Population Fluctuations of the Deer Mouse (Peromyscus maniculatus) in Old-Field and Bunchgrass–Sagebrush Habitats: The Role of Agricultural Setting and Optimum Habitat
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Deer Mouse Populations
2.3. Population Data Analyses
2.4. Statistical Analyses
3. Results
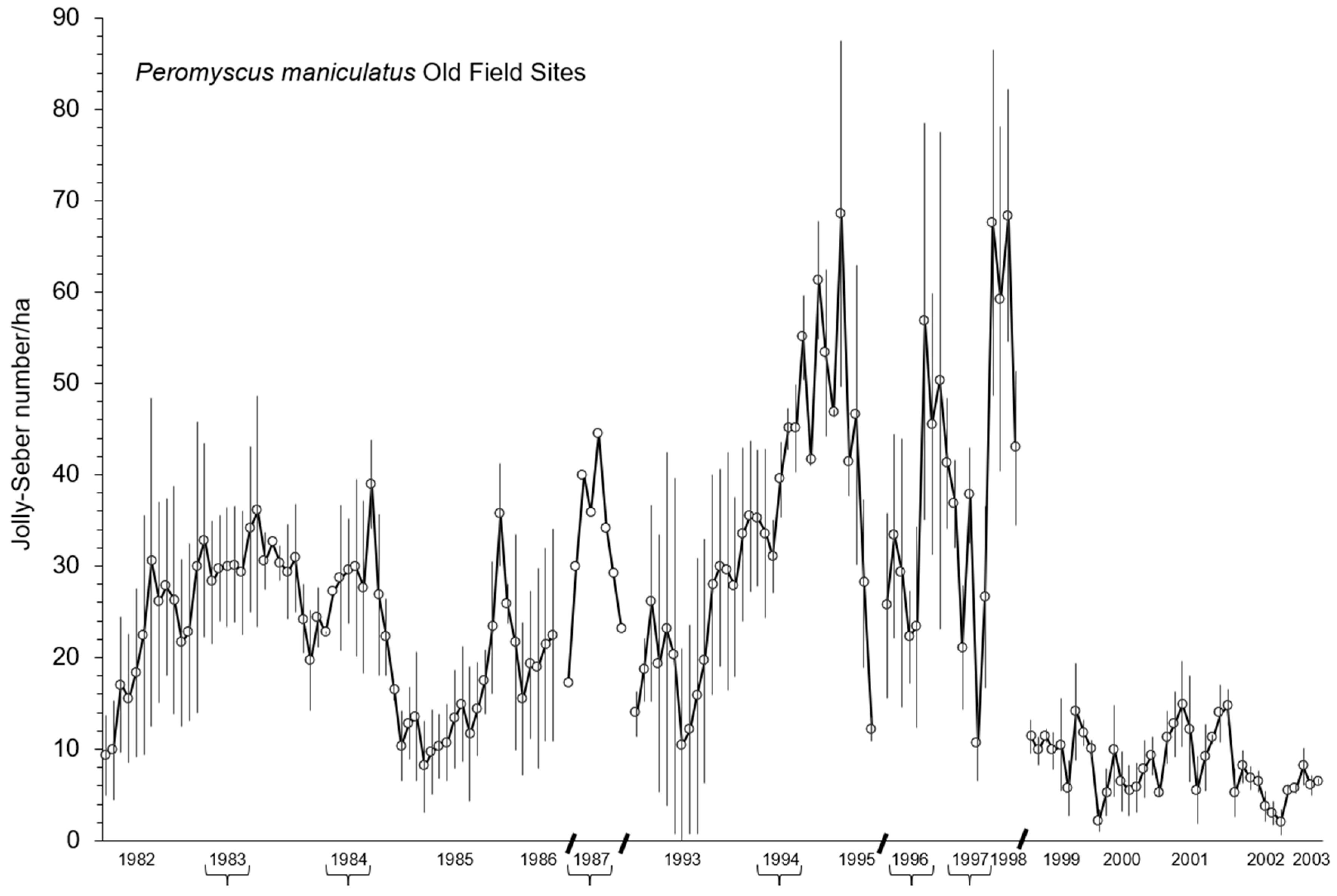
3.1. Population Changes in Old-Field Sites
3.2. Population Changes in Grass–Sagebrush Sites
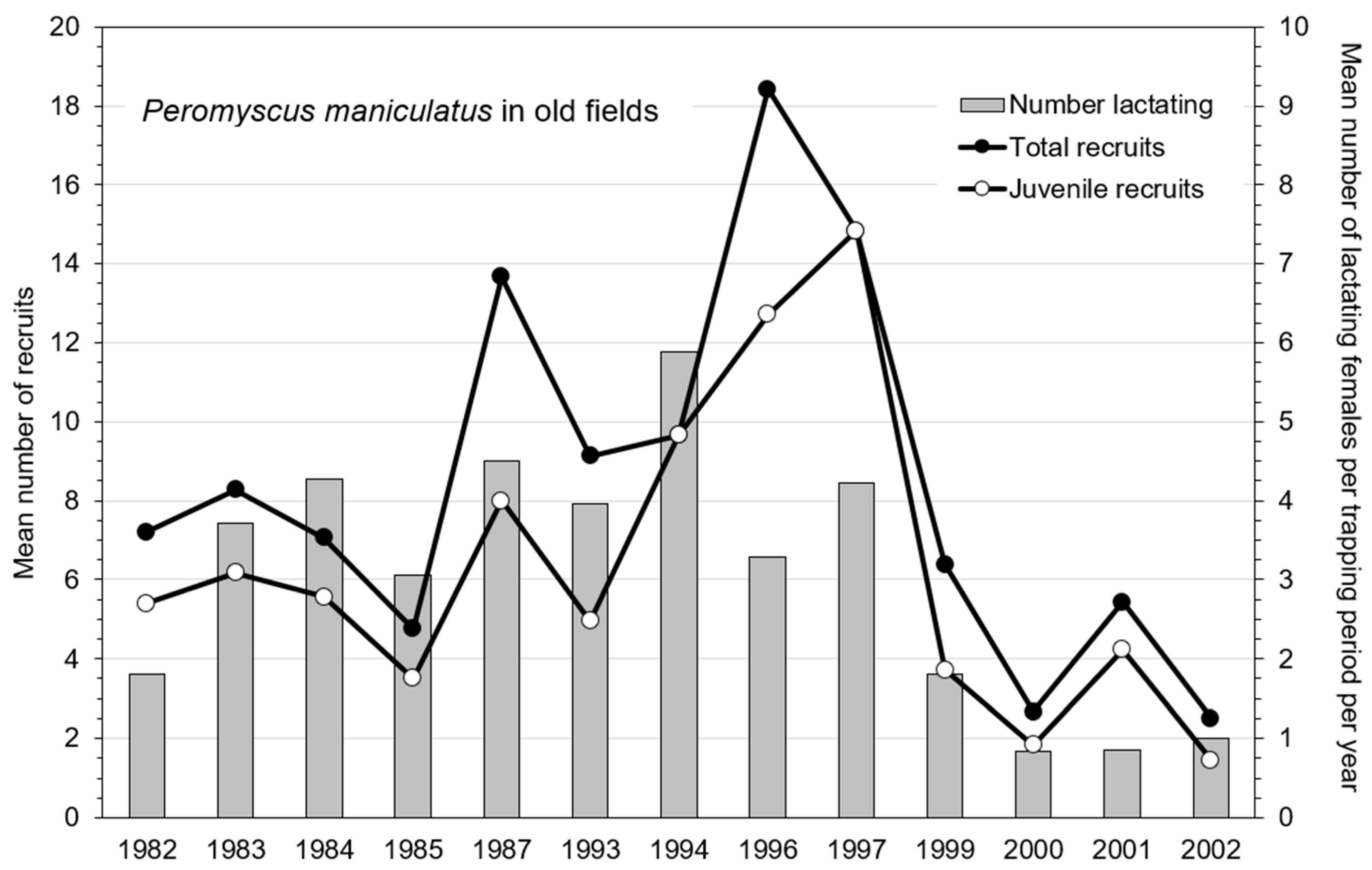
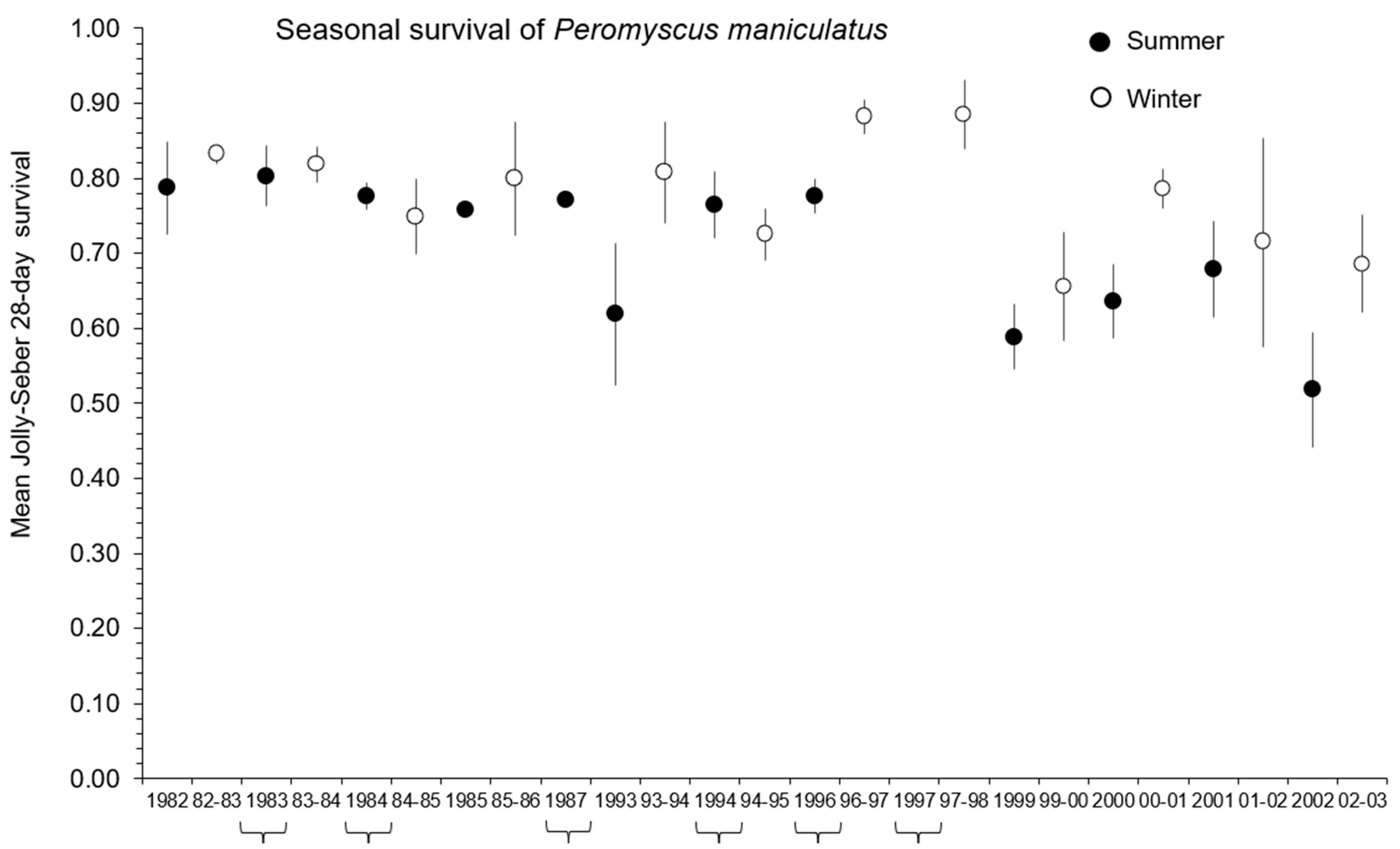
3.3. Demographic Responses in Old-Field Sites
3.4. Demographic Responses in Grass–Sagebrush Sites
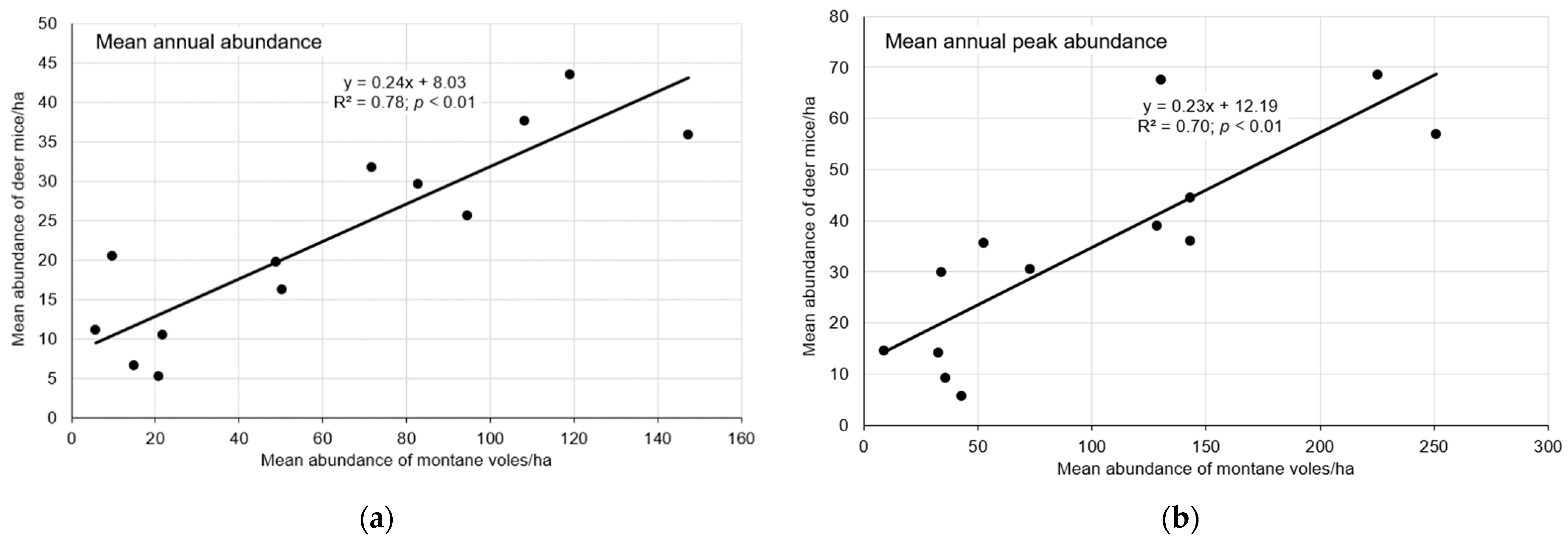
3.5. Association with Montane Voles
4. Discussion
4.1. Multiannual Population Changes
4.2. Spring and Summer Reorganization Events
4.3. Association with Montane Voles
4.4. Role of Agricultural Setting and Optimum Habitat
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harper, W.L.; Lea, E.C.; Maxwell, R.E. Biodiversity Inventory in the South Okanagan. In Our Living Legacy: Proceedings of a Symposium on Biological Diversity; Fenger, M.A., Miller, E.H., Johnson, J.A., Williams, E.J.R., Eds.; Royal British Columbia Museum: Victoria, BC, Canada, 1993. [Google Scholar]
- Fleischner, T.L. Ecological costs of livestock grazing in western North America. Conserv. Biol. 1994, 8, 629–644. [Google Scholar] [CrossRef]
- Pearson, D.E.; Ortega, Y.K.; McKelvey, K.S.; Ruggiero, L.F. Small mammal communities and habitat selection in northern Rocky Mountain bunchgrass: Implications for exotic plant invasions. Northwest Sci. 2001, 75, 107–117. [Google Scholar]
- Sullivan, T.P.; Sullivan, D.S. Plant and small mammal diversity in orchard versus non-crop habitats. Agri. Ecosyst. Environ. 2006, 116, 235–243. [Google Scholar] [CrossRef]
- Baker, R.H. Habitats and distribution. In Biology of Peromyscus (Rodentia); King, J.A., Ed.; Special Publication No. 2; The American Society of Mammalogists: Stillwater, OK, USA, 1968; pp. 98–126. [Google Scholar]
- Banfield, A.W.F. The Mammals of Canada; University of Toronto Press: Toronto, ON, Canada, 1974. [Google Scholar]
- Naughton, D. The Natural History of Canadian Mammals; University of Toronto Press: Toronto, ON, Canada, 2012. [Google Scholar]
- Nagorsen, D.W. Rodents and Lagomorphs of British Columbia; Royal BC Museum Handbook: Victoria, BC, Canada, 2005. [Google Scholar]
- Sieg, C.H.; Uresk, D.W.; Hansen, R.M. Seasonal diets of deer mice on bentonite mine spoils and sagebrush grasslands in Southeastern Montana. Northwest Sci. 1986, 60, 81–89. [Google Scholar]
- Bellocq, M.I.; Smith, S.M. Arthropods preferred as food by Sorex cinereus (masked shrew) and Peromyscus maniculatus (deer mouse): An experimental approach. Mammalia 1994, 53, 391–396. [Google Scholar] [CrossRef]
- Vickery, W.; Daoust, J.L.; El Wartiti, A.; Peletier, J. The effect of energy and protein content on food choice by deer mice Peromyscus maniculatus (Rodentia). Anim. Behav. 1994, 47, 55–64. [Google Scholar] [CrossRef]
- Lackey, J.A.; Huckaby, D.G.; Ormiston, B.G. Peromyscus leucopus. Mamm. Species 1985, 247, 1–10. [Google Scholar]
- Terman, C.R. Population dynamics. In Biology of Peromyscus (Rodentia); King, J.A., Ed.; Special Publication No. 2; The American Society of Mammalogists: Stillwater, OK, USA, 1968; pp. 412–450. [Google Scholar]
- Petticrew, B.G.; Sadleir, R.M.F.S. The ecology of the deer mouse Peromyscus maniculatus in a coastal coniferous forest. I. Population dynamics. Can. J. Zool. 1974, 52, 107–118. [Google Scholar] [CrossRef]
- Grant, P.R. An 11-year study of small mammal populations at Mont St. Hilaire, Quebec. Can. J. Zool. 1976, 54, 2156–2173. [Google Scholar] [CrossRef]
- Fryxell, J.M.; Falls, J.B.; Falls, E.A.; Brooks, R.J. Long-term dynamics of small-mammal populations in Ontario. Ecology 1998, 79, 213–225. [Google Scholar] [CrossRef]
- Lewellyn, T.H.; Vessey, S.H. The effect of density dependence and weather on population size of a polyvoltine species. Ecol. Monogr. 1998, 68, 571–594. [Google Scholar] [CrossRef]
- Merritt, J.F.; Lima, M.; Bozinovc, F. Seasonal regulation in fluctuating small mammal populations: Feedback structure and climate. Oikos 2001, 94, 505–514. [Google Scholar] [CrossRef]
- Sullivan, T.P.; Sullivan, D.S.; Boonstra, R.; Krebs, C.J.; Vyse, A. Population regulation in the deer mouse (Peromyscus maniculatus) in old-growth coniferous forests of southern British Columbia: Insights from a long-term study. Mam. Res. 2023, 68, 37–51. [Google Scholar] [CrossRef]
- Gilbert, B.S.; Krebs, C.J. Population dynamics of Clethrionomys and Peromyscus in southwestern Yukon 1973–1989. Holarctic. Ecol 1991, 14, 250–259. [Google Scholar] [CrossRef]
- Millar., J.S.; McAdam, A.G. Life on the edge: The demography of short-season populations of deer mice. Oikos 2001, 93, 69–76. [Google Scholar] [CrossRef]
- Van Horne, B. Niches of adult and juvenile deer mice (Peromyscus maniculatus) in seral stages of coniferous forest. Ecology 1982, 63, 992–1003. [Google Scholar] [CrossRef]
- Gilbert, B.S.; Krebs, C.J. Competition between Peromyscus maniculatus and other small rodents in the boreal forest of the southern Yukon Territory. Acta Zool. Fenn. 1984, 172, 51–56. [Google Scholar]
- Krebs, C.J.; Boonstra, R.; Kenney, A.J.; Gilbert, B.S. Hares and small rodent cycles: A 45-year perspective on predator-prey dynamics in the Yukon boreal forest. Aust. Zool. 2018, 39, 724–732. [Google Scholar] [CrossRef]
- Krebs, C.J.; Boonstra, R.; Gilbert, B.S.; Kenney, A.J.; Boutin, S. Impact of climate change on the small mammal community of the Yukon boreal forest. Integr. Zool. 2019, 14, 528–541. [Google Scholar] [CrossRef]
- Gashwiler, J.S. Tree seed abundance vs. deer mouse populations in Douglas-Fir clearcuts. In Division of Forest-Wildlife Management; Society of American Foresters: Detroit, MI, USA, 1965; pp. 219–222. [Google Scholar]
- Gashwiler, J.S. Deer mouse reproduction and its relationship to the tree seed crop. Amer. Midl. Nat. 1979, 102, 95–104. [Google Scholar] [CrossRef]
- Wolff, J.O. Population fluctuations of mast-eating rodents are correlated with acorn production. J. Mammal. 1996, 77, 850–856. [Google Scholar] [CrossRef]
- McCracken, K.E.; Witham, J.W.; Hunter, M.L., Jr. Relationships between seed fall of three tree species and Peromyscus leucopus and Clethriononmys gapperi during 10 years in an oak-pine forest. J. Mammal. 1999, 80, 1288–1296. [Google Scholar] [CrossRef]
- Elias, S.P.; Witham, J.W.; Hunter, M.L. Peromyscus leucopus abundance and acorn mast: Population fluctuation patterns over 20 years. J. Mammal. 2004, 85, 743–747. [Google Scholar] [CrossRef]
- Falls, J.B.; Falls, E.A.; Fryxell, J.M. Fluctuations of deer mice in Ontario in relation to seed crops. Ecol. Monogr. 2007, 77, 19–32. [Google Scholar] [CrossRef]
- Lobo, N.; Millar, J.S. Indirect and mitigated effects of pulsed resources on the population dynamics of a northern rodent. J. Animal Ecol. 2013, 82, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, T.P.; Sullivan, D.S. Long-term demographic changes in deer mouse (Peromyscus maniculatus) populations in a forested landscape with cumulative clearcutting. For. Ecol. Manag 2022, 517, 120255. [Google Scholar] [CrossRef]
- Vessey, S.H. Long-term population trends in white-footed mice and the impact of supplemental food and shelter. Am. Zool. 1987, 27, 879–890. [Google Scholar] [CrossRef]
- Brady, M.J.; Slade, N.A. Long-term dynamics of a grassland rodent community. J. Mammal. 2004, 85, 552–561. [Google Scholar] [CrossRef]
- Drost, C.A.; Fellers, G.M. Density cycles in an island population of deer mice, Peromyscus maniculatus. Oikos 1991, 60, 351–364. [Google Scholar] [CrossRef]
- Boonstra, R.; Krebs, C.J. Population dynamics of red-backed voles (Myodes) in North America. Oecologia 2012, 168, 601–620. [Google Scholar] [CrossRef]
- Sadleir, R.M.F.S. The relationship between agonistic behaviour and population changes in the deer mouse Peromyscus maniculatus (Wagner). J. Animal. Ecol 1965, 34, 331–352. [Google Scholar] [CrossRef]
- Fairbairn, D.J. The spring decline in deer mice: Death or dispersal? Can. J. Zool. 1977, 55, 84–92. [Google Scholar] [CrossRef]
- Galindo, C.; Krebs, C.J. Population regulation in deer mice: The role of females. J. Animal. Ecol. 1987, 56, 11–23. [Google Scholar] [CrossRef]
- Redfield, J.A.; Krebs, C.J.; Taitt, M.J. Competition between Peromyscus maniculatus and Microtus townsendii in grasslands of coastal British Columbia. J. Animal. Ecol. 1977, 46, 607–616. [Google Scholar] [CrossRef]
- Dueser, R.D.; Wilson, M.L.; Rose, R.K. Attributes of dispersing meadow voles in open-grid populations. Acta Theriol. 1981, 26, 139–162. [Google Scholar] [CrossRef]
- Schweiger, E.W.; Diffendorfer, J.E.; Holt, R.D.; Pierotti, R.; Gaines, M.S. The interaction of habitat fragmentation, plant, and small mammal succession in an old field. Ecol. Monogr. 2000, 70, 383–400. [Google Scholar] [CrossRef]
- Manson, R.H.; Ostfeld, R.S.; Canham, C.D. Long-term effects of rodent herbivores on tree invasion dynamics along forest-field edges. Ecology 2001, 82, 3320–3329. [Google Scholar]
- Grant, P.R. Experimental studies of competitive interaction in a two species system. III. Microtus and Peromyscus species in enclosures. J. Anim. Ecol. 1971, 40, 323–350. [Google Scholar] [CrossRef]
- Abramsky, Z.; Dyer, M.I.; Harrison, P.D. Competition among small mammals in experimentally perturbed areas of shortgrass prairie. Ecology 1979, 60, 530–536. [Google Scholar] [CrossRef]
- Hallett, J.G.; O’Connell, M.A.; Honeycutt, R.L. Competition and habitat selection: Test of a theory using small mammals. Oikos 1983, 40, 175–181. [Google Scholar] [CrossRef]
- Swihart, R.K.; Slade, N.K. Long-term dynamics of an early successional small mammal community. Am. Midl. Nat. 1990, 123, 372–382. [Google Scholar] [CrossRef]
- Sullivan, T.P.; Sullivan, D.S.; Hogue, E.J.; Lautenschlager, R.A.; Wagner, R.G. Population dynamics of small mammals in relation to vegetation management in orchard agroecosystems: Compensatory responses in abundance and biomass. Crop Prot. 1998, 17, 1–11. [Google Scholar] [CrossRef]
- Manson, R.H.; Ostfeld, R.S.; Canham, C.D. Responses of a small mammal community to heterogeneity along forest-old-field edges. Landsc. Ecol. 1999, 14, 355–367. [Google Scholar] [CrossRef]
- Galindo, C.; Krebs, C.J. Habitat use and abundance of deer mice: Interactions with meadow voles and red-backed voles. Can. J. Zool. 1985, 63, 1870–1879. [Google Scholar] [CrossRef]
- Wolff, J.O.; Dueser, R.D. Noncompetitive coexistence between Peromyscus species and Clethrionomys gapperi. Can. Field Nat. 1986, 100, 186–191. [Google Scholar]
- Sullivan, T.P.; Sullivan, D.S.; Boonstra, R.; Krebs, C.J. Population changes and limitation in the montane vole (Microtus montanus) in perennial old-field grasslands: Insights from a long-term study. J. Mammal. 2021, 102, 404–415. [Google Scholar] [CrossRef]
- Meidinger, D.; Pojar, J. Ecosystems of British Columbia; Special Report Series No 6; Research Branch Ministry of Forests: Victoria, BC, Canada, 1991.
- Hurlbert, S.H. Pseudoreplication and the design of ecological field experiments. Ecol. Monogr. 1984, 54, 187–211. [Google Scholar] [CrossRef]
- Merritt, J.F.; Merritt, J.M. Seasonal home ranges and activity of small mammals of a Colorado subalpine forest. Acta Theriol. 1978, 23, 195–202. [Google Scholar] [CrossRef]
- Krebs, C.J.; Keller, B.L.; Tamarin, R.H. Microtus population biology: Demographic changes in fluctuating populations of M. ochrogaster and M. pennsylvanicus in southern Indiana. Ecology 1969, 50, 587–607. [Google Scholar] [CrossRef]
- Sikes, R.S.; Gannon, W.L. The Animal Care and Use Committee of the American Society of Mammalogists. Guidelines of the American Society of Mammalogists for the use of wild mammals in research. J. Mammal. 2016, 97, 663–688. [Google Scholar] [CrossRef]
- Seber, G.A.F. The Estimation of Animal Abundance and Related Parameters, 2nd ed.; Charles Griffin and Co. Ltd.: London, UK, 1982. [Google Scholar]
- Krebs, C.J. Ecological Methodology; Addison Wesley Longman, Inc.: Menlo Park, CA, USA, 1999. [Google Scholar]
- Krebs, C.J.; Boonstra, R.; Gilbert, S.; Reid, D.; Kenney, A.J.; Hofer, E.J. Density estimation for small mammals from livetrapping grids: Rodents in northern Canada. J. Mammal. 2011, 92, 974–981. [Google Scholar] [CrossRef]
- Krebs, C.J.; Boonstra, R. Trappability estimates for mark-recapture data. Can. J. Zool. 1984, 62, 2440–2444. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis; Prentice-Hall, Inc.: Englewood Cliffs, NJ, USA, 1999; 663p. [Google Scholar]
- Taitt, M.J.; Krebs, C.J. Population dynamics and cycles. In Biology of New World Microtus; Tamarin, R.H., Ed.; Special Publication No. 8; The American Society of Mammalogists: Stillwater, OK, USA, 1985; pp. 567–620. [Google Scholar]
- Terman, C.R. Population fluctuations of Peromyscus maniculatus and other small mammals as revealed by the North American census of small mammals. Am. Midl. Nat. 1966, 76, 419–426. [Google Scholar] [CrossRef]
- Sullivan, T.P.; Krebs, C.J. An irruption of deer mice after logging of coastal coniferous forest. Can. J. For. Res. 1981, 11, 586–592. [Google Scholar] [CrossRef]
- Sullivan, T.P.; Lautenschlager, R.A.; Wagner, R.G. Clear-cutting and burning of northern spruce-fir forests: Implications for small mammal communities. J. Appl. Ecol. 1999, 36, 327–344. [Google Scholar] [CrossRef]
- Boonstra, R. Effect of adult Townsend voles on survival of young. Ecology 1978, 59, 242–248. [Google Scholar] [CrossRef]
- Boonstra, R. Demography of Microtus pennsylvanicus in southern Ontario: Enumeration versus Jolly-Seber compared. Can. J. Zool. 1985, 63, 1174–1180. [Google Scholar] [CrossRef]
- Krebs, C.J.; Boonstra, R. Demography of the spring decline in populations of the vole Microtus townsendii. J. Anim. Ecol. 1978, 47, 1007–1015. [Google Scholar] [CrossRef]
- Rintamaa, D.L.; Mazur, P.A.; Vessey, S.H. Reproduction during two annual cycles in a population of Peromyscus leucopus noveboracensis. J. Mammal. 1976, 57, 593–595. [Google Scholar] [CrossRef]
- Wolff, J.O. Comparative population ecology of Peromyscus leucopus and Peromyscus maniculatus. Can. J. Zool. 1985, 63, 1548–1555. [Google Scholar] [CrossRef]
- Crowell, K.L. Islands—Insight or artifact? Population dynamics of habitat utilization in insular rodents. Oikos 1983, 41, 442–454. [Google Scholar] [CrossRef]
- Dueser, R.D.; Porter, J.H. Habitat use by insular small mammals: Relative effects of competition and habitat structure. Ecology 1986, 67, 195–201. [Google Scholar] [CrossRef]
- Sullivan, T.P. Demography and dispersal in island and mainland populations of the deer mouse, Peromyscus maniculatus. Ecology 1977, 58, 964–978. [Google Scholar] [CrossRef]
- Tamarin, R.H.; Reich, L.M.; Moyer, C.A. Meadow vole cycles within fences. Can. J. Zool. 1984, 62, 1796–1804. [Google Scholar] [CrossRef]
- Sullivan, T.P.; Sullivan, D.S.; Thistlewood, H.M. Abundance and diversity of small mammals in response to various linear habitats in semi-arid agricultural landscapes. J. Arid Environ. 2012, 83, 54–61. [Google Scholar] [CrossRef]
- Grant, W.E.; Birney, E.C.; French, N.R.; Swift, D.M. Structure and reproductivity of grassland small mammal communities related to grazing-induced changes in vegetative cover. J. Mammal. 1982, 63, 248–260. [Google Scholar] [CrossRef]
- MacCracken, J.G.; Uresk, D.W.; Hansen, R.M. Rodent-vegetation relationships in southeastern Montana. Northwest Sci. 1985, 59, 272–278. [Google Scholar]
- Hales, G.S. Small Mammal Communities in Two Grassland Ecosystems in British Columbia, Canada. Master’s Thesis, Thompson Rivers University, Kamloops, BC, Canada, 2011. [Google Scholar]
- Randall, J.A.; Johnson, R.E. Population densities and habitat occupancy by Microtus longicaudus and M. montanus. J. Mammal. 1979, 60, 217–219. [Google Scholar] [CrossRef]
- Belk, M.C.; Smith, H.D.; Lawson, J. Use and partitioning of montane habitat by small mammals. J. Mammal. 1988, 69, 688–695. [Google Scholar] [CrossRef]
- Millar, J.S.; Innes, G.L.; Loewen, V.A. Habitat use by non-hibernating small mammals of the Kananaskis Valley, Alberta. Can. Field-Nat. 1985, 99, 196–204. [Google Scholar]
- Sullivan, T.P.; Sullivan, D.S. Population dynamics of the heather vole (Phenacomys intermedius) in commercial forest landscapes of south-central British Columbia. Canada. J. Mammal. 2021, 102, 1186–1201. [Google Scholar] [CrossRef]
- Pearson, D.E.; McKelvey, K.S.; Ruggiero, L.F. Non-target effects of an introduced biological control agent on deer mouse ecology. Oecologia 2000, 122, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Sera, W.E.; Early, C.N. Microtus montanus. Mamm. Species 2003, 716, 1–10. [Google Scholar] [CrossRef]
- Kothe, S.; Bakanov, N.; Bruhl, C.A.; Gemeinholzer, B.; Hörren, T.; Mühlethaler, R.; Sorg, M.; Sumser, H.; Swenson, S.J.; Lehmann, G.U. Negative spill-over effects of agricultural practices on plant species conservation in nature reserves. Ecol. Indic. 2023, 149, 110170. [Google Scholar] [CrossRef]
- Sala, O.E.; Parton, W.J.; Joyce, L.A.; Lauenroth, W.K. Primary production of the central grasslands region of the United States. Ecology 1988, 69, 40–45. [Google Scholar] [CrossRef]
- Lauenroth, W.K.; Sala, O.E. Long-term forage production of North American shortgrass steppe. Ecol. Appl. 1992, 2, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Shenbrot, G.; Krasnov, B.; Burdelov, S. Long-term study of population dynamics and habitat selection of rodents in the Negev Desert. J. Mammal. 2010, 91, 776–786. [Google Scholar] [CrossRef]
- Thibault, K.M.; Ernest, S.K.M.; White, E.P.; Brown, J.H.; Goheen, J.R. Long-term insights into the influence of precipitation on community dynamics in desert rodents. J. Mammal. 2010, 91, 787–797. [Google Scholar] [CrossRef]
- Reed, A.W.; Kaufman, G.A.; Sandercock, B.K. Demographic response of a grassland rodent to environmental variability. J. Mammal. 2007, 88, 982–988. [Google Scholar] [CrossRef]
- IPCC. IPCC Fifth Assessment Report: Climate Change 2013: The Physical Science Basis. In 2014, Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar] [CrossRef]






| Parameter | High Years (6) | Low Years (7) | Overall (13) |
|---|---|---|---|
| Annual peak abundance/ha * | 52.1 ± 5.8 | 19.9 ± 4.5 | 34.8 ± 5.8 |
| Annual abundance/ha * | 33.9 ± 2.6 | 12.8 ± 2.3 | 22.6 ± 3.5 |
| Annual low abundance/ha * | 18.3 ± 2.8 | 6.7 ± 1.1 | 12.0 ± 2.2 |
| Mean abundance | |||
| Spring season decline (%) | 20.5 ± 8.3 (5) | 16.7 ± 6.0 (8) | 18.2 ± 4.7 (13) |
| Summer decline (%) | 13.7± 4.4 (5) | 34.3 ± 7.0 (7) | 25.7 ± 5.3 (12) |
| Breeding cessation midsummer (%) | 77.7 (9) | 61.1 (18) | 66.7 (27) |
| Breeding season (weeks) | 25.5 ± 0.5 (4) | 23.0 ± 2.0 (6) | 24.0 ± 1.3 (10) |
| Number of lactating females * | 4.3 ± 0.4 | 1.9 ± 0.5 | 3.0 ± 0.4 |
| Total recruits | 12.0 ± 2.1 | 5.6 ± 0.9 | 8.5 ± 1.4 |
| Juvenile recruits | 8.0 ± 1.5 | 3.6 ± 0.5 | 5.6 ± 0.9 |
| Index of juvenile productivity | 2.29 ± 0.29 | 2.66 ± 0.25 | 2.49 ± 0.19 |
| Proportion of young-of-year breeding | |||
| Males | 0.41 ± 0.07 | 0.59 ± 0.06 | 0.51 ± 0.05 |
| Females | 0.14 ± 0.02 | 0.29 ± 0.04 | 0.22 ± 0.03 |
| Summer J–S survival * (rate per 28 days) | 0.78 ± 0.01 | 0.66 ± 0.03 | 0.71 ± 0.03 |
| Winter J–S survival (rate per 28 days) | 0.81 ± 0.03 | 0.75 ± 0.03 | 0.78 ± 0.02 |
| Number of years | n = 6 | n = 7 | n = 13 |
| Parameter | High Years (3) | Low Years (6) | Overall (9) |
|---|---|---|---|
| Annual peak abundance/ha | 32.0 ± 2.3 | 21.7 ± 3.9 | 25.2 ± 3.1 |
| Annual abundance/ha * | 22.8 ± 0.5 | 10.8 ± 1.7 | 14.8 ± 2.3 |
| Annual low abundance/ha | 10.3 ± 3.3 | 4.4 ± 1.5 | 6.4 ± 1.7 |
| Mean abundance | |||
| Spring season decline (%) | 2.3 ± 2.3 (2) | 13.2 ± 5.0 (7) | 11.1 ± 4.2 (9) |
| Summer decline (%) | 33.4± 9.7 (3) | 55.5 ± 12.0 (6) | 48.2 ± 9.0 (9) |
| Breeding cessation midsummer (%) | 0.0 (5) | 85.7 (14) | 63.2 (19) |
| Breeding season (weeks) | 25.5 ± 0.5 (4) | 23.0 ± 2.0 (6) | 24.0 ± 1.3 (10) |
| Number of lactating females * | 3.5 ± 0.3 | 1.4 ± 0.3 | 2.1 ± 0.4 |
| Total recruits | 8.2 ± 2.3 | 2.9 ± 0.3 | 4.7 ± 1.1 |
| Juvenile recruits | 5.6 ± 1.2 | 1.9 ± 0.3 | 3.2 ± 0.7 |
| Index of juvenile productivity | 1.31 ± 0.17 | 0.95 ± 0.27 | 1.07 ± 0.19 |
| Proportion of young-of-year breeding | |||
| Males | 0.72 ± 0.13 | 0.55 ± 0.12 | 0.61 ± 0.09 |
| Females | 0.10 ± 0.05 | 0.29 ± 0.08 | 0.23 ± 0.06 |
| Summer J–S survival (rate per 28 days) | 0.80 ± 0.02 | 0.69 ± 0.05 | 0.73 ± 0.04 |
| Winter J–S survival (rate per 28 days) | 0.82 ± 0.07 | 0.84 ± 0.02 | 0.83 ± 0.02 |
| Number of years | n = 3 | n = 6 | n = 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sullivan, T.P.; Sullivan, D.S. Population Fluctuations of the Deer Mouse (Peromyscus maniculatus) in Old-Field and Bunchgrass–Sagebrush Habitats: The Role of Agricultural Setting and Optimum Habitat. Ecologies 2023, 4, 406-425. https://doi.org/10.3390/ecologies4020026
Sullivan TP, Sullivan DS. Population Fluctuations of the Deer Mouse (Peromyscus maniculatus) in Old-Field and Bunchgrass–Sagebrush Habitats: The Role of Agricultural Setting and Optimum Habitat. Ecologies. 2023; 4(2):406-425. https://doi.org/10.3390/ecologies4020026
Chicago/Turabian StyleSullivan, Thomas P., and Druscilla S. Sullivan. 2023. "Population Fluctuations of the Deer Mouse (Peromyscus maniculatus) in Old-Field and Bunchgrass–Sagebrush Habitats: The Role of Agricultural Setting and Optimum Habitat" Ecologies 4, no. 2: 406-425. https://doi.org/10.3390/ecologies4020026
APA StyleSullivan, T. P., & Sullivan, D. S. (2023). Population Fluctuations of the Deer Mouse (Peromyscus maniculatus) in Old-Field and Bunchgrass–Sagebrush Habitats: The Role of Agricultural Setting and Optimum Habitat. Ecologies, 4(2), 406-425. https://doi.org/10.3390/ecologies4020026





