different Soil Types on Abundance and Seasonal Dynamics of Vesicular Arbuscular Mycorrhizal Fungi in Arable Soils of North Germany. Mycorrhiza 1991, 1, 39–44. [Google Scholar] [CrossRef]
- Karaarslan, E.; Uyanöz, R. Occurrence of Arbuscular Mycorrhizal Fungi in Some Native Plants Grown on Saline Soils Around the Lake Tuz in Turkey and Its Relations with Some Physical and Chemical Properties of Soil. Sci. Res. Essays 2011, 6, 4238–4245. [Google Scholar]
- Titus, J.H.; Titus, P.J.; Nowak, R.S.; Smith, S.D. Arbuscular Mycorrhizae of Mojave Desert Plants. West. N. Am. Nat. 2002, 62, 327–334. [Google Scholar]
- Khan, A.G. The Occurrence of Mycorrhizas in Halophytes, Hydrophytes and Xerophytes, and of Endogone Spores in Adjacent Soils. Microbiology 2000, 81, 7–14. [Google Scholar] [CrossRef]
- Guadarrama, P.; Álvarez-Sánchez, F.J. Abundance of Arbuscular Mycorrhizal Fungi Spores in Different Environments in a Tropical Rain Forest, Veracruz, Mexico. Mycorrhiza 1999, 8, 267–270. [Google Scholar] [CrossRef]
- Adil, S.; Muneer, A.; Imran, M.; Munir, M.Z.; Nisa, Z.; Elahi, H.; Gillani, S.M.N.; Wang, P.; Saifullah, N.-A.; Chaudhry, M.S. Seasonality of Arbuscular Mycorrhiza and Dark Septate Endophytes in Some Grasses under Arid Climatic Conditions. J. Agric. Res. 2017, 55, 601–610. [Google Scholar]
- Lingfei, L.; Anna, Y.; Zhiwei, Z. Seasonality of Arbuscular Mycorrhizal Symbiosis and Dark Septate Endophytes in a Grassland Site in Southwest China. FEMS Microbiol. Ecol. 2005, 54, 367–373. [Google Scholar] [CrossRef]
- Lomolino, M.V.; Riddle, B.R.; Whittaker, R.J. Biogeography: Biological Diversity Across Space and Time; Oxford University Press: Oxford, UK, 2017; ISBN 9781605354729. [Google Scholar]
- Janowski, D.; Leski, T. Factors in the Distribution of Mycorrhizal and Soil Fungi. Diversity 2022, 14, 1122. [Google Scholar] [CrossRef]
- Xu, D.; Yu, X.; Chen, J.; Liu, H.; Zheng, Y.; Qu, H.; Bao, Y. Arbuscular Mycorrhizae Fungi Diversity in the Root–Rhizosphere–Soil of Tetraena Mongolica, Sarcozygium Xanthoxylon, and Nitraria Tangutorum Bobr in Western Ordos, China. Agronomy 2023, 13, 1485. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Z.; He, Y.; Li, G.; Lv, X.; Zhuang, L. High-Throughput Sequencing Analysis of the Rhizosphere Arbuscular Mycorrhizal Fungi (AMF) Community Composition Associated with Ferula Sinkiangensis. BMC Microbiol. 2020, 20, 335. [Google Scholar] [CrossRef] [PubMed]
- Quilliam, R.S.; Jones, D.L. Fungal Root Endophytes of the Carnivorous Plant Drosera Rotundifolia. Mycorrhiza 2010, 20, 341–348. [Google Scholar] [CrossRef]
- Schenck, N.C.; Perez-Collins, Y. Manual for the Identification of Va Mycorrhizal Fungi; Synergistic Publications: London, UK, 1990. [Google Scholar]
- Phillips, J.M.; Hayman, D.S. Improved Procedures for Clearing Roots and Staining Parasitic and Vesicular-Arbuscular Mycorrhizal Fungi for Rapid Assessment of Infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161, IN16–IN18. [Google Scholar] [CrossRef]
- Al-Qarawi, A.A.; Mridha, M.A.U.; Alghamdi, O.M. Diversity of Structural Colonization and Spore Population of Arbuscular Mycorrhizal Fungi in Some Plants from Riyadh, Saudi Arabia. J. Pure Appl. Microbiol. 2012, 6, 1119–1125. [Google Scholar]
- Okalebo, J.R.; Gathua, K.W.; Woomer, P.L. Laboratory Methods of Soil and Plant Analysis: A Working Manual, 2nd ed.; Sustainable Agriculture Centre for Research and Development in East Africa: Nairobi, Kenya, 2002. [Google Scholar]
- Klute, A.; Page, A.L. Methods of Soil Analysis: Chemical and Microbiological Properties; ASA/SSSA. Agronomy; American Society of Agronomy: Madison, WI, USA, 1982; ISBN 9780891180722. [Google Scholar]
- Steel, R.G.D.; Torrie, J.H.; Dickey, D.A. Principles and Procedures of Statistics: A Biometrical Approach, 3rd ed.; McGraw Hill Book Co.: New York, NY, USA, 1997; ISBN 978-0-07-061028-6. [Google Scholar]
- Matekwor Ahulu, E.; Nakata, M.; Nonaka, M. Arum—And Paris—Type Arbuscular Mycorrhizas in a Mixed Pine Forest on Sand Dune Soil in Niigata Prefecture, Central Honshu, Japan. Mycorrhiza 2005, 15, 129–136. [Google Scholar] [CrossRef]
- Tshwene-Mauchaza, B.; Aguirre-Gutiérrez, J. Climatic Drivers of Plant Species Distributions Across Spatial Grains in Southern Africa Tropical Forests. Front. For. Glob. Chang. 2019, 2, 69. [Google Scholar] [CrossRef]
- Jakobsen, T.; Erik Nielsen, N. Vesicular—Arbuscular Mycorrhiza in Field—Grown Crops: I. Mycorrhizal Infection in Cereals and Peas At Various Times and Soil Depths. New Phytol. 1983, 93, 401–413. [Google Scholar] [CrossRef]
- Smith, T. A Note on the Effect of Soil Tillage on the Frequency and Vertical Distribution of Spores of Vesicular-Arbuscular Endophytes. Soil Res. 1978, 16, 359. [Google Scholar] [CrossRef]
- Sutton, J.C. Development of Vesicular-Arbuscular Mycorrhizae in Crop Plants. Can. J. Bot. 1973, 51, 2487–2493. [Google Scholar] [CrossRef]
- Sutton, J.C.; Barron, G.L. Population Dynamics of Endogone Spores in Soil. Can. J. Bot. 1972, 50, 1909–1914. [Google Scholar] [CrossRef]
- Koide, R.T.; Mooney, H.A. Spatial Variation in Inoculum Potential of Vesicular—Arbuscular Mycorrhizal Fungi Caused By Formation of Gopher Mounds. New Phytol. 1987, 107, 173–182. [Google Scholar] [CrossRef]
- Zajicek, J.M.; Hetrick, B.A.D.; Owensby, C.E. The Influence of Soil Depth on Mycorrhizal Colonization of Forbs in the Tallgrass Prairie. Mycologia 1986, 78, 316. [Google Scholar] [CrossRef]
- Higo, M.; Isobe, K.; Yamaguchi, M.; Drijber, R.A.; Jeske, E.S.; Ishii, R. Diversity and Vertical Distribution of Indigenous Arbuscular Mycorrhizal Fungi under Two Soybean Rotational Systems. Biol. Fertil. Soils 2013, 49, 1085–1096. [Google Scholar] [CrossRef]
- Muleta, D.; Assefa, F.; Nemomissa, S.; Granhall, U. Distribution of Arbuscular Mycorrhizal Fungi Spores in Soils of Smallholder Agroforestry and Monocultural Coffee Systems in Southwestern Ethiopia. Biol. Fertil. Soils 2008, 44, 653–659. [Google Scholar] [CrossRef]
- Säle, V.; Aguilera, P.; Laczko, E.; Mäder, P.; Berner, A.; Zihlmann, U.; van der Heijden, M.G.A.; Oehl, F. Impact of Conservation Tillage and Organic Farming on the Diversity of Arbuscular Mycorrhizal fungi. Soil Biol. Biochem. 2015, 84, 38–52. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).