Abstract
Plant–pollinator interactions research can assist in the development of more ecologically friendly crop breeding methods, leading to enhanced global food security. In the present study, we have aimed to assess fifteen floral traits as insect attractancies of six bitter vetch (Vicia ervilia (L.) Willd.) landraces, a neglected crop. Four traits related to seed yield were also measured. Abundance and foraging behavior of potential insect pollinators on bitter vetch flowers were recorded, and their species were identified. Differences among landraces regarding floral and yield traits were statistically significant in most cases. A total number of four insect species were recorded as positively visiting flowers and constituting potential pollinators of bitter vetch. At a landrace level, there was a positive correlation between potential insect pollinators’ foraging activity and the number of open flowers, especially for the landrace ERV65-Kastania, Korinthia (p ≤ 0.01). Floral tube length, as well as standard petal length, was also positively correlated in some cases with potential insect pollinator species abundance and their visitation frequency. A positive correlation was also recorded between seed yield-related traits, which varied among landraces, and potential insect pollinators’ foraging activity. The results showed that bitter vetch flowers can attract and receive positive visits from insects, despite their mainly self-pollination reproductive system. Bitter vetch flower traits, such as the number of open flowers, floral tube length, and standard petal length, could, therefore, be useful as breeding tools, aiming to develop varieties with insect pollinator-friendly traits that could lead to enhanced seed yield production and help to conserve wild insect species biodiversity in the context of sustainable agriculture.
1. Introduction
Pollinators provide a crucial ecosystem service through their role in the sexual reproduction of both wild and cultivated plant species [1,2]. Insect flower visitors in many cases mediate increased seed set and improvement of food quality, even though the latter is not always clear [3,4,5], as well as the variability of native plants and the conservation of ecological balance [6,7]. On the other hand, insects can benefit from the foraging and nesting resources provided by various plant species. As a result, plant–insect pollinator interactions form a key component for the maintenance of both plant and insect groups in different ecological environments [8].
The rapid intensification of modern agriculture has negatively affected insect pollinator populations and their abundance [9,10,11]. For this reason, nowadays, farming systems and approaches are implemented to mitigate the negative impacts of intensified agriculture on the ecosystem and to support more sustainable agricultural models [10]. One of the approaches that is included within the context of sustainable agriculture is the development of plant breeding strategies that aim to preserve and enhance potential insect pollinators, through the provision of suitable floral and nesting resources [6], while in parallel, to lead to conservation of crops heterogeneity, through enhancement of outcrossing rates whenever is desirable [12].
Potential insect pollinators’ foraging preferences are affected by variability present in floral traits [13,14], the chemical composition of the nectar and the pollen, or even the physical contact that insect pollinators have with the flowers [15]. Therefore, pollinator-mediated breeding strategies are focused on the investigation and selection of genotypes/varieties that express desirable traits that could attract insect pollinators. So far, many floral traits have been proved useful in enhancing insect pollinators’ visits, such as operative strength [16], standard and keel petal dimensions, central color patch, and spots existence [17,18,19], while some of them were utilized in the development of hybrid seed production technologies and open pollination improvement [6,12].
Bitter vetch (Vicia ervilia L. Willd.) is a bushy legume crop and one of the eight Neolithic Founder Crops [20,21]. Nowadays, it is cultivated on a small scale around the Mediterranean, as in North Africa, in the Near East, and in the U.S.A. [22,23,24]. Bitter vetch is a multi-purpose legume, cultivated for its grain, straw, and hay production, as well as for protein-based edible films production and its potential as a source of bioactive components [25,26]. Even though nowadays it is considered a neglected crop [27], an increased interest in this crop with multiple uses has been recorded [28]. Therefore, bitter vetch genetic material, including landraces, has been recently investigated, characterized, and evaluated under various aspects [24,29,30,31,32,33,34]. Landraces are constituting a valuable genetic resource for plant breeding programs. On-farm conservation allows continuous selection by farmers and the maintenance of the availability of genetic variability hidden in landraces material [29,35,36].
The bitter vetch pollination system is reported to be autogamous or predominantly autogamous [37,38], as for most legume species [17]. However, legume flowers are capable of outcrossing, depending on the genetic material used, the climatic factors that prevail in different locations, and the available insect fauna in each location [13,39,40,41,42]. Positive potential insect pollinators’ visitations have also been found to increase the proportion of seed set and/or the quality of the produced legume seeds [13].
Bitter vetch accessions have been assessed for their floral phenology traits, including flowering time and flowering duration and design, such as flower color [33,36,43]. Their color is mainly white with a violet or/and pink pattern or/and lines (colored veins) in the inner side of the standard petal [33]. However, to date, to our knowledge, there is no report with the objective of studying bitter vetch floral traits and investigating their ability to attract potential insect pollinators or recording any positive insect pollinator species foraging on its flowers. For this purpose, in this present study, we aim to examine bitter vetch floral traits and their attractiveness to potential insect pollinator species, so as to increase the knowledge of the crop’s pollination system and assess the possibility of the studied flower traits being implemented for the development of insect pollinator-friendly varieties.
2. Materials and Methods
2.1. Experimental Site and Growth Conditions
The experiment was carried out at an experimental field of the Agricultural University of Athens, Greece (Ν 37°59′10″, Ε 23°42′29″, altitude 24 m), in 2016. The environmental conditions of the location correspond to a Mediterranean climate, with an average rainfall of about 400 mm and sandy loam (SL) soil with pH 7.84. Plants were supplied with 800 kg ha−1 of mineral fertilizer (NPK 11-15-15) as base dressing. Meteorological data of the location throughout the flowering period are presented in Table 1.

Table 1.
Meteorological data recorded—mean, max, and min air temperature (Tair mean (°C), Tair max (°C), Tair min (°C)); total precipitation (mm); and average wind speed (km h−1)—during each one of the six weeks of bitter vetch flowering and on the six days that potential insect pollinators’ visitation was recorded.
The seeds were manually sown, so as to have one plant per position. The experimental field experiences dense vegetation of weeds, such as Sinapis alba L., Papaver rhoeas L., Capsella bursa-pastoris (L.) Medik., Urtica urens L., Chrysanthemum coronarium L., Convolvulus arvensis L., Sissymbrium altissimum L., Parietaria judaica L., and Chenopodium album L. Manual weeding was, therefore, performed inside the experimental plots, while weeding was not applied in the spaces between plots, so that each plot would be bordered by weeds. Harvesting was carried out by hand. During the plants’ growing period, no irrigation or chemical control means were applied.
2.2. Experimental Layout
Sowing took place on 9th of December 2016. Six bitter vetch landraces of Greek origin were used that had previously been assessed and presented differences regarding their mean phenotypic diversity () [33]. Two of the landraces were collected from local farms of Greece (AUA2 from Alexandreia, Imathia, Northern Greece, and AUA5 from Lefkada Island), during expeditions of the laboratory of Plant Breeding and Biometry of the Agricultural University of Athens [44] (Figure 1). The rest were landrace accessions of Greek origin (ERV35-Mina, Lakonia, ERV45-Goura, Korinthia, ERV53-Ampelouzos, Herakleio, ERV65-Kastania, Korinthia), obtained from the Genebank of the Leibniz Institute of Plant Genetics and Crop Plant Research (IPK) in Germany (Figure 1). A Randomized Complete Block Design (RCBD) was used with four replications. Each plot consisted of forty plants per plot. Spacing between rows was 50 cm and within each row 50 cm.
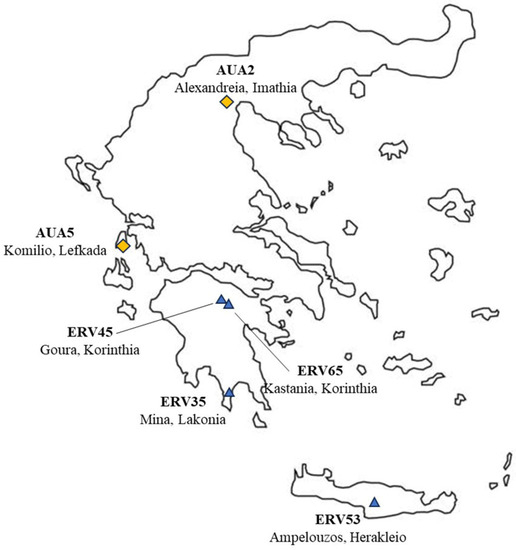
Figure 1.
Bitter vetch landraces codes and collection sites. Blue triangles indicate accessions obtained from the Genebank of Leibniz Institute of Plant Genetics and Crop Plant Research (IPK), while yellow rhombuses indicate landraces collected on-farm.
2.3. Measurements
2.3.1. Flower Traits
Floral characteristics were recorded on ten central plants per experimental plot and then were grouped, in accordance with the protocol proposed by Suso and Maalouf [45], into three groups: (i) phenology traits, (ii) display traits, and (iii) design traits. Phenology traits included beginning of flowering (BOF) and end of flowering (EOF) (measured as days after sowing, DAS), which were recorded with the appearance of the first flower and when there were no open flowers left in each plant, respectively. Flowering duration (FD) was also calculated as the subtraction of the beginning (BOF) from the end of flowering (EOF) per plant.
Display traits recorded included the time of opening and closure of flowers within a day (24 h) time period for each plant, as well as the flower color-pattern (standard petal with or without pigmentation, veins with or without color), the number of flowers per plant, and the number of open flowers per plant. Measurements of opening and closure of flowers, number of flowers per plant, and number of open flowers per plant were taken once per week, on days with favorable weather (not windy or rainy). Standard petal color and pattern (colored veins or not) observations were taken once during morning hours (10 a.m.–12 a.m.).
Design traits included (i) floral advertisement characteristics, namely, standard petal length (cm) (STL) and width (cm) (STW); (ii) female sexual dimensions, namely, style length (cm) (SL) and ovary length (cm) (OL); and (iii) vector matching, namely, keel length (cm) (KL), keel width (cm) (KW), and floral tube length (cm) (FTL). All design traits were measured in three completely open flowers per plant in each experimental plot, using Digimizer Image Analysis Software, version 5.7. (MedCalc Software Ltd., Ostend, Belgium bvba© 2005–2018).
2.3.2. Yield-Related Traits
Traits related to seed yield were also recorded, namely, number of pods per plant (PPP), number of seeds per pod (SPPD), number of seeds per plant (SPPL), and hundred-seed weight (g) (HSW). Measurements were taken from the 10 central plants per plot in which the flower traits had been previously measured.
2.3.3. Potential Insect Pollinators’ Visitation Record
Recording of potential insect pollinators’ visitation took place during the days when display flower traits were measured. Each experimental plot was observed for 5 min, and when a potential insect pollinator was noticed positively foraging in a completely open flower, its visitation and its species was recorded. Positive visits were considered the ones during which potential insect pollinators foraged positively on a flower, entering through the flowers’ natural opening [46]. The potential insect pollinator species (insect species that probably have the ability to pollinate bitter vetch flowers) abundance (number of positively foraging insect species per plant per species) and their foraging behavior (number of positive insect visits per species per plant) were then calculated.
2.3.4. Statistical Analysis
Flower and yield data were subjected to one-way Analysis of Variance (one-way ANOVA), followed by the Least Significant Difference (LSD) means comparison method, using the JMP statistical package, version 8.0.0. [47]. The frequencies of flower color-pattern were calculated for each landrace and cross-tabulation analysis performed to investigate a possible correlation between flower color and potential insect pollinators’ abundance and foraging. Pearson correlation coefficients were computed to assess the relation between potential insect pollinators’ abundance and their foraging behavior, as well as their relation with flower traits and yield-related traits recorded. All the above analyses were performed using Statgraphics Centurion 16 [48].
3. Results
3.1. Flower Traits
Statistically significant differences were found among landraces for all phenological traits studied (p ≤ 0.001) (Table 2). Flowering of the earliest bitter vetch accessions (ERV35-Mina, Lakonia; ERV45-Goura, Korinthia; AUA2-Alexandreia, Imathia) started on 28th of March, while the latest flowering accessions (ERV53-Ampelouzos, Herakleio; ERV65-Kastania, Korinthia; AUA5-Komilio, Lefkada) did not flower until 12th of May.

Table 2.
Flower phenology traits for each bitter vetch landrace. Values are given as means ± SE of four replications. Means in columns with different letters are significantly different by Least Significant Difference (LSD).
More specifically, the mean beginning of flowering ranged from 110.49 DAS (ERV35-Mina, Lakonia) to 134.00 DAS (AUA5-Komilio, Lefkada) (Table 2), while the flowering duration ranged on average from 10.04 days (AUA5-Komilio, Lefkada) to 56.64 days (ERV35-Mina, Lakonia). Among landraces, ERV35 had the earliest flowering and the longest flowering duration, while AUA5 had the latest flowering and the shortest flowering duration, both displaying statistically significant differences from the rest of the landraces. ERV53-Ampelouzos, Herakleio, along with AUA5-Komilio, Lefkada, were the landraces whose flowering ended statistically significantly later than the other ones, with 151.52 and 148.75 DAS, respectively (Table 2). Flowering lasted in total for a six-week period (28th of March–12th of May).
Regarding flower display traits, a total number of six records (28th of March, 4th of April, 11th of April, 20th of April, 28th of April, and 4th of May) took place for the number of flowers and number of open flowers per plant, each one corresponding to one of the six weeks that landraces were flowering. Flowers were recorded to open at 10.00 a.m. in the morning and to close at 19.00 p.m. in the afternoon, while on mornings with partly cloudy weather, a 1 to 2 h delay of the flowers’ opening was observed. The total mean number of flowers and number of open flowers during morning and midday–afternoon hours varied significantly among landraces (p ≤ 0.001) across the six weeks of flowering (Table 3), while a greater number of flowers were recorded to be open during midday hours (15.00–17.00 p.m.) for all landraces in comparison to the morning hours. The time of opening and closure of flowers of all landraces were slightly modified from day to day, depending on the air temperature recorded and the cloudiness.

Table 3.
Mean number of flowers per week (NF1, NF2, NF3, NF4, NF5, NF6), mean number of open flowers during morning–midday hours (NFI1, NFI2, NFI3, NFI4, NFI5, NFI6), and mean number of open flowers during midday–afternoon hours (NFII1, NFII2, NFII3, NFII4, NFII5, NFII6) during each one of the six weeks of flowering, for the six landraces, respectively. Means for the total number of flowers (MNF) and the number of open flowers during morning hours (MNFI) and midday–afternoon hours (MNFII) for each landrace across all weeks. Values are given as mean ± SE of four replicates. Means in columns with different letters are significantly different at 0.001 level by Least Significant Difference (LSD).
The mean number of flowers (MNF) per plant for the landraces that were flowering in each week, across all weeks, ranged from 7.85 flowers per plant (AUA2) to 68.89 (ERV65). AUA2 and ERV35 had an earlier peak of flowering in comparison to ERV65 and AUA5, which flowered later and reached the highest number of flowers (MNF) and number of open flowers (MNFI, MNFII) during the 5th week of flowering (Table 3).
Five flower color and standard petal pattern (standard petal with or without pigmentation, veins with or without color) combination types were recorded, namely, white flower–purple veined (WP), white flower–pink veined and pigmented (WPIP), white flower (W), white flower–purple veined and pink pigmented (WPPIP), and white flower–purple veined and purple pigmented (WPPP) (Figure 2). White–purple veined and pigmented flowers and white–pink veined and pigmented flowers were the most frequent ones, while white flowers were the least frequent, regarding all six landraces (Supplementary Material S1).

Figure 2.
Flower color and standard petal pattern (standard petal with or without pigmentation, veins with or without color) combination types recorded: (a) white flower–purple veined (WP), (b) white flower–pink veined and pigmented (WPIP), (c) white flower (W), (d) white flower–purple veined and pink pigmented (WPPIP), (e) white flower–purple veined and purple pigmented (WPPP).
Statistically significant differences were observed among landraces for all floral design traits studied (p ≤ 0.001), except for keel petal length (KL) and width (KW) (Table 4). ERV65 (Kastania, Korinthia) had the largest standard petal length (STL) (0.730 cm) and width (STW) (0.557 cm), while the shortest standard petal length (0.525 cm) was observed in AUA2 (Alexandreia, Imathia) and the shortest standard petal width (0.305 cm) in AUA5 (Komilio, Lefkada). Style length (SL) ranged from 0.196 cm (AUA2-Alexandreia, Imathia) to 0.250 cm (ERV53-Ampelouzos, Herakleio), while ovary length (OL) ranged from 0.381 (AUA5-Komilio, Lefkada) to 0.514 cm (ERV65-Kastania, Korinthia). AUA2 (Alexandreia, Imathia) and AUA5 (Komilio, Lefkada) had a shorter ovary, with a statistically significant difference from the rest of the landraces (p ≤ 0.001). AUA5 (Komilio, Lefkada) had the longest floral tube (0.386 cm), while AUA2 (Alexandreia, Imathia) displayed the shortest one (0.346 cm) (Table 4).

Table 4.
Flower design traits measured in six bitter vetch landraces. Values are given as means ± SE of four replications. Means in columns with different letters are significantly different by Least Significant Difference (LSD).
3.2. Yield-Related Traits
Among traits related to seed yield, the number of pods per plant (PPP), seeds per plant (SPPL), and hundred-seed weight (HSW) varied significantly among landraces (p ≤ 0.05) (Table 5). ERV35 (Mina, Lakonia) and ERV45 (Goura, Korinthia) had a significantly higher mean number of pods per plant (56.64 and 42.25, respectively) and mean number of seeds per plant (146.31 and 121.26, respectively) than the other landraces. Hundred-seed weight (HSW) was significantly higher in ERV65 (Kastania, Korinthia) (3.93 g) in comparison to most of the landraces studied. Generally, AUA5 (Komilio, Lefkada) received the smallest values for all studied yield traits (Table 5).

Table 5.
Seed yield-related traits for each bitter vetch landrace. Values are given as means ± SE of four replications. Means in columns with different letters are significantly different by Least Significant Difference (LSD).
3.3. Potential Insect Pollinators Record
During the first three weeks of flowering, potential insect pollinators’ visits were not recorded on bitter vetch landraces since the potential insect pollinators were visiting weed and tree flowers that were adjacent to the experimental field and were in bloom at the same time. The main other species that attracted potential insect pollinators of bitter vetch were Sinapis alba L., Sisymbrium altissimum L., Papaver rhoeas L., Matricaria recutita L., and Cercis siliquastrum L. The first positive visits on bitter vetch flowers were recorded during the 4th week of flowering (20th of April) by two solitary Megachilidae bee species, which were recognized as Megachile parietina (Geoffroy, 1785) (black mud bee) and Megachile ericetorum (Lepeletier, 1841) (banded mud bee) (Figure 3a,b). During the two last weeks of flowering (28th of April–12th of May), bitter vetch flowers received visits from another solitary bee species, Andrena flavipes (Panzer, 1799) (mining bee) (Figure 3c), and two butterfly species Pieris rapae (Linnaeus, 1758) and Pieris napi (Linnaeus, 1758). Insect visits were recorded only on four out of six bitter vetch landraces, with the exceptions being AUA2 (Alexandreia, Imathia) and ERV35 (Mina, Lakonia).
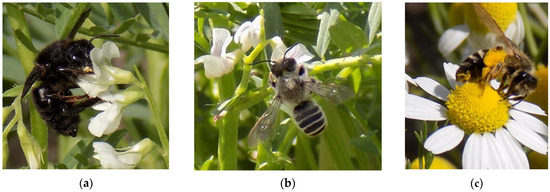
Figure 3.
Bee insect species recorded visiting bitter vetch flowers: (a) Megachile parietina, (b) Megachile ericetorum, (c) Andrena flaviceps.
The mean number of visits per plant of M. parietina ranged from 1.00 to 15.67 for AUA5 (Komilio, Lefkada) and ERV65 (Kastania, Korinthia), respectively (Table 6). ERV65 was the only landrace that was visited by M. ericetorum, while AUA5 (Komilio, Lefkada) and ERV45 (Goura, Korinthia) were visited by Andrena flavipes, with a rate of 3.00 and 8.00 mean number of visits per plant, respectively. Visits from the two pierid butterflies were observed in all four landraces (AUA5, ERV45, ERV53, ERV65) and are presented as a summary (Table 6).

Table 6.
Mean number of visits of potential insect pollinator species on the AUA5, ERV45, ERV53, and ERV65 landraces. Values are given as means ± SE of four replications. Means in columns with different letters are significantly different by Least Significant Difference (LSD).
3.4. Effect of Flower Traits on Potential Insect Pollinator Visits
The effect of bitter vetch floral traits on potential insect pollinator visits was estimated from the 4th to the 6th week and for landraces AUA5, ERV45, ERV53, and ERV65, for which visits by potential insect pollinators were recorded. There were no statistically significant differences regarding abundance and foraging activity among the four landraces, although ERV65 (Kastania, Korinthia) received five times more visits than AUA5 (Komilio, Lefkada) and two times more visits than ERV45 (Goura, Korinthia) (Table 7).

Table 7.
Potential insect pollinators’ abundance, foraging activity, and correlation coefficients (Corr. Coeff.) of abundance and foraging activity with the number of open flowers for landraces AUA5, ERV45, ERV53, and ERV65, during the 4th–6th week. Values are given as means ± SE of four replications.
Analysis showed a significant correlation between potential insect pollinators’ abundance and the number of open flowers of AUA5 (Komilio, Lefkada) (Corr. Coeff. = 0.92, p ≤ 0.01). A positive correlation was also recorded between potential insect pollinators’ abundance and the number of open flowers of ERV65 (Kastania, Korinthia) (Corr. Coeff. = 0.89, p ≤ 0.01) (Table 7). The correlation between the number of open flowers and foraging activity recorded for ERV65 (Kastania, Korinthia) was significant (Corr. Coeff. = 0.93, p ≤ 0.01), whereas on AUA5 (Komilio, Lefkada), a weaker correlation was found (Corr. Coeff. = 0.72, p ≤ 0.01). The number of open flowers of ERV45 (Goura, Korinthia) also displayed a positive, significant correlation with abundance, although not as strong. The ERV53 (Ampelouzos, Herakleio) number of open flowers displayed no significant correlation with abundance and foraging activity (Table 7). The other floral display traits recorded did not show significant correlations either with potential insect pollinators’ abundance or with their foraging habit.
Regarding floral design traits, a statistically significant positive correlation was also found between length of standard petal and potential insect pollinators’ abundance (Corr. Coeff. = 0.92, p ≤ 0.05), as well as between the length and width of standard petal with foraging activity (Corr. Coeff. = 0.95, p ≤ 0.05, and 0.99, p ≤ 0.01, respectively) in the case of ERV65 (Kastania, Korinthia). A negative significant correlation was recorded for this landrace between its floral tube length and abundance of potential insect pollinators’ (Corr. Coeff. = −0.95, p ≤ 0.05), while a significant positive correlation was found between its keel petal width and potential insect pollinators’ foraging activity (Corr. Coeff. = −0.99, p ≤ 0.01).
In AUA5 (Komilio, Lefkada), a statistically significant positive correlation was found between length of floral tube and potential insect pollinators’ abundance (Corr. Coeff. = 0.97, p ≤ 0.05), while negative correlations were exhibited between potential insect pollinators’ abundance and both standard petal length and keel width (Corr. Coeff. = −0.99, p ≤ 0.001, and −0.96, p ≤ 0.05, respectively). None of the floral design traits studied correlated significantly with the foraging activity of the potential insect pollinators recorded. Furthermore, in the cases of ERV65 and AUA5, potential insect pollinators’ abundance and foraging activity did not express a significant correlation with each other. On the contrary, in the ERV45 (Goura, Korinthia) and ERV53 (Ampelouzos, Herakleio) cases, a significant positive correlation was exhibited between potential insect pollinators’ abundance and foraging activity (Corr. Coeff. = 0.95, p ≤ 0.05, and 0.99, p ≤ 0.01, respectively).
The only significant correlation regarding ERV45 (Goura, Korinthia) was that between ovary length and foraging activity (Corr. Coeff. = 0.95, p ≤ 0.05). In the case of ERV53 (Ampelouzos, Herakleio), a significant positive correlation exhibited between style length and both potential insect pollinators’ abundance and foraging activity (Corr. Coeff. = 0.96, p ≤ 0.05 and 0.98, p ≤ 0.05, respectively), while a significant negative correlation was exhibited between keel width and both potential insect pollinators’ abundance and foraging activity (Corr. Coeff. = −0.99, p ≤ 0.01 and −0.99, p ≤ 0.05, respectively).
3.5. Effect of Potential Insect Pollinators Visits on Yield-Related Traits
There was a positive correlation between hundred-seed weight, pods per plant, and seeds per plant with both potential insect pollinators’ abundance and foraging activity for three of the four studied landraces for which visits were recorded (Table 8). Abundance was significantly correlated with the number of pods per plant (PPP) (p ≤ 0.01) and number of seeds per plant (SPPL) (p ≤ 0.01) of AUA5 (Komilio, Lefkada), and with the hundred-seed weight (HSW) of ERV45 (Goura, Korinthia) (p ≤ 0.01), as well as with the number of pods per plant (p ≤ 0.001) and number of seeds per plant (SPPL) (p ≤ 0.01) of ERV53 (Ampelouzos, Herakleio) (Table 8).

Table 8.
Pearson correlation coefficients between potential insect pollinators’ abundance and foraging activity for each one of the four landraces for which visits were recorded, as well as among seed yield-related traits, and potential insect pollinators’ abundance and foraging activity.
Foraging activity was significantly correlated with the number of pods per plant (PPP) of AUA5 (Komilio, Lefkada) (p ≤ 0.05) and ERV53 (Ampelouzos, Herakleio) (p ≤ 0.001), and with the hundred-seed weight (HSW) of ERV45 (Goura, Korinthia) (p ≤ 0.01) and of ERV53 (Ampelouzos, Herakleio) (p ≤ 0.05), as well as with the number of seeds per plant (SPPL) of ERV53 (p ≤ 0.01) (Table 8).
4. Discussion
4.1. Flower and Insect Observations
Breeding for sustainable agriculture is linked to the development of environmental, non-food services [49]. Plant–pollinator interactions can be optimized by breeding strategies that focus on both plant yield and insect pollinators needs, through the selection of plant traits that are favorable to insect pollinators [50].
In our study, bitter vetch landraces presented statistically significant differences for most of their studied floral traits, allowing in this way the assessment of these traits regarding their attractiveness to potential insect pollinators. Bitter vetch flowers of four out of the six studied landraces received frequent positive visits from solitary bees and butterflies, despite the self-pollinated or mainly self-pollinated mating system reported for the species. Therefore, its flowers can be suitable for the foraging needs of various insects that could further serve as pollinators.
To assess potential insect pollinators’ visitation rate, a protocol described by Suso et al. [17,46], which has been also used for pollinator-mediated studies of different legume species, such as faba bean (Vicia faba L.), cowpea (Vigna unguiculata (L.) Walp.), and Andean lupin (Lupinus mutabilis Sweet.), was followed [7,41,51]. During our study, several plants of early-flowering landraces, ERV35 (Mina, Lakonia) and AUA2 (Alexandreia, Imathia), displayed some flowers with completely open standard and keel petals, exposing their stigma and stamens (Figure 4; Supplementary Material S2). However, no potential insect pollinators’ visit was recorded on these plants, suggesting that this phenomenon might not be due to a visit of a bigger and heavier insect, as recorded for cowpea [51].

Figure 4.
Bitter vetch flower with exposed stigma and stamens.
AUA2 and ERV35 have also been previously recorded as expressing lower mean phenotypic diversity within each population across all trait () values than the other four studied landraces [33], which enhances the possibility of this phenomenon not serving as a secondary pollen transfer mechanism [51].
Style and stamens dimensions are reported to not be easily affected by drought; however, differences in flower morphology traits, such as in petal length, have been previously reported to occur due to limited water conditions [52,53], which could affect the stigma and stamens position and lead to their exposure. Therefore, flowers of these two landraces were probably affected by drought that led to an exposed stigma and stamens phenomenon, as no irrigation was implemented, and no precipitation occurred during the plants flowering stage. AUA2 and ERV35 were also the earliest flowering landraces, while AUA2 flowering lasted only 15.65 days on average; therefore, these landraces might be forced to complete their biological cycle earlier. Moreover, they were possibly forced to enter the reproduction phase, as they were the earliest flowering landraces; however, only AUA2 was characterized by a short flowering duration. Additional research is needed regarding drought’s effect on bitter vetch flowers morphology and its ability to modify species’ pollination system. Modifications of the protocol followed may also be needed. Bitter vetch is a bushy legume with smaller flower size and a greater number of flowers than other legume species to which this protocol has been previously applied [7,41,51]. Bitter vetch flowers also remain open for many hours during the day, and therefore, the flower visitation record is complex and difficult. For these reasons, amendments are probably needed regarding the frequency of the observations recording and the time that should be spent for a plot observation.
Among potential insect pollinator species recorded as visiting bitter vetch flowers, two of them belong to the genus Megachile (Latreile, 1802), which is known to include oligolectic species that are common visitors and pollinators of legume species [54]. Another potential insect pollinator species recorded belongs to the genus Andrena (Fabricius, 1775), which is reported to comprise species that are feeding on different flower species or families (polylectic), depending on the species, location, fauna, and crop/tree availability in each season [55,56,57].
During the beginning of bitter vetch plants flowering, no potential pollinator insects’ visitation was recorded on them while potential insect pollinators were visiting frequently the weed species flowering around and in the corridors of the experimental field. Weed species left on purpose, as strips of flowering plants around field crops have been reported to provide food and habitat to pollinators [58]. Weeds have also been left to grow frequently around the field, as in some crop species, they have been found to enhance pollinators visitation on cultivated species [59,60].
Nevertheless, in our case, weeds were found to be strong competitors of bitter vetch regarding potential pollinating insects’ visitation, especially during the first three weeks of bitter vetch flowering. Potential insect pollinators’ preference for weed flowers could be attributed to the higher pollen and nectar concentration of weed species in comparison to that of bitter vetch flowers. No effect on pollinators’ visit rate or a negative impact of flowering weeds have been previously reported on other cultivated crops and orchards [59,61]. There is also the possibility that the smaller number of bitter vetch flowers in total and open flowers available during the first three weeks of the trial’s flowering, as only three of the six landraces were flowering, were not adequate to attract potential insect pollinators, providing poor floral advertisement, since flower rewards available depend on the number of open flowers [62]. Legumes are reported also to provide a high pollen protein content, which is useful to potential insect pollinators to cover their needs during their reproductive and larva feeding periods; however, they cannot meet all the requirements that they have [63,64]. Therefore, the fact that potential insect pollinators’ visitation on bitter vetch flowers started later than their visitation on weed species could probably reveal their increased need for pollen rich in protein content during specific life cycle periods.
The two species of the genus Megachile that were recorded in the present study showed a particular preference for the flowers of bitter vetch over the neighboring weeds, while the Andrena species visited bitter vetch few times and preferred nearby weeds flowers, especially that of Matricaria recutita L. Different needs and preferences for bitter vetch flower resources were expressed among potential insect pollinator species. The visits of these species were from the physical opening of bitter vetch flowers and were considered as positive. No visits for nectar robbing were recorded. More research is needed aiming to identify if these bee species constitute pollinators of bitter vetch and if they promote self- or cross-pollination.
Even though there were abundant honeybees (Apis mellifera L.), Xylocopa spp. and Bombus spp. in the experimental field, no visitations to bitter vetch flowers were recorded by them; instead, they preferred and were visiting tree and weed species that were around the experimental field. The bitter vetch small flower size, as well as floral display traits, might be the reasons that they were not being visited by these species [65]. In general, legumes are not considered to solely sufficiently provide insects the essential nutrients needed and, therefore, are suggested to be used in multicultures to favor pollinators [64].
Two butterfly species belonging to the genus Pieris (Schrank, 1801) were also recorded while visiting bitter vetch flowers. Pieridae (Lepidoptera: Papilionoidea) butterflies are reported to feed as larvae on plants belonging to legumes, among others [66]. On the other hand, Pieridae are considered among the butterfly species that visit flowers for nectar, as they have long, thin proboscis and sometimes are able to transfer pollen [67,68]. However, in the present study, the two species recorded were not able to trigger bitter vetch flowers, and therefore, they have a reduced probability of constituting pollinators of the species.
4.2. Flower Traits Effect on Potential Insect Pollinators’ Visitation
Early-flowering landraces, ERV35 and AUA2, received no visits from potential insect pollinators, in comparison to late-flowering landraces, and therefore, they were considered as the least preferable for selection for breeding insect pollinator-friendly varieties adapted to the specific climatic conditions of this location. On the other hand, most potential insect pollinators’ visits were recorded for ERV65 (Kastania, Korinthia), along with AUA5 (Komilio, Lefkada), landraces that both were among the late flowering ones and with a relatively short flowering duration. These findings are in agreement with those of Rodríguez-Pérez and Traveset [69], who supported that, in plant species with long flowering periods, flowers that are developing outside the flowering peak may have lower chances to be pollinated by insects, since plants and the experimental field are overall less advertised in a specific period of time. Therefore, synchronization of flowering time with the insect pollinators’ emergence and flight period abundance in an area, as everything can change seasonally, is of main importance when the aim is to breed high-yielding, insect pollinator-friendly varieties [70].
The implementation of agronomic practices, such as applying different sowing dates (e.g., in the case of early flowering landraces AUA2 and ERV35), could effectively mediate this synchronization and, therefore, optimize bitter vetch and potential insect pollinators’ interactions. This has been achieved on the highly pollinator-dependent niger (Guizotia abyssinica (L.f.) Cas.), leading to an increase of pollinator visits and, consequently, to higher seed yield [71]. Studies on the genetic control of flowering could also further assist in synchronizing flowering with potential insect pollinators’ flight period, as it is the key factor for the adaptation of many species [72]. In this way, genotypes could be selected based on the desirable sequences, as most of the flowering regulatory genes are known [73].
The number of open flowers of the landrace ERV65 (Kastania, Korinthia) had a positive correlation with the potential insect pollinators’ foraging activity. Although not comparable, a high significant correlation between floral display size and pollinators’ activity has been previously reported by Kudo and Harder [65], who stated that bumblebees visited more flowers per inflorescence in legume species that displayed a larger number of flowers. Our results are also consistent with the statements of Ohashi and Yahara [74] that bee species tend to visit individual plants with large displays more frequently than plants of the same species with smaller displays. Particularly, in faba bean, the number of available flowers had a positive regression with a higher number of bee visits [46]. A significant relation between the number of open flowers and pollinators visitation has also been recorded for other plant species [75,76]. However, in the present study, AUA5 (Komilio, Lefkada), despite having a similar number of open flowers to that of ERV65, received fewer visits than any other late-flowering landrace. Probably, this happened because AUA5 was the landrace that presented a significantly smaller size of flowers in comparison to the other landraces used in the present study.
Flowers with a venation pattern are reported to be preferred by insect pollinators more often than colored ones, because of their perceived utility as nectar guides [18]. Flower color has also been reported to affect pollination insects’ visitation [77], as innate flower color preferences were proved to exist [78]. However, color preference is not always reward-dependent [78,79,80]. In the case of bitter vetch, no differences were observed in potential insect pollinators’ preferences among the various types of recorded flower color-patterns. So, in bitter vetch, color-pattern preference should be taken into consideration in combination with other floral traits, as it has been stated that color alone is maybe not adequate to affect plant–insect pollinators’ interactions [78,81]. Floral display, for example, was reported to attract insect pollinators to a greater extent than flower color [82].
With regard to floral design traits studied, significant positive or negative correlations were recorded between keel width and potential insect pollinators’ abundance and foraging activity. However, this trait did not present statistically significant differences among the landraces and, therefore, could not be used solely by breeders for the creation of insect pollinator-friendly varieties. ERV53 (Ampelouzos, Herakleio) presented a significant positive correlation of style length with potential insect pollinators’ abundance and foraging activity and, in parallel, presented a statistically significantly higher mean value of this trait than the other landraces. ERV65 (Kastania, Korinthia) also presented a positive correlation of standard petal width with foraging activity and had the largest mean standard petal length among the landraces studied. These traits could, therefore, constitute interesting traits for breeding insect pollinator-friendly varieties.
Potential insect pollinators’ abundance and foraging activity correlated significantly with floral tube length, as well as standard petal length, either positively (AUA5 and ERV65, respectively, for the two flower traits) or negatively (ERV65 and AUA5, respectively, for the two flower traits). Therefore, aiming to enhance potential insect pollinators’ visitation rates, bitter vetch genotypes or varieties/landraces with short floral tube length and large standard petal length should be selected. In faba bean, outcrossing rates were lower in genotypes with long floral tubes [18], while in cowpea, floral design traits did not enhance potential insect pollinators’ visitation [51]. Generally, long floral tube flowers, although they profit pollinators with more pollen [83], are becoming available for less insect pollinator species, as the latter should have a long proboscis [84].
ERV65 (Kastania, Korinthia) received the largest number of visits by Megachilidae bee species. The larger values of floral design traits that this landrace expressed probably were the ones that attracted more intensively these species. On the other hand, Andrena flavipes (Panzer, 1799) was recorded visiting only the AUA5 (Komilio, Lefkada) and ERV45 (Goura, Korinthia) landraces. Andrena flavipes is a polylectic bee species—its presence could favor pollination of these two landraces; however, an insect species more specialized on legume species, like Megachile parietina (Geoffroy, 1785), could, in general, enhance more the pollination ability of the species [85].
4.3. Potential Insect Pollinators’ Visitation Effect on Yield-Related Traits
A significant effect of potential insect pollinators’ abundance and foraging activity on most studied seed yield-related traits was recorded, depending on the landrace. Out of the four bitter vetch landraces that received pollinator visits, ERV53 (Ampelouzos, Herakleio) showed positive correlations of potential insect pollinators’ species and foraging behavior with pods per plant and seeds per plant. This landrace exhibited large-sized flowers with a medium-low floral display size and was visited only by M. parietina. AUA5 (Komilio, Lefkada), which had received visits mainly by A. flavipes, presented also positive correlations between potential insect pollinators’ abundance and foraging and the number of pods and seeds per plant, while ERV45 (Goura, Korinthia) presented a significant positive correlation with hundred-seed weight and was visited by both aforementioned insect species. These two insect species could, therefore, be positive pollinators of bitter vetch flowers promoting either cross- or self-pollination and contribute at the same time to seed yield increase. It is unlikely there was not an impact of potential insect pollinators’ presence and activity on yield traits of ERV65 (Kastania, Korinthia), despite the fact that it was the landrace that received most visits and exhibited a larger standard petal size.
The significant impact of insect pollinators’ presence and foraging activity on yield traits has been previously recorded in sesame (Sesamum indicum L.), in cotton (Gossypium hirsutum L.), and in rapeseed (Brassica napus L.), in which an increase in seed weight was recorded, as well as in the number of pods and seeds per plant in faba bean (Vicia faba L.) and cowpea (Vigna unguiculata (L.) Walp.) [17,86,87,88]. The positive correlation of landraces’ yield traits with potential insect pollinators’ visitation observed gives an early insight that insects can enhance bitter vetch seed yield, while floral traits, such as the flower size and number of open flowers, can be regarded as attractants to pollinators, giving in parallel valuable information that could lead to successful development of insect pollinator-friendly, high-yielding cultivars, such as in pigeon-pea (Cajanus cajan L.), for which a successful hybrid was developed [89]; in soybean (Glycine max L.); and in faba bean (Vicia faba L.) [90,91]. On the other hand, outcrossing and increased heterogeneity is not desirable in many breeding procedures of various crops, such as in pure lines creation where five to six generations of inbreeding are needed, in multilines, and in hybrids creation [92,93,94]. Further experimentation is needed, as the yield and pollinators abundance and impact on crops varies among locations, and environmental conditions prevail in each area [46,95], affecting also the breeding processes applied.
5. Conclusions
Bitter vetch flowers received visits from potential insect pollinators and, therefore, are capable of providing suitable food resources, despite the species’ primarily self-pollination system. The high statistically significant positive correlation between the number of open flowers and potential insect pollinators’ abundance and foraging activity revealed, as well as the the statistically significant positive correlation between floral design traits, such as floral tube length and standard petal length, with potential insect pollinators’ abundance and visitation rates in many cases, showed that these traits can be regarded as tools for breeders aiming to develop insect pollinator-friendly varieties. Landrace ERV65 (Kastania, Korinthia) received more visits and, therefore, is considered the most insect-friendly landrace of the present study, although its yield-related traits were not enhanced by its increased visitation. AUA5 (Komilio, Lefkada) and ERV53 (Ampelouzos, Herakleio) were, on the other hand, the landraces that presented a statistically significant correlation of their seed yield components with potential insect pollinators’ abundance and foraging activity. Further investigation is needed to confirm if the insect species recorded are effective pollinators of bitter vetch.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ecologies4030039/s1, Supplementary Material S1. Flower color-pattern types (standard petal with or without pigmentation- veins with or without color) frequencies (%) within each landrace and total percentages of each type observed. Prevalent flower type and pattern per landrace is indicated in bold.; Supplementary Material S2. Bitter vetch flower with exposed stigma and stamens.
Author Contributions
Conceptualization, P.J.B.; methodology, P.J.B. and E.L.; validation, V.F., E.L. and P.J.B.; formal analysis, V.F. and E.L.; investigation, V.F., E.L. and F.J.O.-S.; resources, P.J.B.; data curation, E.L. and V.F.; writing—original draft preparation, V.F. and E.L.; writing—review and editing, M.J.S., F.J.O.-S., A.T. and P.J.B.; visualization, E.L., M.J.S. and P.J.B.; supervision, P.J.B. (supervised V.F.’s Master’s thesis, was in charge of overall coordination and planning, and prepared the final version of the manuscript with input from all authors); project administration, P.J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are contained within this article.
Acknowledgments
We would like to thank the farmers for providing the on-farm conserved bitter vetch material and IPK Gatersleben for the provision of ERV35, ERV45, ERV53, and ERV65 landrace accessions used in this study. P.J.B. would like to thank her students for their assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vanbergen, A.J.; Insect Pollinators Initiative. Threats to an ecosystem service: Pressures on pollinators. Front. Ecol. Environ. 2013, 11, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Katumo, D.M.; Liang, H.; Ochola, A.C.; Lv, M.; Wang, Q.-F.; Yang, C.-F. Pollinator diversity benefits natural and agricultural ecosystems, environmental health, and human welfare. Plant Divers. 2022, 44, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Klatt, B.K.; Holzschuh, A.; Westphal, C.; Clough, Y.; Smit, I.; Pawelzik, E.; Tscharntke, T. Bee pollination improves crop quality, shelf life and commercial value. Proc. R. Soc. B 2014, 281, 20132440. [Google Scholar] [CrossRef]
- Adamidis, G.C.; Cartar, R.V.; Melanthopoulos, A.P.; Pernal, S.F.; Hoover, S.E. Pollinators enhance crop yield and shorten the growing season by modulating plant functional characteristics: A comparison of 23 canola varieties. Sci. Rep. 2019, 9, 14208. [Google Scholar] [CrossRef] [PubMed]
- Kehrberger, S.; Holzschuh, A. How does timing of flowering affect competition for pollinators, flower visitation and seed set in an early spring grassland plant? Sci. Rep. 2019, 9, 15593. [Google Scholar] [CrossRef]
- Palmer, R.G.; Perez, P.T.; Ortiz-Perez, E.; Maalouf, F.; Suso, M.J. The role of crop-pollinator relationships in breeding for pollinator-friendly legumes: From a breeding perspective. Euphytica 2009, 170, 35–52. [Google Scholar] [CrossRef]
- Suso, M.J.; Del Río, R. Faba bean gene-pools development for low-input agriculture: Understanding early stages of natural selection. Euphytica 2014, 196, 77–93. [Google Scholar] [CrossRef]
- Morton, E.M.; Rafferty, N.E. Plant-pollinator interactions under climate change: The use of spatial and temporal transplants. Appl. Plant Sci. 2017, 56, 1600133. [Google Scholar] [CrossRef]
- Kremen, C.; Williams, N.M.; Thorp, R.W. Crop pollination from native bees at risk from agricultural intensification. Proc. Natl. Acad. Sci. USA 2002, 99, 16812–16816. [Google Scholar] [CrossRef]
- Kovács-Hostyánszki, A.; Espíndola, A.; Vanbergen, A.J.; Settele, J.; Kremen, C.; Dicks, L.V. Ecological intensification to mitigate impacts of conventional intensive land use on pollinators and pollination. Ecol. Let. 2017, 20, 673–689. [Google Scholar] [CrossRef]
- Tommasi, N.; Biella, P.; Guzzetti, L.; Lasway, J.V.; Njovu, H.K.; Tapparo, A.; Agostinetto, G.; Peters, M.K.; Steffan-Dewenter, I.; Labra, M.; et al. Impact of land use intensification and local features on plants and pollinators in Sub-Saharan smallholder farms. Agric. Ecosyst. Environ. 2021, 319, 107560. [Google Scholar] [CrossRef]
- Suso, M.J.; Bebeli, P.J.; Christmann, S.; Mateus, C.; Negri, V.; Pinheiro de Carvalho, M.A.A.; Torricelli, R.; Veloso, M.M. Enhancing legume ecosystem services through an understanding of plant–pollinator interplay. Front. Plant Sci. 2016, 7, 333. [Google Scholar] [CrossRef] [PubMed]
- Suso, M.J.; Bebeli, P.J.; Palmer, R.G. Reproductive biology of grain legumes. In Grain Legumes Handbook of Plant Breeding; De Ron, A.M., Ed.; Springer: New York, NY, USA, 2015; pp. 365–399. [Google Scholar]
- Cresswell, C.J.; Cunningham, H.M.; Wilcox, A.; Randall, N.P. What specific plant traits support ecosystem services such as pollination, bio-control and water quality protection in temperate climates? A systematic map. Environ. Evid. 2018, 7, 2. [Google Scholar] [CrossRef]
- Chess, S.K.; Raguso, R.A.; Lebuhn, G. Geographic divergence in floral morphology and scent in Linanthus dichotomus Polemoniaceae. Am. J. Bot. 2008, 95, 1652–1659. [Google Scholar] [CrossRef] [PubMed]
- Córdoba, S.A.; Cocucci, A.A. Flower power: Its association with bee power and floral functional morphology in papilionate legumes. Ann. Bot. 2011, 1085, 919–931. [Google Scholar] [CrossRef]
- Suso, M.J.; Harder, L.; Moreno, M.T.; Maalouf, F. New strategies for increasing heterozygosity in crops: Vicia faba mating system as a study case. Eyphytica 2005, 143, 51–65. [Google Scholar] [CrossRef]
- Whitney, H.M.; Milne, G.; Rands, S.A.; Vignolini, S.; Martin, C.; Glover, B.J. The influence of pigmentation patterning on bumblebee foraging from flowers of Antirrhinum majus. Naturwissenschaften 2013, 100, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Polte, S.; Reinhold, K. The function of the wild carrot’s dark central floret: Attract, guide or deter? Plant Species Biol. 2013, 28, 81–86. [Google Scholar] [CrossRef]
- Ladizinsky, G. Plant Evolution under Domestication; Springer: Dordrecht, The Netherlands, 1998; pp. 1–254. [Google Scholar]
- Zohary, D.; Hopf, M. Current state of the art. In Domestication of Plants in the Old World: The Origin and Spread of Cultivated Plants in West Asia. Europe, and the Nile Valley, 4th ed.; Zohary, D., Hopf, M., Weiss, E., Eds.; Oxford University Press: New York, NY, USA, 2000; pp. 1–7. [Google Scholar]
- Samarah, N.; Allataifeh, N.; Turk, M.A.; Tawaha, A.M. Effect of maturity stage on germination and dormancy of fresh and air-dried seeds of bitter vetch (Vicia ervilia L.). N. Z. J. Agric. Res. 2003, 46, 347–354. [Google Scholar] [CrossRef]
- Sadeghi, G.H.; Pourreza, J.; Samei, A.; Rahmani, H. Chemical composition and some anti-nutrient content of raw and processed bitter vetch (Vicia ervilia) seed for use as feeding stuff in poultry diet. Trop. Anim. Health Prod. 2009, 41, 85–93. [Google Scholar] [CrossRef] [PubMed]
- González-Verdejo, C.I.; Fernández-Aparicio, M.; Córdoba, E.M.; Nadal, S. Identification of Vicia ervilia germplasm resistant to Orobanche crenata. Plants 2020, 9, 1568. [Google Scholar] [CrossRef]
- Porta, R.; Di Pierro, P.; Sabbah, M.; Regalado-Gonzales, C.; Mariniello, L.; Kadivar, M.; Arabestani, A. Blend films of pectin and bitter vetch (Vicia ervilia) proteins: Properties and effect of transglutaminase. Innov. Food Sci. Emerg. Technol. 2016, 36, 245–251. [Google Scholar] [CrossRef]
- Petkova, Z.; Antova, G.; Teneva, O.; Angelova-Romova, M. Bitter vetch seed oil (Vicia ervilia L.)—A new source of bioactive components. J. Indian Chem. Soc. 2020, 97, 2130–2135. [Google Scholar]
- López Bellido, L. Grain legumes for animal feed. In Neglected Crops: 1492 from a Different Perspective; Hernándo Bermejo, J.E., León, J., Eds.; Plant Prod Prot Series No. 26; FAO: Rome, Italy, 1994; pp. 273–288. [Google Scholar]
- Jaenicke, H.; Höschle-Zeledon, I. A Strategic framework for underutilized plant species research and development, with special reference to Asia and the Pacific, and to Sub-Saharan Africa. Acta Hortic. 2009, 818, 333–342. [Google Scholar] [CrossRef]
- Larbi, A.; El-Moneim, A.A.; Nakkoul, H.; Jammal, B.; Hassan, S. Intra-species variations in yield and quality determinants in Vicia species: 1. Bitter vetch (Vicia ervilia L.). Anim. Feed Sci. Technol. 2011, 165, 278–287. [Google Scholar] [CrossRef]
- Córdoba, E.M.; Fernández-Aparicio, M.; González-Verdejo, C.I.; López-Grau, C.; Muñoz-Muñoz, M.V.; Nadal, S. Search for resistant genotypes to Cuscuta campestris infection in two legume species, Vicia sativa and Vicia ervilia. Plants 2021, 10, 738. [Google Scholar] [CrossRef] [PubMed]
- Tarahi, M.; Shahidi, F.; Hedayati, S. A Novel starch from bitter vetch (Vicia ervilia) seeds: A comparison of its physicochemical, structural, thermal, rheological and pasting properties with conventional starches. Int. J. Food Sci. Technol. 2022, 57, 6833–6842. [Google Scholar] [CrossRef]
- El Fatehi, S.E.; Bena, G.; Filali-Maltouf, A.; Ater, M. Genetic diversity of Moroccan bitter vetch Vicia ervilia (L.) Willd. landraces revealed by morphological and SSR markers. Aust. J. Crop Sci. 2016, 10, 717–725. [Google Scholar] [CrossRef]
- Livanios, I.; Lazaridi, E.; Bebeli, P.J. Assessment of phenotypic diversity in bitter vetch (Vicia ervilia (L.) Willd.) populations. Genet. Resour. Crop Evol. 2017, 65, 355–371. [Google Scholar] [CrossRef]
- Hassanpour, F.; Sahhafi, S.R. Genetic variation in some Iranian bitter vetch (Vicia ervilia L.) landraces based on agronomic-morphological traits for use in breeding program in Rafsanjan. Genet. Resour. Crop Evol. 2020, 67, 2087–2100. [Google Scholar] [CrossRef]
- El Fatehi, S.E.; Bena, G.; Sbabou, L.; Filali-Maltouf, A.; Ater, M. Preliminary results for user SSR markers in bitter vetch (Vicia ervilia L. Willd.). Int. J. Res. 2014, 1, 40–46. [Google Scholar]
- Abbasi, M.R.; Vaezi, S.H.; Bachaei, N. Genetic diversity of bitter vetch (Vicia ervilia) collection of the National Plant Gene Bank of Iran based on agro-morphological traits. Iran. J. Rangel. For. Plant Breed. Genet. Res. 2007, 152, 113–128. [Google Scholar] [CrossRef]
- Zhang, X.; Mosjidis, J.A. Rapid prediction of mating system of Vicia species. Crop Sci. 1998, 38, 872–875. [Google Scholar] [CrossRef]
- Zohary, D.; Hopf, M.; Weiss, E. Domestication of Plants in the Old World: The Origin and Spread of Domesticated Plants in Southwest Asia, Europe, and the Mediterranean Basin, 3rd ed.; Oxford University Press: Oxford, UK, 2012; p. 316. [Google Scholar]
- Rubio, J.; Fernandez-Romero, M.D.; Millán, T.; Gil, J.; Suso, M.J. Outcrossing rate and genetic structure on an open-flowering population of Cicer arietinum based on microsatellite markers. In Proceedings of the 5th International Food Legumes Research Conference and of the 7th European Conference on Grain Legumes for Global Health Legume Crops and Products for Food, Feed and Environmental Benefits, Antalya, Turkey, 26 April 2010. [Google Scholar]
- Lioi, L.; Sparvoli, F.; Sonnante, G.; Laghetti, G.; Lupo, F.; Zaccardelli, M. Characterization of Italian grasspea (Lathyrus sativus L.) germplasm using agronomic traits, biochemical and molecular markers. Genet. Res. Crop Evol. 2011, 58, 425–437. [Google Scholar] [CrossRef]
- Bebeli, P.J.; Lazaridi, E.; Chatzigeorgiou, T.; Suso, M.-J.; Hein, W.; Alexopoulos, A.A.; Canha, G.; van Haren, R.J.F.; Jóhannsson, M.H.; Mateos, C.; et al. State and progress of Andean Lupin cultivation in Europe: A review. Agronomy 2020, 10, 1038. [Google Scholar] [CrossRef]
- Barda, M.S.; Chatzigeorgiou, T.; Papadopoulos, G.K.; Bebeli, P.J. Agro-morphological evaluation of Lupinus mutabilis in two locations in Greece and association with insect pollinators. Agriculture 2021, 11, 236. [Google Scholar] [CrossRef]
- Seydoșoğlu, S.; Saruhan, V.; Kökten, K. Researches on determination yield and yield components of some bitter vetch (Vicia ervilia L. Willd.) genotypes in ecological conditions of Diyarbakır. Gaziosmanpașa Üniv. Ziraat Fakültesi Derg. 2015, 32, 107–115. [Google Scholar]
- Thomas, K.; Thanopoulos, R.; Knüpffer, H.; Bebeli, P.J. Plant genetic resources in a touristic island: The case of Lefkada Ionian Islands, Greece. Genet. Resour. Crop Evol. 2013, 60, 2431–2455. [Google Scholar] [CrossRef]
- Suso, M.J.; Maalouf, F. Direct and correlated responses to upward and downward selection for outcrossing in Vicia faba. Field Crop Res. 2010, 116, 116–126. [Google Scholar] [CrossRef][Green Version]
- Suso, M.J.; Pierre, J.; Moreno, M.; Esnault, R.; Le Guen, J. Variation in outcrossing levels in faba bean cultivars: Role of ecological factors. J. Agric. Sci. 2001, 136, 399–405. [Google Scholar] [CrossRef]
- SAS Institute Inc. JMP/Sales Department; SAS Institute Inc.: Cary, NC, USA, 2008. [Google Scholar]
- Statgraphics Centurion XVII, Version 17.2.0.0; StatPoint, Inc.: Herndon, VA, USA, 2016.
- Helenius, J.; Stoddard, F. Agro-ecosystem services from increased usage and novel applications of legumes. In Integrating Legume Biology for Sustainable Agriculture, European association for grain legume research. In Proceedings of the 6th European Conference on Grain Legumes, Lisbon, Portugal, 12 November 2007; pp. 18–19. [Google Scholar]
- Cerrutti, N.; Pontet, C. Differential attractiveness of sunflower cultivars to the honeybee Apis mellifera L. Oilseeds Fats Crops Lipids 2016, 23, D204. [Google Scholar] [CrossRef]
- Lazaridi, E.; Suso, M.J.; Ortiz-Sánchez, F.J.; Bebeli, P.J. Investigation of Cowpea (Vigna unguiculata (L.) Walp.)–Insect pollinator interactions aiming to increase Cowpea yield and define new breeding tools. Ecologies 2023, 4, 124–140. [Google Scholar] [CrossRef]
- Descamps, C.; Quinet, M.; Jacquemart, A.L. The effects of drought on plant–pollinator interactions: What to expect? Environ. Exp. Bot. 2021, 182, 104297. [Google Scholar] [CrossRef]
- Kuppler, J.; Wieland, J.; Junker, R.R.; Ayasse, M. Drought-induced reduction in flower size and abundance correlates with reduced flower visits by bumble bees. AoB Plants 2021, 13, plab001. [Google Scholar] [CrossRef]
- Sinu, P.A.; Bronstein, J.L. Foraging preferences of leafcutter bees in three contrasting geographical zones. Divers. Distrib. 2018, 24, 621–628. [Google Scholar] [CrossRef]
- Wray, J.C.; Neame, L.A.; Elle, E. Floral resources, body size, and surrounding landscape influence bee community assemblages in oak-savannah fragments. Ecol. Entomol. 2014, 39, 83–93. [Google Scholar] [CrossRef]
- Dar, S.A.; Sofi, M.A.; El-Sharnouby, M.; Hassan, M.; Rashid, R.; Mir, S.H.; Al Naggar, Y.; Salah, M.; Gajger, I.T.; Sayed, S. Nesting behaviour and foraging characteristics of Andrena cineraria (Hymenoptera: Andrenidae). Saudi J. Biol. Sci. 2021, 28, 4147–4154. [Google Scholar] [CrossRef]
- Müller, A.; Westrich, P. Morphological specialization for primary nectar robbing in a pollen specialist mining bee (Hymenoptera, Andrenidae). J. Hymenopt. Res. 2023, 95, 215–230. [Google Scholar] [CrossRef]
- Kowalska, J.; Antkowiak, M.; Sienkiewicz, P. Flower strips and their ecological multifunctionality in agricultural fields. Agriculture 2022, 12, 1470. [Google Scholar] [CrossRef]
- Azpiazu, C.; Medina, P.; Adán, Á.; Sánchez-Ramos, I.; del Estal, P.; Fereres, A.; Viñuela, E. The role of annual flowering plant strips on a melon crop in Central Spain. Influence on pollinators and crop. Insects 2020, 11, 66. [Google Scholar] [CrossRef]
- Fountain, M.T. Impacts of wildflower interventions on beneficial insects in fruit crops: A review. Insects 2022, 13, 304. [Google Scholar] [CrossRef]
- Barda, M.; Karamaouna, F.; Kati, V.; Perdikis, D. Do patches of flowering plants enhance insect pollinators in apple orchards? Insects 2023, 14, 208. [Google Scholar] [CrossRef] [PubMed]
- Parachnowitsch, A.L.; Manson, J.S.; Sletvold, N. Evolutionary ecology of nectar. Ann. Bot. 2019, 123, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Kleijn, D.; Raemakers, I. Ecological society of America. A retrospective analysis of pollen host plant use by stable and declining bumble bee species. Ecol. Soc. Am. 2008, 89, 1811–1823. [Google Scholar] [CrossRef]
- Cole, L.J.; Baddeley, J.A.; Robertson, D.; Topp, G.F.E.; Walker, R.L.; Watson, C.A. Supporting wild pollinators in agricultural landscapes through targeted legume mixtures. Agric. Ecosyst. Environ. 2022, 323, 107648. [Google Scholar] [CrossRef]
- Kudo, G.; Harder, L.D. Floral and inflorescence effects on variation in pollen removal and seed production among six legume species. Funct. Ecol. 2005, 19, 245–254. [Google Scholar] [CrossRef]
- Braby, M.F.; Trueman, W.H. Evolution of larval host plant associations and adaptive radiation in pierid butterflies. J. Evol. Biol. 2006, 19, 1677–1690. [Google Scholar] [CrossRef]
- Reddi, C.S.; Bai, G.M. Butterflies and pollination biology. Proc. Indian Acad. Sci. 1984, 93, 391–396. [Google Scholar] [CrossRef]
- Zhang, H.-H.; Wang, W.-L.; Yu, Q.; Xing, D.-H.; Xu, Z.-B.; Duan, K.; Zhu, J.-Q.; Zhang, X.; Li, Y.-P.; Hu, S.-J. Spatial distribution of pollinating butterflies in Yunnan Province, Southwest China with resources conservation implications. Insects 2020, 11, 525. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, J.; Traveset, A. Effects of flowering phenology and synchrony on the reproductive success of a long-flowering shrub. AoB Plants 2016, 8, plw007. [Google Scholar] [CrossRef]
- Gallagher, M.K.; Campbell, D.R. Pollinator visitation rate and effectiveness vary with flowering phenology. Am. J. Bot. 2020, 107, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Kachhela, H.R.; Pastagia, J.J. Effect of abundance of pollinators on yield parameter of Niger, Guizotia abyssinica (L.f.) Cass. grown at different dates of sowing. J. Entomol. Zool. 2018, 6, 2393–2396. [Google Scholar]
- Weller, J.L.; Ortega, R. Genetic control of flowering time in legumes. Front. Plant Sci. 2015, 6, 207. [Google Scholar] [CrossRef]
- Jung, C.; Müller, A.E. Flowering time control and applications in plant breeding. Trends Plant Sci. 2009, 14, 563–573. [Google Scholar] [CrossRef]
- Ohashi, K.; Yahara, T. Behavioral responses of pollinators to variation in floral display size and their influences on the evolution of floral traits. In Cognitive Ecology of Pollination; Chittka, L., Thomson, J.D., Eds.; Cambridge University Press: Cambridge, UK, 2001; pp. 274–296. [Google Scholar] [CrossRef]
- Chen, M.; Zuo, X.; Zhao, X. Comparative floral characters, pollinator limitation, and pollination success in different habitats of Caragana microphylla Lam. Front. Ecol. Evol. 2020, 8, 170. [Google Scholar] [CrossRef]
- Hernández-Villa, V.; Vibrans, H.; Uscanga-Mortera, E.; Aguirre-Jaimes, A. Floral visitors and pollinator dependence are related to floral display size and plant height in native weeds of central Mexico. Flora 2020, 262, 151505. [Google Scholar] [CrossRef]
- Narbona, E.; del Valle, J.C.; Arista, M.; Buide, M.L.; Ortiz, P.L. Major flower pigments originate different colour signals to pollinators. Front. Ecol. Evol. 2021, 9, 743850. [Google Scholar] [CrossRef]
- Reverté, S.; Retana, J.; Gómez, J.M.; Bosch, J. Pollinators show flower colour preferences but flowers with similar colours do not attract similar pollinators. Ann. Bot. 2016, 118, 249–257. [Google Scholar] [CrossRef]
- Giurfa, M.; Núñez, J.; Chittka, L.; Menzel, R. Colour preferences of flower-naive honeybees. J. Comp. Physiol. A 1995, 177, 247–259. [Google Scholar] [CrossRef]
- Streinzer, M.; Neumayer, J.; Spaethe, J. Flower color as predictor for nectar reward quantity in an Alpine flower community. Front. Ecol. Evol. 2021, 9, 721241. [Google Scholar] [CrossRef]
- Garcia, J.E.; Dyer, A.G.; Burd, M.; Shrestha, M. Flower colour and size signals differ depending on geographical location and altitude region. Plant Biol. 2021, 23, 905–914. [Google Scholar] [CrossRef]
- Bauer, A.A.; Clayton, M.K.; Brunet, J. Floral traits influencing plant attractiveness to three bee species: Consequences for plant reproductive success. Am. J. Bot. 2017, 104, 772–781. [Google Scholar] [CrossRef]
- Minnaar, C.; de Jager, M.L.; Anderson, B. Intraspecific divergence in floral-tube length promotes asymmetric pollen movement and reproductive isolation. New Phytol. 2019, 224, 1160–1170. [Google Scholar] [CrossRef]
- Xiang, G.-J.; Lázaro, A.; Dai, X.-K.; Xia, J.; Yang, C.-F. Pollinator proboscis length plays a key role in floral integration of honeysuckle flowers (Lonicera spp.). Plants 2023, 12, 1629. [Google Scholar] [CrossRef] [PubMed]
- Burns, C.; Villalobos, S.; Vamosi, J.C. When less is more: Visitation by generalist pollinators can have neutral or negative effects on plant reproduction. Front. Ecol. Evol. 2022, 10, 1012809. [Google Scholar] [CrossRef]
- Bommarco, R.; Marini, L.; Vaissière, B.E. Insect pollination enhances seed yield, quality, and market value in oilseed rape. Oecologia 2012, 169, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Stein, K.; Coulibaly, D.; Stenchly, K.; Goetze, D.; Porembski, S.; Lindner, A.; Konaté, S.; Linsenmair, E.K. Bee pollination increases yield quantity and quality of cash crops in Burkina Faso, West Africa. Sci. Rep. 2017, 7, 17691. [Google Scholar] [CrossRef]
- Fohouo, F.-N.T.; Ngakou, A.; Kengni, S. Pollination and yield responses of cowpea (Vigna unguiculata L. Walp.) to the foraging activity of Apis mellifera adansonii (Hymenoptera: Apidae) at Ngaoundéré (Cameroon). Afr. J. Biotechnol. 2009, 8, 1988–1996. [Google Scholar]
- Saxena, K.B.; Kumar, R.V.; Tikle, A.N.; Saxena, M.K.; Gautam, V.S.; Rao, S.K.; Khare, D.K.; Chauhan, Y.S.; Saxena, R.K.; Reddy, B.V.S.; et al. ICPH 2671—The world’s first commercial food legume hybrid. Plant Breed. 2013, 132, 479–485. [Google Scholar] [CrossRef]
- Monasterolo, M.; Musicante, M.L.; Valladares, G.R.; Salvo, A. Soybean crops may benefit from forest pollinators. Agric. Ecosyst. Environ. 2015, 202, 217–222. [Google Scholar] [CrossRef]
- Bishop, J.; Jones, H.E.; O’ Sullivan, D.M.; Potts, S.G. Elevated temperature drives a shift from selfing to outcrossing in the insect-pollinated legume, faba bean (Vicia faba). J. Exp. Bot. 2016, 688, 2055–2063. [Google Scholar] [CrossRef] [PubMed]
- Edmands, S. Between a rock and a hard place: Evaluating the relative risks of inbreeding and outbreeding for conservation and management. Mol. Ecol. 2007, 16, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Liu, H.; Wang, H.; Lu, Z.; Wang, Y.; Mullan, D.; Hamblin, J.; Liu, C. Accelerated generation of selfed pure line plants for gene identification and crop breeding. Front. Plant Sci. 2017, 8, 1786. [Google Scholar] [CrossRef] [PubMed]
- Rutkoski, J.E.; Krause, M.R.; Sorrells, M.E. Breeding methods: Line development. In Wheat Improvement. Food Security in a Changing Climate; Reynolds, M.P., Braun, H.-J., Eds.; Springer: Cham, Switzerland, 2022; pp. 69–82. [Google Scholar]
- Jiménez-López, F.J.; Ortiz, P.L.; Talavera, M.; Arista, M. Reproductive assurance maintains red-flowered plants of Lysimachia arvensis in Mediterranean populations despite inbreeding depression. Front. Plant Sci. 2020, 11, 563110. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).