Abstract
Loeselia mexicana (Polemoniaceae) is a Mexican shrub with significant medicinal value since pre-Hispanic times. Despite its ethnobotanical importance and apparent role in supporting pollinator communities, detailed information about its reproductive biology remains limited, hindering conservation efforts for this increasingly harvested species. We investigated the reproductive ecology of L. mexicana across two flowering seasons (2023–2024 and 2024–2025) in central Mexico through an integrated approach examining flowering phenology, floral morphology, sexual maturation sequence, nectar characteristics, floral visitors, and breeding system experiments. Flowering occurs from September to March, peaking in October. Flowers exhibit protandry, with anther dehiscence on days 1–2 and stigma receptivity from day 2 onward (flower lifespan: 2.85 ± 0.11 days). Maximum nectar production (1.46 ± 0.05 µL per flower; 193.13 ± 8.8 mg/mL) coincided with peak visitor activity. Despite possessing classic ornithophilous traits, we recorded 21 floral visitor species (5 hummingbirds, 3 hymenopterans, 13 butterflies) with similar visitation patterns, challenging previous assumptions about pollination specialization. Controlled pollination experiments confirmed self-incompatibility, with cross-pollination producing significantly more seeds than autonomous selfing. Our findings reveal that L. mexicana maintains a generalized pollination system, while protandry and self-incompatibility enforce outcrossing, providing critical baseline information for conservation strategies.
1. Introduction
Plant reproductive biology involves complex interactions among multiple phenological, morphological, and ecological factors that together determine reproductive success and population persistence [,]. The temporal coordination of flowering, sexual maturation, and reward production with pollinator activity represents a critical dimension of plant reproductive strategies that has evolved through selective pressures [,]. For plants with both ecological and ethnobotanical significance, understanding these reproductive mechanisms becomes particularly important, as they can directly influence conservation status and management approaches [,]. The Polemoniaceae family exhibits diverse floral morphologies and pollination systems, with most species pollinated by insects, while some genera such as Loeselia reportedly show adaptations for hummingbird pollination [,]. This is especially relevant for medicinal plants that face harvesting pressure while simultaneously serving as keystone resources for pollinator communities in their native ecosystems [,]. Within the genus Loeselia, distributed from southwestern United States through Central America to Venezuela [], detailed reproductive biology studies remain scarce. While several congeners (e.g., L. glandulosa, L. grandiflora, L. spectabilis) share similar tubular, red corolla morphology suggesting potential hummingbird pollination, empirical pollination studies are lacking for most species.
Loeselia mexicana (Lam.) stands out among the 17 described Loeselia species for its ethnobotanical importance and ecological role [,]. Known locally as “espinosilla” and in Nahuatl as ‘huitzitzilxochitl’ (hummingbird plant), it has been valued for medicinal purposes in Mexico since pre-Hispanic times. Traditional uses involve aerial parts (leaves and stems) for treating anxiety disorders and neurological conditions [,], gastrointestinal ailments including diarrhea [,], respiratory conditions, skin diseases, and renal inflammation [,,]. The plant also has documented antifungal properties [] and substantial commercial potential []. This extensive medicinal importance appears unmatched among its congeners. Beyond its medicinal value, L. mexicana appears to play a crucial ecological role through plant–pollinator interactions across its range in Mexico. Several studies have highlighted its importance for hummingbird communities [,,,], establishing an apparent consensus that hummingbirds are the primary pollinators. Despite its dual importance as a medicinal plant under harvesting pressure and as a keystone resource for pollinators, comprehensive knowledge of its reproductive biology remains surprisingly limited—a critical gap given that pollinator-dependent species require healthy pollinator communities for population persistence [,].
Previous research has offered only fragmented insights into L. mexicana’s reproductive biology. Plitmann and Levin [] examined pollen-ovule ratios suggesting outbreeding, while Plitmann [] found pollen tube growth patterns in herbarium specimens consistent with self-incompatibility mechanisms. However, these studies were based on limited samples without controlled pollination experiments or field observations necessary to confirm the breeding system. Sexual maturation timing, dichogamy, nectar dynamics, and floral visitor documentation—all critical for reproductive success—remain undocumented for this species. Precise determination of floral longevity and sexual maturation sequences is essential for understanding reproductive strategies, as these temporal patterns directly influence the effectiveness of plant–pollinator interactions and the potential for self- versus cross-pollination [,]. This comprehensive perspective is particularly valuable for species like L. mexicana that may depend on specific pollinators while facing anthropogenic pressures, potentially revealing specialized adaptations or unexpected functional redundancies in pollination systems [,,] and inform more effective conservation strategies for plants with multiple values [].
The present study aims to provide the first comprehensive examination of the reproductive biology of L. mexicana in natural populations from central Mexico. Our objectives were to: (1) describe the flowering phenology and identify peak flowering periods; (2) characterize floral morphology, longevity, and sexual maturation timing; (3) quantify nectar production patterns and sugar concentration throughout the day; (4) identify all floral visitors and analyze their visitation patterns; and (5) determine the breeding system through experimental manipulations. Based on the ornithophilous floral morphology and existing literature highlighting its importance for hummingbird communities, we hypothesized that hummingbirds would be the primary pollinators of L. mexicana, showing higher visitation rates and more consistent presence throughout the flowering period compared to other visitor groups. Additionally, we expected to find evidence of adaptations promoting outcrossing (reproduction through cross-pollination between different individuals), such as dichogamy (temporal separation of male and female phases, including protandry) or self-incompatibility, given previous suggestions in the literature.
2. Materials and Methods
2.1. Study Site and Species
This study was conducted over two flowering seasons: from September 2023 to March 2024 (first season), and from September 2024 to February 2025 (second season), along a disturbance gradient in an area where the natural temperate forest habitat has undergone modifications over the years. This gradient reflects the natural habitat preferences of L. mexicana, which characteristically inhabits secondary vegetation derived from oak forests, pine forests, xerophytic shrublands, and tropical deciduous forests, allowing us to study its reproductive ecology across representative environments where the species naturally occurs. The study area covered approximately 50 hectares in the municipality of Ixtacuixtla, Tlaxcala, Mexico, located 14.1 km west of Tlaxcala City (19°19′6″–19°22′30″ N, 98°20′64″–98°23′40″ W) at elevations ranging from 2200 to 2500 m. a.s.l. The climate of the area is considered temperate sub-humid, with rains primarily from May to September, and the warmest months being March to May. The average annual precipitation ranges between 800–1000 mm. The original vegetation in the study sites was mainly comprising Juniperus deppeana and Quercus spp. forests, of which some remnants are still preserved. However, much of this vegetation has been replaced by Eucalyptus and/or modified to create areas of induced grasslands and seasonal agricultural areas with scattered trees around them. In all these environments, patches of Loeselia mexicana plants were present. This partially lignified shrub, one of 17 Loeselia species endemic to Mexico, produces red to pink tubular flowers approximately 1 cm long with exserted reproductive structures [,]. The species typically forms dense patches in disturbed areas and forest edges, with flowering typically beginning in September according to preliminary observations from previous years.
2.2. Flowering Phenology
Phenological monitoring was carried out during the first flowering season from September 2023 to March 2024, when preliminary observations suggested the beginning of flowering. In the vegetation zones mentioned, a total of 34 individual plants were marked and monitored biweekly (twice per month) until the end of flowering. In each monitoring event, the number of flowers per plant was quantified.
2.3. Floral Longevity and Morphology
To determine floral longevity, 48 flower buds prior to opening (from at least 20 plants) were selected in September 2023 (first flowering season). These buds were marked and excluded with tulle bags, and daily observations were made to record the lifespan of the flower until wilting or falling. In these same flowers, anther dehiscence was determined through daily direct observations using a magnifying glass to establish whether the anthers were open (i.e., whether the tissues composing the anther had separated or were dehiscent, allowing the release of pollen).
The progression of sexual maturation and evidence of protandry was documented during these same observations by recording the timing of: (1) anther dehiscence, (2) filament elongation, (3) pistil elongation, and (4) stigmatic receptivity. This sequential monitoring allowed us to determine the temporal separation between male and female phases in the development of individual flowers.
Floral morphology was characterized from a sample of 60 fresh flowers collected from at least 30 plants in November 2024 during the second flowering season, where variables such as width and total length of the corolla, filament length (measured from corolla tube insertion to anther base) and pistil length (measured from the distal part of the ovary to stigma tip) were recorded to assess reproductive organ positioning relative to the corolla tube. Measurements were performed with a digital caliper.
2.4. Stigmatic Receptivity
To determine stigma receptivity, a necessary measure to establish when flowers can be pollinated, 30 flower buds were marked and bagged with tulle in February 2024 (first season). These buds were collected from 15 individual plants. Daily throughout the floral lifespan, three different flowers (each on its respective day of anthesis) were sampled and tested by applying a drop of hydrogen peroxide to the stigma. The bubbling reaction indicated receptivity []. Each tested flower was sacrificed after evaluation, meaning that on the first day, three first-day flowers were tested; on the second day, three second-day flowers were tested; and so on. This procedure continued until positive bubbling reactions were first observed, indicating the onset of stigmatic receptivity. Once receptivity was confirmed (on day 2), the procedure was discontinued as the objective of determining when stigmas become receptive had been achieved.
2.5. Nectar Measurements
To comprehensively characterize the patterns of nectar production and availability, we used two methods. (1) Standing crop involves quantifying the amount of nectar available to pollinators at a given time under natural conditions. Therefore, nectar production was measured in a total of 90 flowers in November 2024 (second flowering season), which were collected from 30 different individuals. Calibrated 5 μL microcapillaries were used to collect the nectar, and its concentration in sucrose equivalents was quantified using a portable refractometer (Atago, Tokyo, Japan) with a concentration range of 0–32° Brix. The concentration was then converted to mg/mL using the sucrose calibration curve described by Kearns and Inouye []. This sampling was conducted at three different times of day: 09:00 h (morning), 12:00 h (midday), and 15:00 h (afternoon). (2) The accumulated nectar method allows measuring the actual nectar production capacity of the plant by excluding visitors. Therefore, on 22 January 2025 (second flowering season), 37 bagged flowers were evaluated for total nectar production after preventing pollinator visits by covering them prior to opening. Just before the end of the flower’s lifespan, the volume and concentration of nectar in these flowers were measured. The combined use of these methods allows quantifying the intrinsic nectar secretion capacity and evaluating its effective availability under natural foraging conditions, allowing characterization of the functional dynamics of the plant–pollinator system.
2.6. Floral Visitors
During both flowering seasons (September 2023–March 2024 and September 2024–February 2025), floral visitors were identified through direct observations and/or with binoculars (10 × 42 mm) on focal plants, in three time periods: 09:00–11:00, 11:00–13:00, and 13:00–15:00 h, in each sampling event. A sampling event consisted of one full day of observations across all three time periods. A total of 10 sampling days were conducted throughout the study period (5 days per flowering season), resulting in 60 h of observation (10 days × 6 h per day). During the observations, the type of visitor (hummingbird or insect), arrival time, and flowers visited were recorded. When it was not possible to identify visitors in the field, in the case of insects, they were collected with entomological nets for taxonomic determination of the species.
2.7. Breeding System
To determine the mechanism through which reproduction of this species occurs, two pollination treatments were carried out under natural conditions during January–February 2025 (second flowering season), which are detailed below:
Autogamy and/or self-compatibility: 37 flower buds about to open from at least 10 plants were marked. These buds were covered with tulle bags and did not receive any manual pollination treatment, allowing for potential autonomous self-pollination.
Cross-pollination: A total of 37 flower buds about to open from a different set of at least 10 plants were marked. Both plant sets were selected from the same disturbance gradient areas to ensure comparable growing conditions and genetic backgrounds. These buds were covered with tulle bags, and after the flower opened, the anthers were cut (emasculated) to avoid the possibility of self-pollination. After this, the flower was bagged again. On the second day of opening, when the flowers were already receptive, they were uncovered again and manually pollinated using pollen from anthers collected from other individuals. After this treatment, the flower was covered again.
For both treatments, reproductive success was evaluated through two parameters: (1) the proportion of flowers that produced fruits (fruiting rate), and (2) the number of seeds per fruit for all successfully formed fruits. This dual assessment allowed us to quantify both fruit set success and seed production efficiency for each reproductive pathway.
2.8. Statistical Analyses
All statistical analyses were performed using R statistical software version 4.1.2 [] with a significance level of α = 0.05. The variation throughout the flowering period in the number of open flowers in the marked plants was evaluated using a linear mixed model (LMM) with the “lme4” package (v.1.1-27) [], with sampling time (biweekly) as a fixed effect and individual plant as a random effect. Descriptive statistics (mean and standard error) were applied for floral morphology, longevity, dehiscence, receptivity, total nectar production, and sugar concentration values.
Differences between schedules in the volumes and concentrations of nectar measured by standing crop were evaluated through a linear model using the lm() function. Model assumptions of normality and homoscedasticity were verified through graphical analysis of residuals.
To analyze the arrival time patterns of different floral visitor groups (hummingbirds, bees, and butterflies), Kaplan–Meier survival analysis was performed using the “survival” package (v.3.2-13) [], and the Mantel–Cox test was used to determine if significant differences existed between the temporal visitation patterns. Arrival times represent the elapsed time from the start of each observation period (time zero) until the first pollinator visit occurred to each flower. As the observation period progresses, remaining unvisited flowers have progressively shorter potential arrival times. This approach allowed us to assess whether different pollinator guilds exhibited temporal segregation in resource use or similar foraging patterns throughout the observation periods.
To compare visitation patterns among different floral visitor groups, we analyzed the number of flowers visited per individual visitor using a linear model with the lm() function, with visitor type (hummingbirds, bees, and butterflies) as the fixed factor. We also evaluated temporal variation in visitation intensity by comparing the number of visits recorded during three daily observation periods (morning: 09:00–11:00 h, midday: 11:00–13:00 h, and afternoon: 13:00–15:00 h) using a linear model. When significant differences were detected, post hoc Tukey’s tests were performed using the “multcomp” package (v.1.4-17) [] to identify which specific time periods differed significantly. These analyses determined whether there were preferences among different pollinator guilds and whether visitation patterns aligned with nectar production dynamics throughout the day.
To determine the breeding system of L. mexicana, we compared reproductive success between the two pollination treatments (autonomous self-pollination and cross-pollination) using two parameters. First, the proportion of flowers that successfully produced fruits in each treatment was analyzed using Fisher’s exact test to assess whether fruiting rates were independent of pollination treatment. Second, for those flowers that successfully developed into fruits, we compared the number of seeds per fruit among treatments. We included a control group of 28 fruits from open pollination to provide ecological context. As data did not meet the assumptions of normality, we used a Kruskal–Wallis test for the overall comparison of the three treatments, followed by Dunn’s test for specific pairwise comparisons using the “dunn.test” package (v.1.3.5) []. Our breeding system experiments represent a preliminary assessment using only autonomous selfing and cross-pollination treatments. Complete diagnosis would require additional treatments including hand self-pollination (emasculated + bagged + hand self) and open pollination controls (natural exposure) to distinguish between different incompatibility mechanisms and quantify autogamy, geitonogamy, and natural pollination success. This comprehensive approach allowed us to evaluate both the initial reproductive success (fruit set) and the quality of reproduction (seed production) under different pollination scenarios, providing robust evidence regarding the breeding system of this species and the effectiveness of natural pollinators.
3. Results
3.1. Flowering Phenology
The flowering of Loeselia mexicana was very abundant in the locality of Ixtacuixtla, and we observed that during the first flowering season, the flowering period began in late September 2023, extending until mid-March 2024. That is, the flowering of this species spans up to 6 months. The biweekly evaluations carried out on the marked plants demonstrated that the largest quantity of mature flowers was presented in October 2023 (LMM: χ211 = 473.0, p < 0.001), as shown in Figure 1.
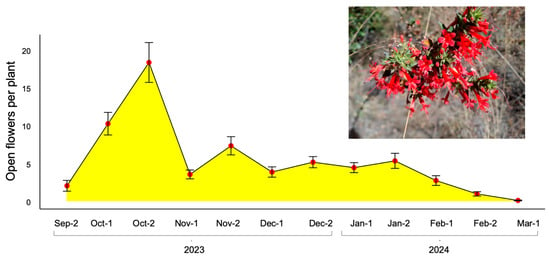
Figure 1.
Flowering phenology of Loeselia mexicana in central Mexico. The main graph shows the mean number of open flowers per plant (±standard error) monitored biweekly during the first flowering season (September 2023 to March 2024). The x-axis labels indicate the first (1) and second (2) biweekly sampling periods within each month. Peak flowering occurred in mid-October, with a secondary peak in late November, and flowering extending over a six-month period. Inset: Photograph showing the typical inflorescence during peak flowering (Photo credit: Carlos Lara).
3.2. Floral Morphology and Longevity
Of the 48 flower buds that were marked to determine the average lifespan, we found that the flowers remain open for 2 to 4 days (mean ± standard error: 2.85 ± 0.11 days). Morphometric measurements from 60 flowers (collected from 30 plants during the second flowering season, November 2024) that had been open for at least 2 days revealed that the corolla length averaged 21.76 ± 0.44 mm, with a relatively narrow aperture of 3.38 ± 0.47 mm. The filaments were the longest structures at 24.64 ± 0.40 mm, while pistils measured 18.35 ± 0.50 mm. These measurements, along with observations of various flowers during our sampling, evidenced temporal changes in the length of the pistil and filaments throughout the life of the flowers. This finding demonstrated that the plant exhibits protandry, meaning that after the opening of the flowers, the elongation of filaments, development, and dehiscence of the anthers occurs first (between days 1 and 2 of life), while the pistil elongates and is receptive from day 2 until its fall.
The sequential monitoring of floral development revealed a consistent pattern of protandry (Figure 2). On day 1 after anthesis, filaments rapidly elongated and the majority of anthers had dehisced by the end of the first day. In contrast, the pistil remained relatively short during this initial phase and showed no signs of receptivity. By day 2, the pistil had elongated significantly, surpassing the position of the anthers, and stigmas became receptive as confirmed by the hydrogen peroxide tests. This clear temporal separation between male and female phases provides an effective mechanism for promoting outcrossing in this species.

Figure 2.
Sequential floral development showing protandry in Loeselia mexicana. (A–F). Chronological flower development: (A,B) Day 1, male phase with dehiscent anthers and immature pistil. (C,D) Transition between phases. (E,F) Day 2–3, female phase with elongated and receptive pistil surpassing the position of anthers. This temporal separation between male and female functions promotes cross-pollination.
3.3. Stigmatic Receptivity
To determine stigmatic receptivity, we evaluated bagged flowers throughout their opening period during the first flowering season (February 2024). When drops of hydrogen peroxide were applied to the stigmas, only 2-day-old flowers showed bubbling, evidencing their receptivity. This timing of receptivity directly coincided with the elongation of the pistil observed in our morphological assessment, confirming that the female phase begins on day 2 after anthesis and continues until flower abscission.
3.4. Nectar Measurements
3.4.1. Standing Crop
The volumes harvested in the flowers collected at different times during the second flowering season (November 2024) showed significant variation (linear model: F2,87 = 3.40, p = 0.0184). On average, flowers collected during the 11:00–13:00 h schedule had significantly more nectar volume than those from the 9:00–11:00 and 13:00–15:00 periods (Tukey’s test, p < 0.05), suggesting that maximum nectar availability occurs at midday for floral visitors (Figure 3A).
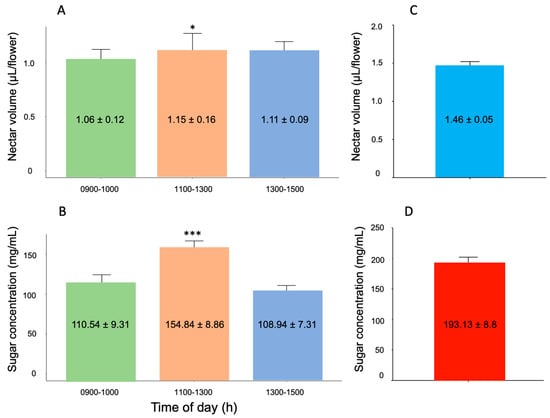
Figure 3.
Nectar dynamics in Loeselia mexicana flowers during the second flowering season. (A) Mean nectar volume (μL ± standard error) available throughout the day measured using standing crop method (November 2024, n = 30 flowers per time period). (B) Mean sugar concentration (mg/mL ± standard error) of nectar measured at different times of day (n = 30 flowers per time period). Both nectar volume and sugar concentration peaked at midday (1100–1300 h), coinciding with maximum pollinator activity. (C) Total nectar production measured using the accumulated nectar method in bagged flowers (January 2025, n = 37 flowers), showing the maximum production capacity per flower (1.46 ± 0.05 μL). (D) Sugar concentration in accumulated nectar from bagged flowers (193.13 ± 8.8 mg/mL, n = 37 flowers). Asterisks indicate statistical significance levels based on Tukey’s test: * p < 0.05, *** p < 0.001.
The same pattern was found when evaluating the sugar concentration present in the collected nectar volumes (linear model: F2,87 = 10.35, p = 0.0002). Thus, in the midday period, flowers presented significantly more concentrated nectar than in the other evaluated schedules (Tukey’s test, p < 0.05) (Figure 3B).
3.4.2. Accumulated Nectar
The total nectar production in L. mexicana, evaluated through the accumulated nectar method in bagged flowers until their abscission on 22 January 2025 (second flowering season), reached an average volume of 1.46 ± 0.05 µL (Figure 3C). The analysis of sugar concentration in these samples revealed an average content of 193.13 ± 8.8 mg/mL (equivalent to approximately 19.3% sugar on a weight/weight basis) (Figure 3D). The higher sugar concentration observed in accumulated nectar likely reflects water evaporation during the accumulation period, which concentrates the remaining sugars. These measurements, made in 37 flowers that completed their 4-day life cycle under controlled conditions during the second flowering season, represent the maximum nectar secretion capacity per flower.
3.5. Floral Visitors
A total of 1399 records of 21 species of floral visitors were obtained across both flowering seasons (2023–2024 and 2024–2025). Among the visitors, we had 5 species of hummingbirds (Trochilidae): Archilochus colubris, Basilinna leucotis, Calothorax lucifer, Cynanthus latirostris, and Saucerottia beryllina. Likewise, 3 species of hymenopterans were recorded: Apis mellifera, Bombus sonorus, and Xylocopa sp. Finally, the most abundant group of visitors comprises 13 species of butterflies (Lepidoptera), including: Battus pilenor, Danaus gilipus, Dione juno, Dione moneta, Dione vanillae, Eutoiepta claudia, Nathalis iole, Leptophobia aripa, Papilio garamas, Papilio polyxenes, Phoebis agarithe, Phoebis sennae, and Zerene cesonia.
No significant differences were found in the number of flowers visited among the three groups of floral visitors (linear model: F2,1396= 1.60, p > 0.05). Although bees showed a slightly higher average of visits per individual, this difference was not statistically significant (Figure 4A).
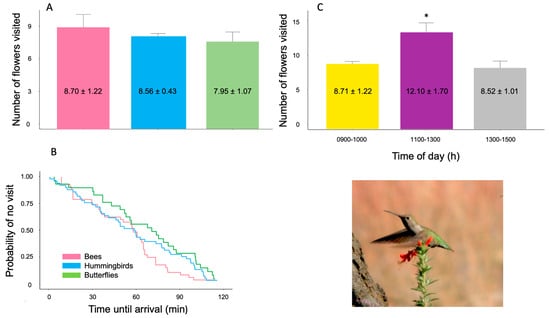
Figure 4.
Floral visitation patterns in Loeselia mexicana across both flowering seasons (2023–2024 and 2024–2025). (A) Mean number of flowers visited (±standard error) per individual visitor across different pollinator groups: Bees (pink bars), Hummingbirds (blue bars), and Butterflies (green bars), showing no significant differences (p > 0.05). (B) Arrival time patterns of the three visitor groups shown as survival curves; no significant differences were detected (p = 0.452), indicating similar temporal foraging patterns. (C) Temporal variation in flower visitation throughout the day, with peak activity occurring at midday (1100–1300 h), coinciding with maximum nectar volume and sugar concentration. Asterisk indicates significant difference (p < 0.05) based on Tukey’s test. Inset photo: Archilochus colubris (Ruby-throated Hummingbird) visiting a flower of L. mexicana (Photo credit: Sergio Díaz Infante).
The temporal arrival patterns of the different groups of floral visitors were similar throughout the observation period. The Kaplan–Meier survival analysis and the Mantel–Cox test did not reveal significant differences in arrival times between bees, hummingbirds, and butterflies (χ2 = 0.315, d.f. = 2, p = 0.452). The survival curves showed a similar gradual decrease in the probability of arrival for the three groups during the 120 min of observation, suggesting that there is no temporal segregation in the use of the floral resource among these visitors (Figure 4B).
When jointly analyzing the visits of the three groups and evaluating their intensity during the three recording periods (linear model: F2,1396 = 4.74, p = 0.031), a significantly higher frequency was observed at midday (Tukey’s test, p < 0.05; Figure 4C). Correlation analysis confirmed significant positive relationships between nectar volume and pollinator visitation frequency (r = 0.97, p < 0.05) and between sugar concentration and visitation rates (r = 0.89, p < 0.05) across the three time periods, providing statistical support for temporal coordination between resource availability and foraging activity.
3.6. Breeding System
The experimental treatments to determine the breeding system of L. mexicana conducted during the second flowering season (January–February 2025) strongly suggest self-incompatibility. Of the 37 flowers subjected to the autogamy treatment, only 2 (5.4%) developed into fruits, while 28 of the 37 cross-pollinated flowers (75.7%) successfully formed fruits. This difference in fruit set between treatments was highly significant (Fisher’s exact test, p < 0.001). However, complete breeding system diagnosis would require additional experimental treatments to definitively distinguish among incompatibility mechanisms.
Further examination of seed production in the successfully formed fruits strengthened the evidence for self-incompatibility. For a comprehensive analysis, we selected 28 fruits from open pollination during the second flowering season as a control group to compare with our experimental treatments. The analysis revealed significant differences in the number of seeds per fruit among the three pollination treatments (Kruskal–Wallis test, H = 8.03, df = 2, p < 0.05). Post hoc comparisons using Dunn’s test showed that the two fruits that developed from the autogamy treatment contained significantly fewer seeds (Median = 1.5, Range = 1–2; mean ± SE: 1.5 ± 0.5 seeds per fruit) compared to both cross-pollination (Median = 4, Range = 3–7; mean ± SE: 4.82 ± 0.26 seeds per fruit; p = 0.009) and open pollination treatments (Median = 4, Range = 2–7; mean ± SE: 4.11 ± 0.27 seeds per fruit; p = 0.045). No significant difference was found between cross-pollination and open pollination treatments (p = 0.091), suggesting that there was no detectable difference in effectiveness between natural pollinators and manual cross-pollination in this system.
4. Discussion
This study provides the first comprehensive characterization of the reproductive biology of Loeselia mexicana, integrating multiple facets from flowering phenology to breeding system. Our findings reveal several noteworthy aspects of its reproductive strategy that challenge previous assumptions while confirming others, highlighting the importance of such integrated approaches for understanding plant reproductive ecology [,]. The species flowers for six months with October showing maximum bloom, demonstrates clear protandry with sequential maturation of male and female organs, secretes highest nectar volumes at midday, and attracts a diverse assemblage of pollinators including hummingbirds, bees, and butterflies whose foraging coincides with resource availability. L. mexicana enforces outcrossing through complementary mechanisms of strong self-incompatibility and dichogamy, evidenced by significantly higher reproductive success in cross-pollination compared to autogamy treatments.
The extended flowering period of L. mexicana from late September to mid-March, with peak flowering in October, contributes significantly to pollinator resource availability during autumn and winter months when floral resources may be limited in temperate environments of central Mexico. However, individual flower lifespan (2.85 ± 0.11 days) is relatively short compared to many temperate species, suggesting the extended flowering period results from sequential flower production rather than prolonged individual flower longevity. This strategy likely involves significant resource allocation trade-offs, as extended flowering periods can deplete plant resources []. This prolonged flowering strategy may compensate for these costs by maximizing exposure to potential pollinators over time and may represent an adaptation to seasonal variation in pollinator abundance or activity []. Such extended flowering phenology is relatively uncommon in temperate zones and deserves further investigation in relation to climatic conditions and the phenology of other flowering plants in the community. Further research comparing resource allocation patterns and individual flower longevity with related species would help clarify the costs and benefits of this extended flowering strategy.
The floral morphology and functional traits documented for L. mexicana align with classic ornithophilous syndrome features [,], including red to pink tubular corollas and exserted reproductive structures. However, our findings on protandry and temporal patterns of nectar production add important nuances to this characterization. The protandrous development we observed, with anther dehiscence occurring on days 1–2 and stigmatic receptivity on day 2 until flower abscission, represents an important temporal separation of male and female functions [] that may primarily optimize pollen presentation timing rather than prevent self-fertilization, given the confirmed self-incompatibility. We acknowledge that our experimental design did not test geitonogamous compatibility (between flowers on the same plant), which would determine whether self-incompatibility operates at the flower or whole-plant level. This reproductive strategy aligns with our experimental confirmation that L. mexicana is indeed self-incompatible, with significantly higher seed production resulting from cross-pollination compared to autonomous selfing. The minimal seed production observed in the autogamy treatment (1.5 ± 0.5 seeds per fruit from only 2 of 37 flowers) likely represents either residual self-compatibility under extreme conditions or potential reproductive assurance during pollinator scarcity, rather than indicating a mixed mating system. While this low level of autonomous selfing may provide minimal reproductive assurance, it is insufficient for population maintenance and confirms the species’ effective dependence on cross-pollination for successful reproduction. These results validate the earlier indirect evidence suggesting self-incompatibility based on pollen-ovule ratios and pollen tube growth patterns [,] and demonstrate the plant’s dependency on pollinators for successful reproduction. Our controlled pollination experiments—comparing autonomous selfing with cross-pollination under standardized field conditions—provide robust and conclusive evidence that directly addresses the limitations of previous indirect approaches, establishing definitively that L. mexicana requires obligate outcrossing for successful reproduction.
The self-incompatibility documented here has important implications for conservation. For medicinal plants under harvesting pressure like L. mexicana, self-incompatibility increases vulnerability to reproductive failure in scenarios of pollinator decline or habitat fragmentation [,]. However, the generalized pollination system documented in this study may provide some buffering capacity against the decline of specific pollinator groups, as multiple taxa can potentially provide pollination services. This functional redundancy could offer greater resilience compared to plants with highly specialized pollination systems that depend on single pollinator species. Nevertheless, the effectiveness of this potential buffering depends on the actual pollination efficiency of different visitor groups, which requires further investigation. This finding underscores the need for conservation strategies that protect not only the plant populations but also their pollinator communities, recognizing the complex web of interactions that sustain these systems [].
Our nectar measurements revealed temporal patterns in nectar availability throughout the day. The accumulated nectar production of 1.46 ± 0.05 μL per flower with a high sugar concentration (193.13 ± 8.8 mg/mL) falls within the range typical for hummingbird-pollinated plants in the Neotropics [,], supporting previous suggestions of ornithophily in this species. Peak nectar volume and sugar concentration at midday coincided with peak visitation intensity across all pollinator groups. This temporal correlation between resource availability and pollinator activity may maximize reproductive opportunities. Similar temporal correlations have been documented in other plant species [,], though the causal mechanisms underlying these patterns remain to be determined.
Perhaps the most surprising finding of our study is the diversity and relative importance of floral visitors observed. With 21 species spanning hummingbirds, hymenopterans, and lepidopterans all showing similar visitation patterns, L. mexicana appears to support a more generalized pollination system than previously assumed based on floral morphology alone. This finding challenges previous assumptions about this species being exclusively or primarily hummingbird-pollinated, based on specific studies of this species [,], rather than assumptions about the entire genus. While confirming the continued ecological importance of hummingbirds as documented in those studies, our results align with growing evidence that many plants with apparently specialized floral traits may interact with diverse pollinator assemblages [,]. This challenge to traditional assumptions represents a crucial advance in understanding plant–pollinator interactions by demonstrating the complexities beyond simple syndrome predictions. Our comprehensive multimodal visitor data—encompassing detailed behavioral observations, temporal patterns, and quantitative visitation records across multiple pollinator guilds—was essential for revealing this ecological complexity that would have been missed by studies focusing solely on morphological traits or single visitor groups.
Although we found no significant differences in visitation frequency among visitor groups, our field observations suggest potential variation in pollination effectiveness. Hummingbirds, with their long bills and hovering behavior, consistently contacted both anthers and stigmas while probing for nectar, positioning them as potentially more efficient pollinators. Bees, particularly larger species like Xylocopa sp., also made frequent contact with reproductive structures, while butterflies often fed without substantial contact with the stigma due to their long proboscides and tendency to perch on peripheral parts of the flower. However, without quantitative analysis of pollen loads and transfer efficiency, these remain preliminary observations. Future research should also distinguish between different bee types based on morphological compatibility, as smaller bees like Apis mellifera with shorter mouthparts may be less effective pollinators than larger, long-tongued species (Bombus sonorus, Xylocopa sp.), despite similar visitation frequencies. Single-visit effectiveness experiments examining pollen deposition would help separate legitimate pollinators from nectar thieves within the hymenopteran guild. This methodological approach would help clarify whether L. mexicana exhibits ecological generalization but functional specialization in its pollination system, potentially providing functional redundancy that could buffer against fluctuations in specific pollinator populations [,].
This apparent generalization in pollinator use despite seemingly specialized floral traits raises interesting questions about the evolution of plant–pollinator interactions in L. mexicana, patterns that parallel recent findings in Salvia where morphological specialization can coexist with pollinator generalization [,]. Therefore, the link between floral traits and reproductive outcomes should not be overestimated. One possibility is that the system represents an “adaptive generalization” [] where floral traits that appear to target one pollinator group (hummingbirds) simultaneously accommodate other effective pollinators (bees and butterflies). Alternatively, the current visitation patterns may reflect recent ecological changes, such as altered pollinator communities resulting from anthropogenic disturbance [,]. The generalized pollination system observed may result from several factors: (1) the disturbed habitat context of our study site, where altered pollinator communities may have led to broader visitor assemblages; (2) floral traits that, while appearing specialized for hummingbirds, are accessible to multiple visitor types; or (3) an inherently generalized strategy that provides resilience against pollinator fluctuations. Based on our data, the floral accessibility hypothesis (factor 2) receives the strongest support, as evidenced by the consistent visitation across all three pollinator groups and the morphological compatibility between the 25–35 mm corolla tubes and the diverse range of visitor mouthpart lengths we documented. However, distinguishing definitively among these hypotheses would require comparative studies across habitat gradients and pollinator effectiveness experiments. Future studies comparing pollinator effectiveness, rather than just visitation frequency, would be valuable for determining whether all visitor groups contribute equally to reproductive success [,]. Our findings of a generalized pollination system in L. mexicana raise intriguing questions about pollination strategies across the genus Loeselia. While morphological similarities among red-flowered congeners might suggest convergent evolution toward ornithophilous syndromes, the lack of empirical pollination data for other species prevents meaningful comparative analysis.
The coincidence of peak visitation intensity with maximal nectar availability at midday demonstrates the importance of temporal dynamics in plant–pollinator interactions [,]. This synchronization likely increases pollination efficiency by concentrating pollen movement during optimal periods []. For L. mexicana, this temporal pattern may be particularly important given its protandrous development and self-incompatibility, as it could maximize opportunities for effective cross-pollination when stigmas are receptive.
The ecological significance of L. mexicana extends beyond its own reproduction. As a plant flowering during autumn and winter months in temperate zones of Mexico, it likely represents a crucial resource for resident pollinators during resource-limited periods. The autumn-winter flowering phenology of L. mexicana contrasts with most Mexican temperate forest species, which typically bloom during the rainy season (May–October), including Lamiaceae (Salvia elegans), Rubiaceae (Bouvardia ternifolia), and Plantaginaceae (Penstemon roseus) []. This temporal separation may reduce competition for pollinators while providing crucial resources during resource-limited dry months. While detailed phenological data for other Loeselia species remain lacking, this winter flowering strategy represents a key ecological adaptation that contributes to the species’ role as a keystone resource for diverse pollinator communities during periods when few other nectar sources are available. Previous studies have highlighted this role for hummingbirds [,,], but our findings indicate that this ecological support extends to multiple pollinator guilds, including important bee and butterfly species. The relatively high nectar rewards offered may be particularly important for energetically constrained pollinators during dry and colder months, potentially influencing community structure and population dynamics of these animal groups [,]. The timing of L. mexicana flowering (September-March) coincides with the presence of winter migratory hummingbirds like Archilochus colubris in central Mexican forests [], while also supporting multiple year-round resident species (Basilinna leucotis, Calothorax lucifer, Cynanthus latirostris, Saucerottia beryllina) during resource-limited months. This temporal alignment may contribute to the species’ importance for maintaining hummingbird community diversity.
From a conservation perspective, our findings highlight the importance of protecting L. mexicana populations for multiple reasons. First, as a self-incompatible species dependent on cross-pollination for reproduction, it requires healthy pollinator communities for population persistence [,]. Second, while Martínez-Roldán et al. [] documented its importance specifically for hummingbird communities across diverse habitat types in central Mexico, our findings suggest its role as a floral resource extends to diverse pollinator groups during resource-limited seasons, making it potentially important for maintaining pollinator diversity in its ecosystems [,]. Third, its medicinal value creates anthropogenic pressure that must be balanced with ecological considerations [,]. The conservation implications of our findings merit urgent attention given documented population decline in L. mexicana. Field studies conducted over a decade ago reported that populations were scarce and declining due to extensive commercialized medicinal extraction []. The absence of updated conservation assessments since then represents a critical knowledge gap. Our demonstration of dependence on diverse pollinator communities suggests that continued extraction pressure and habitat modification could significantly impact reproductive success through pollinator community disruption, potentially compounding population decline trends documented in 2013.
The integrated approach employed in this study—examining phenology, floral longevity, sexual development, nectar dynamics, pollinator interactions, and breeding system—has provided insights that would not have been apparent from studying any single aspect in isolation. Such comprehensive approaches are increasingly recognized as necessary for understanding the complex interplay of factors influencing plant reproductive success [,] and for developing effective conservation strategies for plant species with multiple values [,].
Our study had some limitations that suggest directions for future research. First, we documented visitation patterns of different pollinator groups, limiting our ability to establish direct causal relationships between floral morphology and reproductive success [,]. Future studies using single-visit efficiency experiments would clarify the relative contributions of different visitors to reproductive success. Second, although our successful cross-pollination experiments indirectly confirmed pollen viability, direct tests of pollen viability throughout flower development would provide more detailed insights into male-phase dynamics in this protandrous species. Third, our nectar sampling design did not stratify flowers by developmental stage (days 1, 2, or 3), which may have confounded our temporal nectar dynamics since nectar secretion can vary with floral age and sexual phase in protandrous species. Additionally, emasculation experiments removing anthers before dehiscence would help determine whether the transition to female phase is controlled by intrinsic timing or influenced by anther presence. Fourth, our breeding system experiments did not include manual self-pollination tests, which would have more definitively distinguished between self-incompatibility and mechanical barriers to autonomous selfing. Moreover, we did not test for apomixis, and the experiments did not assess geitonogamous self-pollination, leaving unclear whether the incompatibility system prevents fertilization between flowers on the same individual plant. Fifth, our study was conducted at a single location characterized by significant anthropogenic disturbance, where natural temperate forest has been largely replaced by Eucalyptus plantations, induced grasslands, and seasonal agricultural areas. The observed generalized pollination system may therefore represent a response to altered pollinator communities resulting from this habitat modification, rather than solely an inherent trait of L. mexicana. Comparative studies across the species’ range would reveal potential geographic variation in reproductive biology and pollinator relationships []. Finally, longer-term studies spanning multiple flowering seasons would provide insights into year-to-year variation in phenology, nectar production, and visitor assemblages in relation to climatic variability [].
5. Conclusions
This study provides the first detailed characterization of the reproductive biology of Loeselia mexicana from an integrated perspective. Our findings reveal a protandrous, self-incompatible flowering system with an extended blooming period, substantial nectar rewards, and a diverse assemblage of floral visitors including hummingbirds, bees, and butterflies. The temporal patterns of nectar production and pollinator visitation suggest a synchronized system that maximizes reproductive opportunities while supporting a diverse pollinator community during autumn and winter months. These results challenge the traditional assumption that L. mexicana is primarily hummingbird-pollinated, suggesting instead a more generalized pollination system despite ornithophilous floral traits. The confirmed self-incompatibility of this species highlights its dependency on pollinators for successful reproduction and its potential vulnerability to pollinator declines. Collectively, these findings contribute to our understanding of the ecological role of this medicinally important plant and provide valuable baseline information for developing comprehensive conservation and management strategies that consider both its ethnobotanical value and ecological functions.
Author Contributions
L.M.-H.: Writing—review and editing, Conceptualization. C.L.: Writing—review and editing, Writing—original draft, Visualization, Supervision, Software, Resources, Project administration, Investigation, Funding acquisition, Formal analysis, Conceptualization. M.C.: Writing—review and editing, Conceptualization. U.M.-L.: Writing—review and editing, Conceptualization. K.L.-V.: Writing—review and editing, Investigation. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Once accepted the manuscript these data will be public in the digital repository Open Science Framework (https://osf.io) (accessed on 13 October 2025).
Acknowledgments
We thank Hellen Martínez-Roldán and Mauro Piedras for help in fieldwork. This research has been funded by a scholarship to Liliana Mora-Henández as ayudante SNI 3 (I1200/051/2023) from the Secretaría de Ciencia, Humanidades, Tecnología e Innovación (Secihti).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Willmer, P. Pollination and Floral Ecology; Princeton University Press: Princeton, NJ, USA, 2011; 778p. [Google Scholar]
- Barrett, S.C.H. Sexual interference of the floral kind. Heredity 2002, 88, 154–159. [Google Scholar] [CrossRef]
- Rathcke, B.; Lacey, E.P. Phenological patterns of terrestrial plants. Annu. Rev. Ecol. Syst. 1985, 16, 179–214. [Google Scholar] [CrossRef]
- Fenster, C.B.; Armbruster, W.S.; Wilson, P.; Dudash, M.R.; Thomson, J.D. Pollination syndromes and floral specialization. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 375–403. [Google Scholar] [CrossRef]
- Ashman, T.L.; Knight, T.M.; Steets, J.A.; Amarasekare, P.; Burd, M.; Campbell, D.R.; Dudash, M.R.; Johnston, M.O.; Mazer, S.J.; Mitchell, R.J.; et al. Pollen limitation of plant reproduction: Ecological and evolutionary causes and consequences. Ecology 2004, 85, 2408–2421. [Google Scholar] [CrossRef]
- Kearns, C.A.; Inouye, D.W.; Waser, N.M. Endangered mutualisms: The conservation of plant-pollinator interactions. Annu. Rev. Ecol. Syst. 1998, 29, 83–112. [Google Scholar] [CrossRef]
- Wilken, D.H. Polemoniaceae. In Flora of the Great Plains; McGregor, R.L., Barkley, T.M., Brooks, R.E., Schofield, E.K., Eds.; University Press of Kansas: Lawrence, KS, USA, 1986; pp. 666–677. [Google Scholar]
- Rzedowski, J.; Calderón de Rzedowski, G. Flora del Bajio y de Regiones Adyacentes; Instituto de Ecologia: Xalapa, Mexico, 1995; Volume 33, pp. 28–32. [Google Scholar]
- Vodouhè, F.G.; Coulibaly, O.; Greene, C.; Sinsin, B. Estimating the local value of non-timber forest products to Pendjari Biosphere Reserve dwellers in Benin. Econ. Bot. 2011, 65, 369–383. [Google Scholar] [CrossRef]
- Hamilton, A.C. Medicinal plants, conservation and livelihoods. Biodivers. Conserv. 2004, 13, 1477–1517. [Google Scholar] [CrossRef]
- Porter, J.M.; Johnson, L.A. A phylogenetic classification of Polemoniaceae. Aliso 2000, 19, 55–91. [Google Scholar] [CrossRef]
- Porter, J.M.; Steinmann, V.W. Two New Loeselia (Polemoniaceae) Species from Michoacán, Mexico. Syst. Bot. 2009, 34, 730–736. [Google Scholar] [CrossRef]
- Herrera-Ruiz, M.; González-Carranza, A.; Zamilpa, A.; Jiménez-Ferrer, E.; Huerta-Reyes, M.; Navarro-García, V.M. The standardized extract of Loeselia mexicana possesses anxiolytic activity through the α-amino butyric acid mechanism. J. Ethnopharmacol. 2011, 138, 262–267. [Google Scholar] [CrossRef]
- Guzmán-Gutiérrez, L.; Reyes-Chilpa, R.; Bonilla-Jaime, H. Medicinal plants for the treatment of “nervios”, anxiety, and depression in Mexican Traditional Medicine (Review). Braz. J. Pharmacogn. 2014, 24, 591–608. [Google Scholar] [CrossRef]
- Rojas, A.; Bah, M.; Rojas, J.I.; Serrano, V.; Pacheco, S. Spasmolytic activity of some plants used by the Otomi Indians of Querétaro (México) for the treatment of gastrointestinal disorders. Phytomedicine 1999, 6, 367–371. [Google Scholar] [CrossRef]
- Pérez, G.S.; Pérez, G.C.; Zavala, S.M.A. A study of the antidiarrheal properties of Loeselia mexicana on mice and rats. Phytomedicine 2005, 12, 670–674. [Google Scholar] [CrossRef] [PubMed]
- FAO [Food and Agriculture Organization of the United Nations]. Estado de la Información Forestal en México. Información para el Desarrollo Forestal Sostenible; Technical Report GCP/RLA/133/EC; FAO: Santiago, Chile, 2002; Volume 11, pp. 179–279. [Google Scholar]
- BDMTM [Biblioteca Digital de la Medicina Tradicional Mexicana]. Atlas de las Plantas de la Medicina Tradicional Mexicana: Espinosilla; Universidad Nacional Autónoma de México: México City, México, 2009; Available online: http://www.medicinatradicionalmexicana.unam.mx/apmtm/termino.php?l=3&t=loeselia-mexicana (accessed on 25 June 2023).
- CONABIO [Comisión Nacional para el uso de la Biodiversidad]. Polemoniaceae, Loeselia mexicana. In Malezas de México; CONABIO: México City, México, 2009; Available online: http://www.conabio.gob.mx/malezasdemexico/polemoniaceae/loeselia-mexicana/fichas/ficha.htm (accessed on 12 February 2024).
- Navarro-García, V.; Rojas, G.; Avilés, M.; Fuentes, M.; Zepeda, G. In vitro antifungal activity of coumarin extracted from Loeselia mexicana Brand. Mycoses 2011, 54, e569–e571. [Google Scholar] [CrossRef] [PubMed]
- SEDEREC [Secretaría de Desarrollo Rural y Equidad para las Comunidades]. Programa para la recuperación de la medicina tradicional y la herbolaria en la Ciudad de México. Gac. Of. Del Dist. Fed. 2012, Tomo II, 106–115. [Google Scholar]
- Freeman, C.E.; Worthington, R.D.; Corral, R.D. Some floral nectar-sugar compositions from Durango and Sinaloa, Mexico. Biotropica 1985, 17, 309–313. [Google Scholar] [CrossRef]
- Lara-Rodríguez, N.Z.; Díaz-Valenzuela, R.; Martínez-García, V.; Mauricio-Lopéz, E.; Anaid-Díaz, S.; Valle, O.I.; Fisher de León, A.D.; Lara, C.; Ortiz-Pulido, R. Redes de interacción colibrí-planta del centro-este de México. Rev. Mex. Biodivers. 2012, 83, 569–577. [Google Scholar] [CrossRef]
- López-Segoviano, G.; Bribiesca, R.; Arizmendi, M.C. The role of size and dominance in the feeding behaviour of coexisting hummingbirds. Ibis 2018, 160, 283–292. [Google Scholar] [CrossRef]
- Martínez-Roldán, H.; Pérez-Crespo, M.J.; Lara, C. Unraveling habitat-driven shifts in alpha, beta, and gamma diversity of hummingbirds and their floral resource. PeerJ 2024, 12, e17713. [Google Scholar] [CrossRef]
- Vázquez, D.P.; Blüthgen, N.; Cagnolo, L.; Chacoff, N.P. Uniting pattern and process in plant–animal mutualistic networks: A review. Ann. Bot. 2009, 103, 1445–1457. [Google Scholar] [CrossRef]
- Aguilar, R.; Ashworth, L.; Galetto, L.; Aizen, M.A. Plant reproductive susceptibility to habitat fragmentation: Review and synthesis through a meta-analysis. Ecol. Lett. 2006, 9, 968–980. [Google Scholar] [CrossRef]
- Plitmann, U.; Levin, D.A. Breeding systems in the Polemoniaceae. Plant Syst. Evol. 1990, 170, 205–214. [Google Scholar] [CrossRef]
- Plitmann, U. Assessing functional reproductive traits from herbarium material: The test case of pollen tubes in pistils of Polemoniaceae. Taxon 1994, 43, 63–69. [Google Scholar] [CrossRef]
- Dafni, A.; Kevan, P.G.; Husband, B.C. (Eds.) Practical Pollination Biology; Enviroquest Ltd.: Cambridge, ON, Canada, 2005; 590p. [Google Scholar]
- Harder, L.D.; Barrett, S.C. (Eds.) Ecology and Evolution of Flowers; Oxford University Press: Oxford, UK, 2006; 496p. [Google Scholar]
- Faegri, K.; van der Pijl, L. The Principles of Pollination Ecology, 3rd ed.; Pergamon Press: Oxford, UK, 1979; 244p. [Google Scholar]
- Ollerton, J.; Alarcón, R.; Waser, N.M.; Price, M.V.; Watts, S.; Cranmer, L.; Hingston, A.; Peter, C.I.; Rotenberry, J. A global test of the pollination syndrome hypothesis. Ann. Bot. 2009, 103, 1471–1480. [Google Scholar] [CrossRef]
- Herrera, C.M. Floral traits and plant adaptation to insect pollinators: A devil’s advocate approach. In Floral Biology; Lloyd, D.G., Barrett, S.C.H., Eds.; Springer: Boston, MA, USA, 1996; pp. 65–87. [Google Scholar] [CrossRef]
- Kearns, C.A.; Inouye, D.W. Techniques for Pollination Biologists; University Press of Colorado: Niwot, CO, USA, 1993; 583p. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 13 October 2025).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Therneau, T.M. Survival: A Package for Survival Analysis in R. R Package Version 3.2-13. 2020. Available online: https://CRAN.R-project.org/package=survival (accessed on 13 October 2025).
- Hothorn, T.; Bretz, F.; Westfall, P. multcomp: Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
- Dinno, A. Dunn.Test: Dunn’s Test of Multiple Comparisons Using Rank Sums. R Package Version 1.3.5. 2017. Available online: https://CRAN.R-project.org/package=dunn.test (accessed on 13 October 2025).
- Elzinga, J.A.; Atlan, A.; Biere, A.; Gigord, L.; Weis, A.E.; Bernasconi, G. Time after time: Flowering phenology and biotic interactions. Trends Ecol. Evol. 2007, 22, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Flores, C.I.; Arizmendi, M.D.C. The dynamics of hummingbird dominance and foraging strategies during the winter season in a highland community in Western Mexico. J. Zool. 2016, 299, 262–274. [Google Scholar] [CrossRef]
- Lloyd, D.G.; Webb, C.J. The avoidance of interference between the presentation of pollen and stigmas in angiosperms I. Dichogamy. New Zealand J. Bot. 1986, 24, 135–162. [Google Scholar] [CrossRef]
- Aizen, M.A.; Ashworth, L.; Galetto, L. Reproductive success in fragmented habitats: Do compatibility systems and pollination specialization matter? J. Veg. Sci. 2002, 13, 885–892. [Google Scholar] [CrossRef]
- Baker, H.G. Sugar concentrations in nectars from hummingbird flowers. Biotropica 1975, 7, 37–41. [Google Scholar] [CrossRef]
- Abrahamczyk, S.; Kessler, M.; Hanley, D.; Karger, D.N.; Müller, M.P.; Knauer, A.C.; Keller, F.; Schwerdtfeger, M.; Humphreys, A.M. Pollinator adaptation and the evolution of floral nectar sugar composition. J. Evol. Biol. 2017, 30, 112–127. [Google Scholar] [CrossRef]
- Herrera, C.M. Daily patterns of pollinator activity, differential pollinating effectiveness, and floral resource availability, in a summer-flowering Mediterranean shrub. Oikos 1990, 58, 277–288. [Google Scholar] [CrossRef]
- Stone, G.N.; Gilbert, F.; Willmer, P.; Potts, S.; Semida, F.; Zalat, S. Windows of opportunity and the temporal structuring of foraging activity in a desert solitary bee. Ecol. Entomol. 1999, 24, 208–221. [Google Scholar] [CrossRef]
- Waser, N.M.; Chittka, L.; Price, M.V.; Williams, N.M.; Ollerton, J. Generalization in pollination systems, and why it matters. Ecology 1996, 77, 1043–1060. [Google Scholar] [CrossRef]
- Brosi, B.J.; Briggs, H.M. Single pollinator species losses reduce floral fidelity and plant reproductive function. Proc. Natl. Acad. Sci. USA 2013, 110, 13044–13048. [Google Scholar] [CrossRef]
- Fontaine, C.; Dajoz, I.; Meriguet, J.; Loreau, M. Functional diversity of plant–pollinator interaction webs enhances the persistence of plant communities. PLoS Biol. 2006, 4, e1. [Google Scholar] [CrossRef]
- Xiao, H.W.; Liu, Q.S.; Huang, Y.B.; Ma, Y.P.; Claßen-Bockhoff, R.; Tian, R.N.; Wei, Y.K. Effective hawkmoth pollination in the primarily bee-pollinated Salvia daiguii—An example of adaptive generalization. Plant Species Biol. 2023, 38, 18–26. [Google Scholar] [CrossRef]
- Xiao, H.W.; Huang, Y.B.; Liu, Q.S.; Claßen-Bockhoff, R.; Tian, R.N.; Wei, Y.K. Mixed mating patterns in morphologically diverse bumblebee-pollinated Salvia species from China. Biol. J. Linn. Soc. 2024, 143, blad164. [Google Scholar] [CrossRef]
- Aigner, P.A. Optimality modeling and fitness trade-offs: When should plants become pollinator specialists? Oikos 2001, 95, 177–184. [Google Scholar] [CrossRef]
- Burkle, L.A.; Marlin, J.C.; Knight, T.M. Plant-pollinator interactions over 120 years: Loss of species, co-occurrence, and function. Science 2013, 339, 1611–1615. [Google Scholar] [CrossRef] [PubMed]
- Traveset, A.; Richardson, D.M. Mutualistic interactions and biological invasions. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 89–113. [Google Scholar] [CrossRef]
- Ne’eman, G.; Jürgens, A.; Newstrom-Lloyd, L.; Potts, S.G.; Dafni, A. A framework for comparing pollinator performance: Effectiveness and efficiency. Biol. Rev. 2010, 85, 435–451. [Google Scholar] [CrossRef] [PubMed]
- Ollerton, J. Pollinator diversity: Distribution, ecological function, and conservation. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 353–376. [Google Scholar] [CrossRef]
- Willmer, P.; Stone, G.N. Behavioral, ecological, and physiological determinants of the activity patterns of bees. Adv. Study Behav. 2004, 34, 347–466. [Google Scholar] [CrossRef]
- Lara, C. Temporal dynamics of flower use by hummingbirds in a highland temperate forest in Mexico. Écoscience 2006, 13, 23–29. [Google Scholar] [CrossRef]
- Potts, S.G.; Vulliamy, B.; Dafni, A.; Ne’eman, G.; Willmer, P. Linking bees and flowers: How do floral communities structure pollinator communities? Ecology 2003, 84, 2628–2642. [Google Scholar] [CrossRef]
- Williams, N.M.; Cariveau, D.; Winfree, R.; Kremen, C. Bees in disturbed habitats use, but do not prefer, alien plants. Basic Appl. Ecol. 2012, 13, 215–222. [Google Scholar] [CrossRef]
- Knight, T.M.; Steets, J.A.; Vamosi, J.C.; Mazer, S.J.; Burd, M.; Campbell, D.R.; Dudash, M.R.; Johnston, M.O.; Mitchell, R.J.; Ashman, T.L. Pollen limitation of plant reproduction: Pattern and process. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 467–497. [Google Scholar] [CrossRef]
- Bascompte, J.; Jordano, P. Plant-animal mutualistic networks: The architecture of biodiversity. Annu. Rev. Ecol. Evol. Syst. 2007, 38, 567–593. [Google Scholar] [CrossRef]
- Memmott, J.; Waser, N.M.; Price, M.V. Tolerance of pollination networks to species extinctions. Proc. R. Soc. Lond. B Biol. Sci. 2004, 271, 2605–2611. [Google Scholar] [CrossRef] [PubMed]
- Schippmann, U.; Leaman, D.J.; Cunningham, A.B. Impact of cultivation and gathering of medicinal plants on biodiversity: Global trends and issues. In Biodiversity and the Ecosystem Approach in Agriculture, Forestry and Fisheries. Satellite Event on the Occasion of the Ninth Regular Session of the Commission on Genetic Resources for Food and Agriculture; FAO: Rome, Italy, 2002; pp. 140–167. [Google Scholar]
- Becerra, H.; Varela, G.; Martínez-Cárdenas, M. Fenología de L. mexicana en el Tehutli, Xochimilco; D.F. Universidad Autónoma Metropolitana Congreso Mexicano de Botánica: Tuxtla Gutiérrez, Chiapas, 2013. [Google Scholar]
- Herrera, C.M.; Castellanos, M.C.; Medrano, M. Geographical context of floral evolution: Towards an improved research programme in floral diversification. In Ecology and Evolution of Flowers; Harder, L.D., Barrett, S.C., Eds.; Oxford University Press: Oxford, UK, 2006; pp. 278–294. [Google Scholar]
- Inouye, D.W. Effects of climate change on phenology, frost damage, and floral abundance of montane wildflowers. Ecology 2008, 89, 353–362. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).